tasiR-ARFs Production and Target Regulation during In Vitro Maize Plant Regeneration
Abstract
1. Introduction
2. Results
2.1. Maize TAS3 and tasiR-ARF Components Gene Expression
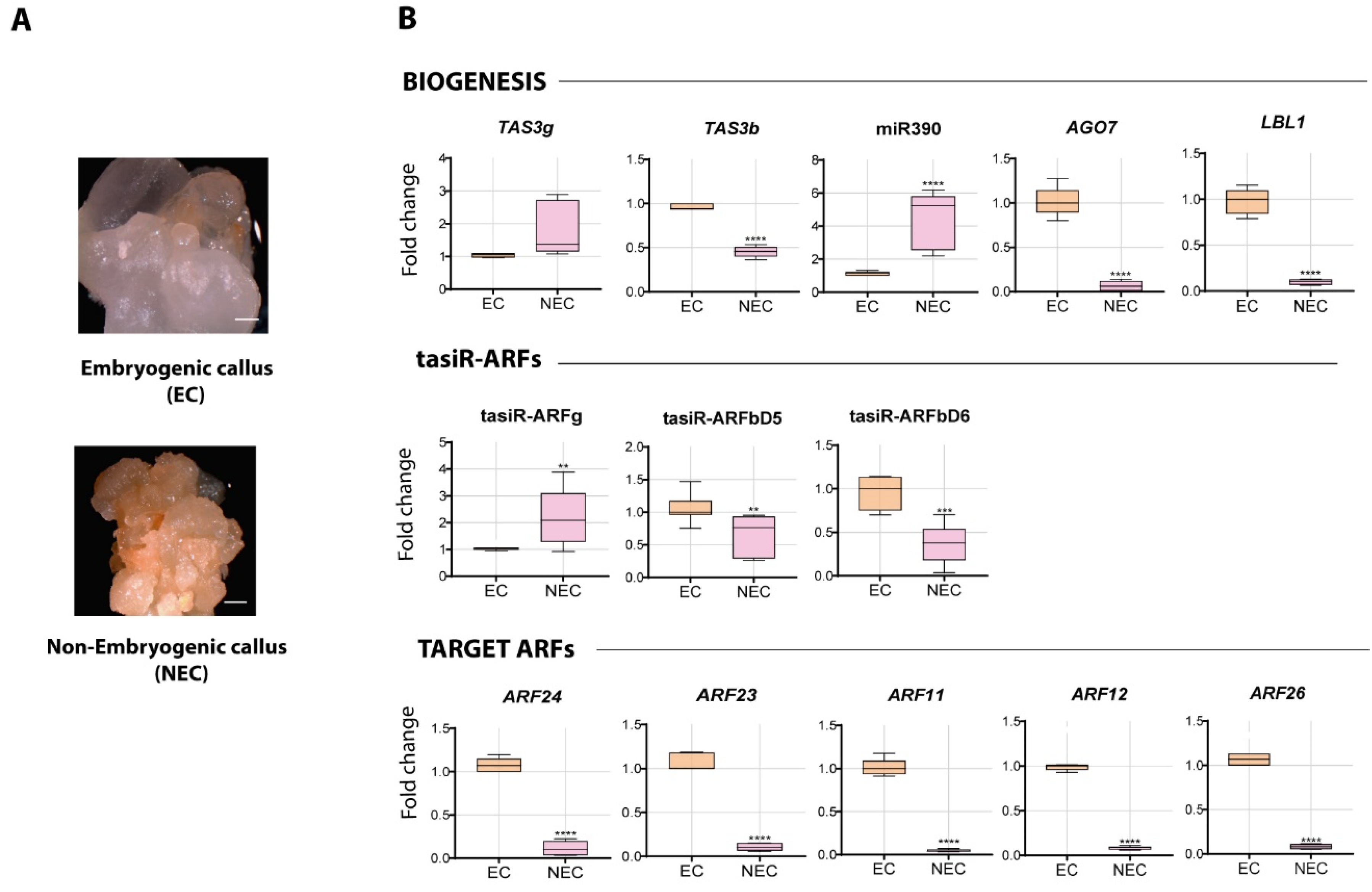
2.2. Differential Abundance of tasiR-ARF Pathway Components in EC and NEC Calli
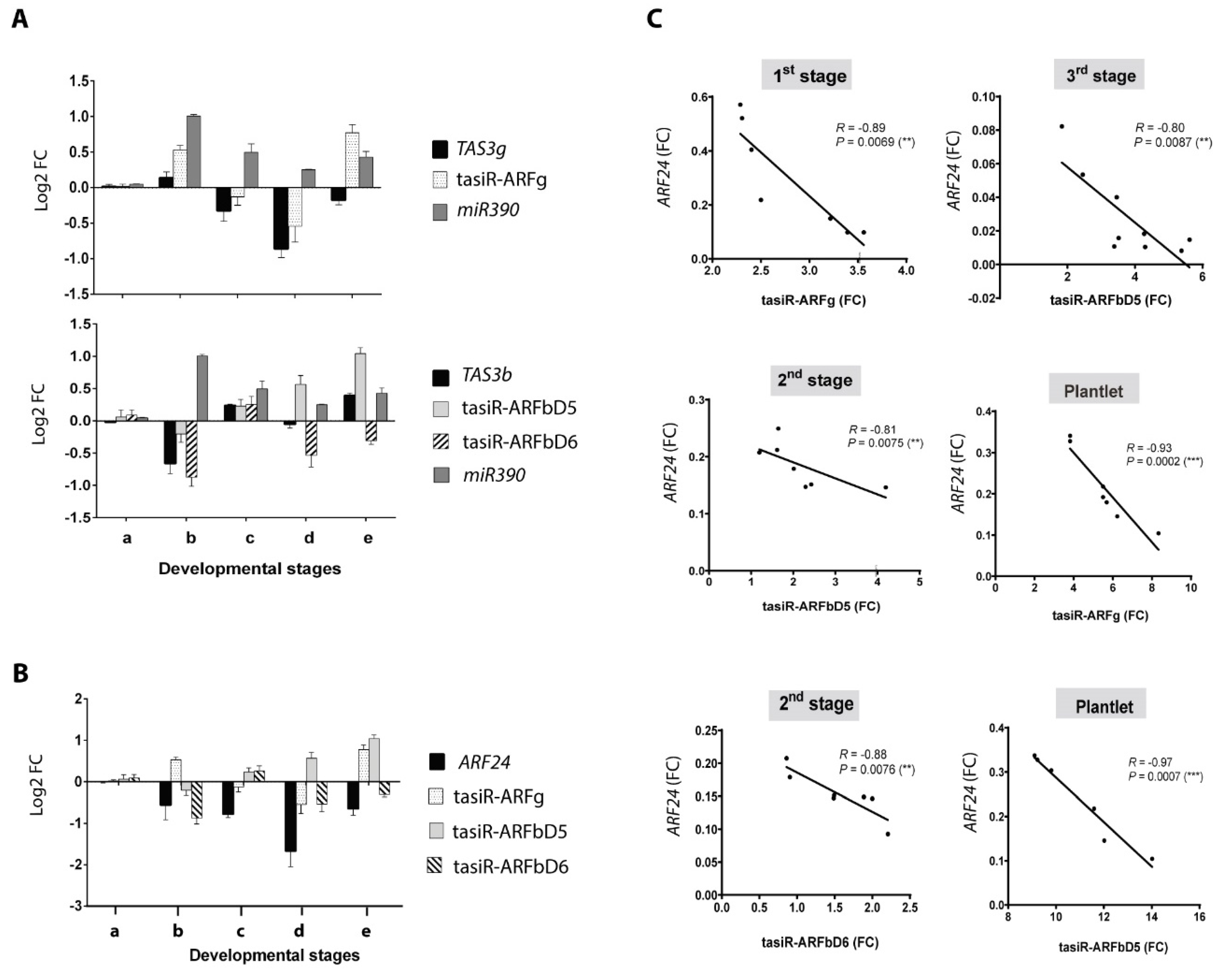
2.3. Readjustment of tasiR-ARF Pathway Component Accumulation Patterns During Plant Regeneration
2.4. Immunolocalization of Auxin
2.5. Immunolocalization of Cytokinin
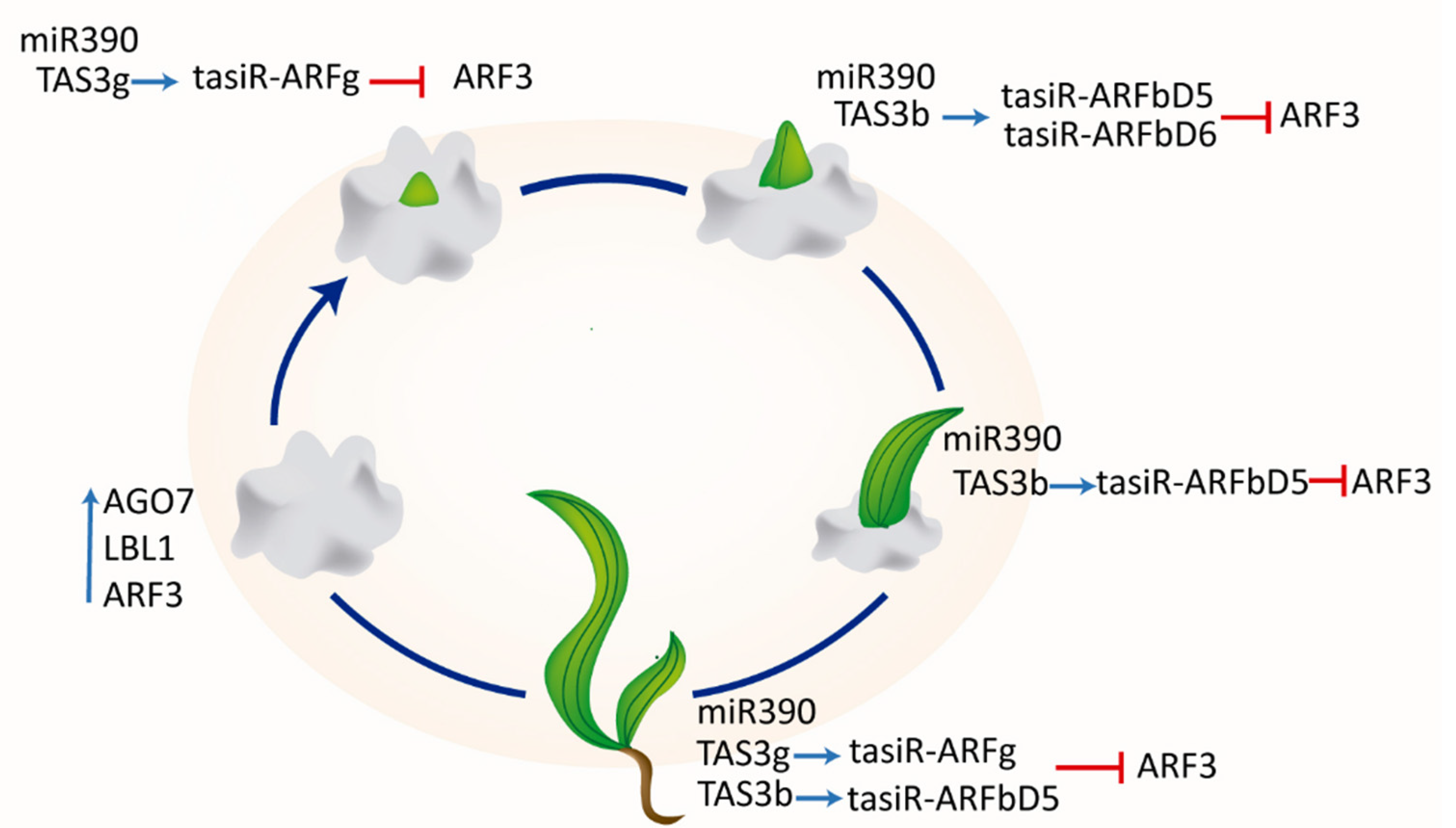
3. Discussion
4. Materials and Methods
4.1. Plant Materials, Culture Conditions and Sample Collection
4.2. RNA Isolation
4.3. qRT-PCR
4.4. Auxin and Cytokinin Immunolocalization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, C.E.; Phillips, R.L. Plant Regeneration from Tissue Cultures of Maize. Crop Sci. 1975. [Google Scholar] [CrossRef]
- Bronsema, F.B.F.; van Oostveen, W.J.F.; van Lammeren, A.A.M. Comparative analysis of callus formation and regeneration on cultured immature maize embryos of the inbred lines A188 and A632. Plant Cell Tissue Organ Cult. 1997, 50, 57–65. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular Mechanisms of Plant Regeneration. Annu. Rev. Plant Biol. 2019, 70, 377–406. [Google Scholar] [CrossRef]
- Márquez-Sánchez, F. From Creole Corn Varieties (Zea mays L.), to Transgenic Hybrids. I: Germplasm Collection and Improved Cultivars. Agric. Soc. Desarro. 2008, 5, 152–166. [Google Scholar]
- Garrocho-Villegas, V.; de Jesús-Olivera, M.T.; Quintanar, E.S. Maize Somatic Embryogenesis: Recent Features to Improve Plant Regeneration. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA, 2012; Volume 877, pp. 173–182. ISBN 978-1-61779-817-7. [Google Scholar]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Huang, X.; Hu, H.; Zhang, Y.; Li, Z.; Zou, C.; Peng, H.; Li, L.; Gao, S.; Pan, G.; et al. Endogenous small interfering RNAs associated with maize embryonic callus formation. PLoS ONE 2017, 12, e0180567. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Hernández, E.C.; Alejandri-Ramírez, N.D.; Juárez-González, V.T.; Dinkova, T.D. Maize miRNA and target regulation in response to hormone depletion and light exposure during somatic embryogenesis. Front. Plant Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J. Classification and Comparison of Small RNAs from Plants. Annu. Rev. Plant Biol. 2013, 64, 137–159. [Google Scholar] [CrossRef]
- Allen, E.; Xie, Z.; Gustafson, A.M.; Carrington, J.C. microRNA-Directed Phasing during Trans-Acting siRNA Biogenesis in Plants. Cell 2005, 121, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef]
- Nogueira, F.T.S.; Chitwood, D.H.; Madi, S.; Ohtsu, K.; Schnable, P.S.; Scanlon, M.J.; Timmermans, M.C.P. Regulation of small RNA accumulation in the maize shoot apex. PLoS Genet. 2009. [Google Scholar] [CrossRef] [PubMed]
- Adenot, X.; Elmayan, T.; Lauressergues, D.; Boutet, S.; Bouché, N.; Gasciolli, V.; Vaucheret, H. DRB4-Dependent TAS3 trans-Acting siRNAs Control Leaf Morphology through AGO7. Curr. Biol. 2006, 16, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Axtell, M.J.; Jan, C.; Rajagopalan, R.; Bartel, D.P. A Two-Hit Trigger for siRNA Biogenesis in Plants. Cell 2006, 127, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, D.; Carbonell, A.; Wang, A.; Lee, A.; Tun, V.; Wang, Z.; Carrington, J.C.; Chang, C.A.; Jin, H. ARGONAUTE PIWI domain and microRNA duplex structure regulate small RNA sorting in Arabidopsis. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Montgomery, T.A.; Howell, M.D.; Cuperus, J.T.; Li, D.; Hansen, J.E.; Alexander, A.L.; Chapman, E.J.; Fahlgren, N.; Allen, E.; Carrington, J.C. Specificity of ARGONAUTE7-miR390 Interaction and Dual Functionality in TAS3 Trans-Acting siRNA Formation. Cell 2008, 133, 128–141. [Google Scholar] [CrossRef]
- Allen, E.; Howell, M.D. miRNAs in the biogenesis of trans-acting siRNAs in higher plants. Semin. Cell Dev. Biol. 2010, 21, 798–804. [Google Scholar] [CrossRef]
- de Felippes, F.F.; Marchais, A.; Sarazin, A.; Oberlin, S.; Voinnet, O. A single miR390 targeting event is sufficient for triggering TAS3-tasiRNA biogenesis in Arabidopsis. Nucleic Acids Res. 2017, 45, 5539–5554. [Google Scholar] [CrossRef]
- Xia, R.; Xu, J.; Meyers, B.C. The Emergence, Evolution, and Diversification of the miR390- TAS3- ARF Pathway in Land Plants. Plant Cell 2017, 29, 1232–1247. [Google Scholar] [CrossRef]
- Dotto, M.C.; Petsch, K.A.; Aukerman, M.J.; Beatty, M.; Hammell, M.; Timmermans, M.C.P. Genome-Wide Analysis of leafbladeless1-Regulated and Phased Small RNAs Underscores the Importance of the TAS3 ta-siRNA Pathway to Maize Development. PLoS Genet. 2014, 10, e1004826. [Google Scholar] [CrossRef]
- Kuhlemeier, C.; Timmermans, M.C.P. The Sussex signal: Insights into leaf dorsiventrality. Development 2016, 143, 3230–3237. [Google Scholar] [CrossRef]
- Hunter, C.; Sun, H.; Poethig, R.S. The Arabidopsis Heterochronic Gene ZIPPY Is an ARGONAUTE Family Member. Curr. Biol. 2003, 13, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Jouannet, V.; Herz, A.; Lokerse, A.S.; Weijers, D.; Vaucheret, H.; Nussaume, L.; Crespi, M.D.; Maizel, A. miR390, Arabidopsis TAS3 tasiRNAs, and Their AUXIN RESPONSE FACTOR Targets Define an Autoregulatory Network Quantitatively Regulating Lateral Root Growth. Plant Cell 2010, 22, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Henderson, D.C.; Zhang, X.; Brooks, L.; Scanlon, M.J. RAGGED SEEDLING2 is required for expression of KANADI2 and REVOLUTA homologues in the maize shoot apex. Genesis 2006, 44, 372–382. [Google Scholar] [CrossRef]
- Sarkar Das, S.; Yadav, S.; Singh, A.; Gautam, V.; Sarkar, A.K.; Nandi, A.K.; Karmakar, P.; Majee, M.; Sanan-Mishra, N. Expression dynamics of miRNAs and their targets in seed germination conditions reveals miRNA-ta-siRNA crosstalk as regulator of seed germination. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Matsui, A.; Mizunashi, K.; Tanaka, M.; Kaminuma, E.; Nguyen, A.H.; Nakajima, M.; Kim, J.-M.; Nguyen, D.V.; Toyoda, T.; Seki, M. tasiRNA-ARF Pathway Moderates Floral Architecture in Arabidopsis Plants Subjected to Drought Stress. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lin, L.; Lai, R.; Liu, W.; Chen, Y.; Zhang, Z.; XuHan, X.; Lai, Z. MicroRNA390-Directed TAS3 Cleavage Leads to the Production of tasiRNA-ARF3/4 During Somatic Embryogenesis in Dimocarpus longan Lour. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Han, S.; Wu, T.; Li, X.; Li, W.; Qi, L. Genome-wide identification of microRNAs in larch and stage-specific modulation of 11 conserved microRNAs and their targets during somatic embryogenesis. Planta 2012, 236, 647–657. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, T.; Li, L.; Han, S.; Li, X.; Zhang, S.; Qi, L. Dynamic expression of small RNA populations in larch (Larix leptolepis). Planta 2013, 237, 89–101. [Google Scholar] [CrossRef]
- Wu, X.-M.; Liu, M.-Y.; Ge, X.-X.; Xu, Q.; Guo, W.-W. Stage and tissue-specific modulation of ten conserved miRNAs and their targets during somatic embryogenesis of Valencia sweet orange. Planta 2011, 233, 495–505. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Yuan, D.; Lindsey, K.; Zhang, X. Small RNA and degradome sequencing reveal complex miRNA regulation during cotton somatic embryogenesis. J. Exp. Bot. 2013, 64, 1521–1536. [Google Scholar] [CrossRef]
- López-Ruiz, B.A.; Juárez-González, V.T.; Sandoval-Zapotitla, E.; Dinkova, T.D. Development-Related miRNA Expression and Target Regulation during Staggered In Vitro Plant Regeneration of Tuxpeño VS-535 Maize Cultivar. Int. J. Mol. Sci. 2019, 20, 2079. [Google Scholar] [CrossRef]
- Juárez-González, V.T.; López-Ruiz, B.A.; Baldrich, P.; Luján-Soto, E.; Meyers, B.C.; Dinkova, T.D. The explant developmental stage profoundly impacts small RNA-mediated regulation at the dedifferentiation step of maize somatic embryogenesis. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Xing, H.; Pudake, R.N.; Guo, G.; Xing, G.; Hu, Z.; Zhang, Y.; Sun, Q.; Ni, Z. Genome-wide identification and expression profiling of auxin response factor (ARF) gene family in maize. BMC Genom. 2011, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Plavskin, Y.; Nagashima, A.; Perroud, P.-F.; Hasebe, M.; Quatrano, R.S.; Atwal, G.S.; Timmermans, M.C.P. Ancient trans-Acting siRNAs Confer Robustness and Sensitivity onto the Auxin Response. Dev. Cell 2016, 36, 276–289. [Google Scholar] [CrossRef]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Perrot-Rechenmann, C. Cellular Responses to Auxin: Division versus Expansion. Cold Spring Harb. Perspect. Biol. 2010, 2, a001446. [Google Scholar] [CrossRef]
- Wu, Q.; Xu, F.; Jackson, D. All together now, a magical mystery tour of the maize shoot meristem. Curr. Opin. Plant Biol. 2018, 45, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Wang, L.; Sun, W.; Zhang, Y.; Zhou, C.; Su, Y.H.; Li, W.; Sun, T.T.; Zhao, X.Y.; Li, X.G.; et al. Pattern of Auxin and Cytokinin Responses for Shoot Meristem Induction Results from the Regulation of Cytokinin Biosynthesis by AUXIN RESPONSE FACTOR3. Plant Physiol. 2013, 161, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Paque, S.; Weijers, D. Q & A: Auxin: The plant molecule that influences almost anything. BMC Biol. 2016, 14. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Hagen, G.; Guilfoyle, T. The Roles of Auxin Response Factor Domains in Auxin-Responsive Transcription. Plant Cell 2003, 15, 533–543. [Google Scholar] [CrossRef]
- Bohn-Courseau, I. Auxin: A major regulator of organogenesis. Comptes Rendus Biol. 2010, 333, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Vernoux, T.; Besnard, F.; Traas, J. Auxin at the Shoot Apical Meristem. Cold Spring Harb. Perspect. Biol. 2010, 2, a001487. [Google Scholar] [CrossRef] [PubMed]
- Boer, D.R.; Freire-Rios, A.; van den Berg, W.A.M.; Saaki, T.; Manfield, I.W.; Kepinski, S.; López-Vidrieo, I.; Franco-Zorrilla, J.M.; de Vries, S.C.; Solano, R.; et al. Structural Basis for DNA Binding Specificity by the Auxin-Dependent ARF Transcription Factors. Cell 2014, 156, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Guilfoyle, T.J. The PB1 Domain in Auxin Response Factor and Aux/IAA Proteins: A Versatile Protein Interaction Module in the Auxin Response. Plant Cell 2015, 27, 33–43. [Google Scholar] [CrossRef]
- Simonini, S.; Mas, P.J.; Mas, C.M.V.S.; Østergaard, L.; Hart, D.J. Auxin sensing is a property of an unstructured domain in the Auxin Response Factor ETTIN of Arabidopsis thaliana. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis Regeneration from Multiple Tissues Occurs via a Root Development Pathway. Dev. Cell 2010, 18, 463–471. [Google Scholar] [CrossRef]
- Perianez-Rodriguez, J.; Manzano, C.; Moreno-Risueno, M.A. Post-embryonic organogenesis and plant regeneration from tissues: Two sides of the same coin? Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Wu, X.-M.; Kou, S.-J.; Liu, Y.-L.; Fang, Y.-N.; Xu, Q.; Guo, W.-W. Genomewide analysis of small RNAs in nonembryogenic and embryogenic tissues of citrus: MicroRNA-and siRNA-mediated transcript cleavage involved in somatic embryogenesis. Plant Biotechnol. J. 2015, 13, 383–394. [Google Scholar] [CrossRef]
- Qiao, M.; Zhao, Z.; Song, Y.; Liu, Z.; Cao, L.; Yu, Y.; Li, S.; Xiang, F. Proper regeneration from in vitro cultured Arabidopsis thaliana requires the microRNA-directed action of an auxin response factor: ARF10 in shoot regeneration in vitro. Plant J. 2012, 71, 14–22. [Google Scholar] [CrossRef]
- Chen, C.-J.; Liu, Q.; Zhang, Y.-C.; Qu, L.-H.; Chen, Y.-Q.; Gautheret, D. Genome-wide discovery and analysis of microRNAs and other small RNAs from rice embryogenic callus. RNA Biol. 2011, 8, 538–547. [Google Scholar] [CrossRef]
- Nardmann, J.; Zimmermann, R.; Durantini, D.; Kranz, E.; Werr, W. WOX gene phylogeny in poaceae: A comparative approach addressing leaf and embryo development. Mol. Biol. Evol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, R.; Werr, W. Pattern formation in the monocot embryo as revealed by NAM and CUC3 orthologues from Zea mays L. Plant Mol. Biol. 2005. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.H.; Nogueira, F.T.S.; Howell, M.D.; Montgomery, T.A.; Carrington, J.C.; Timmermans, M.C.P. Pattern formation via small RNA mobility. Genes Dev. 2009, 23, 549–554. [Google Scholar] [CrossRef]
- Benkovics, A.H.; Timmermans, M.C. Developmental patterning by gradients of mobile small RNAs. Curr. Opin. Genet. Dev. 2014, 27, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Skopelitis, D.S.; Benkovics, A.H.; Husbands, A.Y.; Timmermans, M.C.P. Boundary Formation through a Direct Threshold-Based Readout of Mobile Small RNA Gradients. Dev. Cell 2017, 43, 265–273. [Google Scholar] [CrossRef]
- Wójcikowska, B.; Gaj, M.D. Expression profiling of AUXIN RESPONSE FACTOR genes during somatic embryogenesis induction in Arabidopsis. Plant Cell Rep. 2017. [Google Scholar] [CrossRef]
- López-Ruiz, B.A.; Juárez-González, V.T.; Chávez-Hernández, E.C.; Dinkova, T.D. MicroRNA Expression and Regulation During Maize Somatic Embryogenesis. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: New York, NY, USA, 2018; Volume 1815, pp. 397–410. ISBN 978-1-4939-8593-7. [Google Scholar]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Ruiz, B.A.; Juárez-González, V.T.; Gómez-Felipe, A.; De Folter, S.; Dinkova, T.D. tasiR-ARFs Production and Target Regulation during In Vitro Maize Plant Regeneration. Plants 2020, 9, 849. https://doi.org/10.3390/plants9070849
López-Ruiz BA, Juárez-González VT, Gómez-Felipe A, De Folter S, Dinkova TD. tasiR-ARFs Production and Target Regulation during In Vitro Maize Plant Regeneration. Plants. 2020; 9(7):849. https://doi.org/10.3390/plants9070849
Chicago/Turabian StyleLópez-Ruiz, Brenda Anabel, Vasti Thamara Juárez-González, Andrea Gómez-Felipe, Stefan De Folter, and Tzvetanka D. Dinkova. 2020. "tasiR-ARFs Production and Target Regulation during In Vitro Maize Plant Regeneration" Plants 9, no. 7: 849. https://doi.org/10.3390/plants9070849
APA StyleLópez-Ruiz, B. A., Juárez-González, V. T., Gómez-Felipe, A., De Folter, S., & Dinkova, T. D. (2020). tasiR-ARFs Production and Target Regulation during In Vitro Maize Plant Regeneration. Plants, 9(7), 849. https://doi.org/10.3390/plants9070849






