Comparative Analyses of Four Chemicals Used to Control Black Mold Disease in Tomato and Its Effects on Defense Signaling Pathways, Productivity and Quality Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Chemicals Tested as Inducers
2.2. Soil Analysis
2.3. Fungal Isolates
2.4. Evaluation of Antifungal Activity In Vitro
2.5. Field Studies (In Vivo)
2.6. Effect of the Pre-Harvest Foliar Application of Tested Chemicals on Some Growth Parameters, Yield, and Quality of Tomato Fruits
2.6.1. Vegetative Growth Characters
2.6.2. Fruit Yield and Their Components
2.6.3. Fruit Quality
2.7. Effect of Tested Chemicals as a Pre-Harvest Foliar Application on Tomato Black Mold Rot During Storage under Cold Conditions
2.8. Effect of Pre-Harvest Foliar Application of the Tested Chemicals on the Activities of Catalase and Chitinase in Treated Tomato Fruits
2.8.1. Enzyme Extraction
2.8.2. Chitinase Activity
2.8.3. Catalase Activity
2.9. Effect of the Pre-Harvest Foliar Application of Tested Chemicals on the Expression Signals of Resistance Genes in Treated Tomato Plants
2.9.1. Extraction of RNA and cDNA Synthesis
2.9.2. Gene Expression Analysis Using qRT-PCR
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effect of the Tested Chemicals on the Growth of Alternaria Alternata In Vitro
3.2. Field Studies (In Vivo)
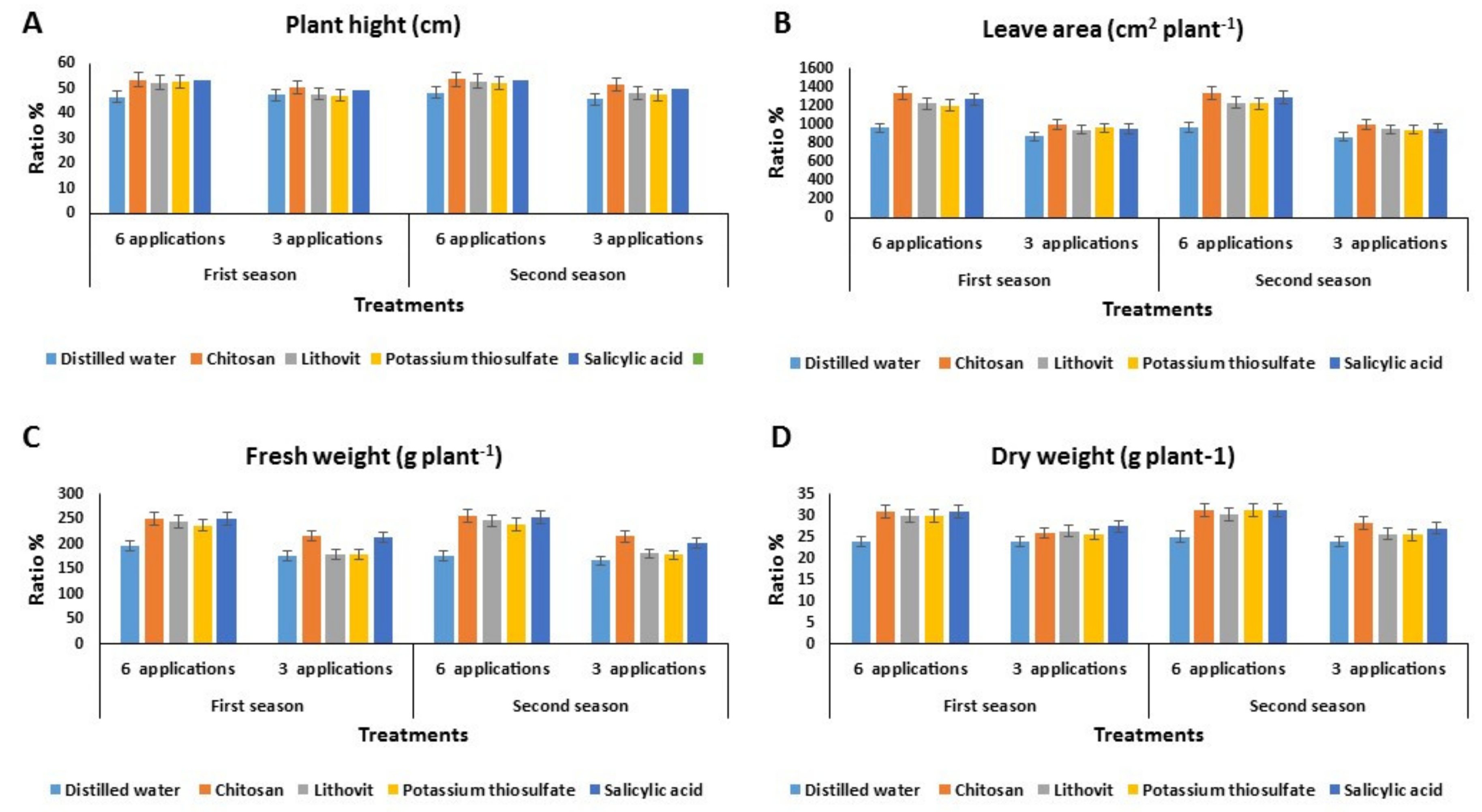
Effect of the Pre-Harvest Foliar Application of Tested Chemicals on the Vegetative Growth Characters of Tomato Plants
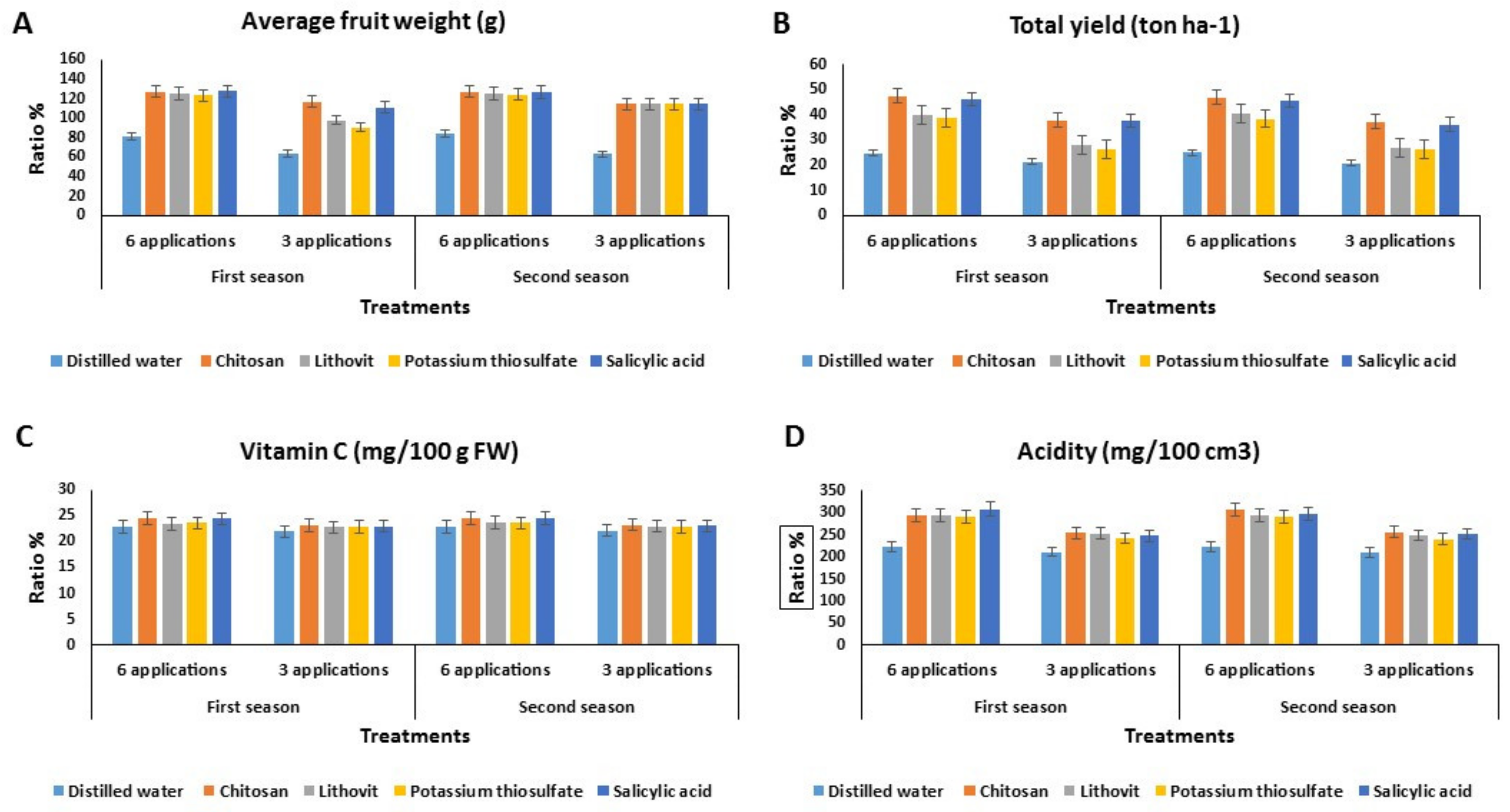
3.3. Effect of the Pre-Harvest Foliar Application of the Tested Chemicals on Yield and Quality of Tomato Fruits
3.4. Effect of the Pre-Harvest Foliar Application of the Tested Chemicals on Tomato Black Rot during Storage under Cold Conditions
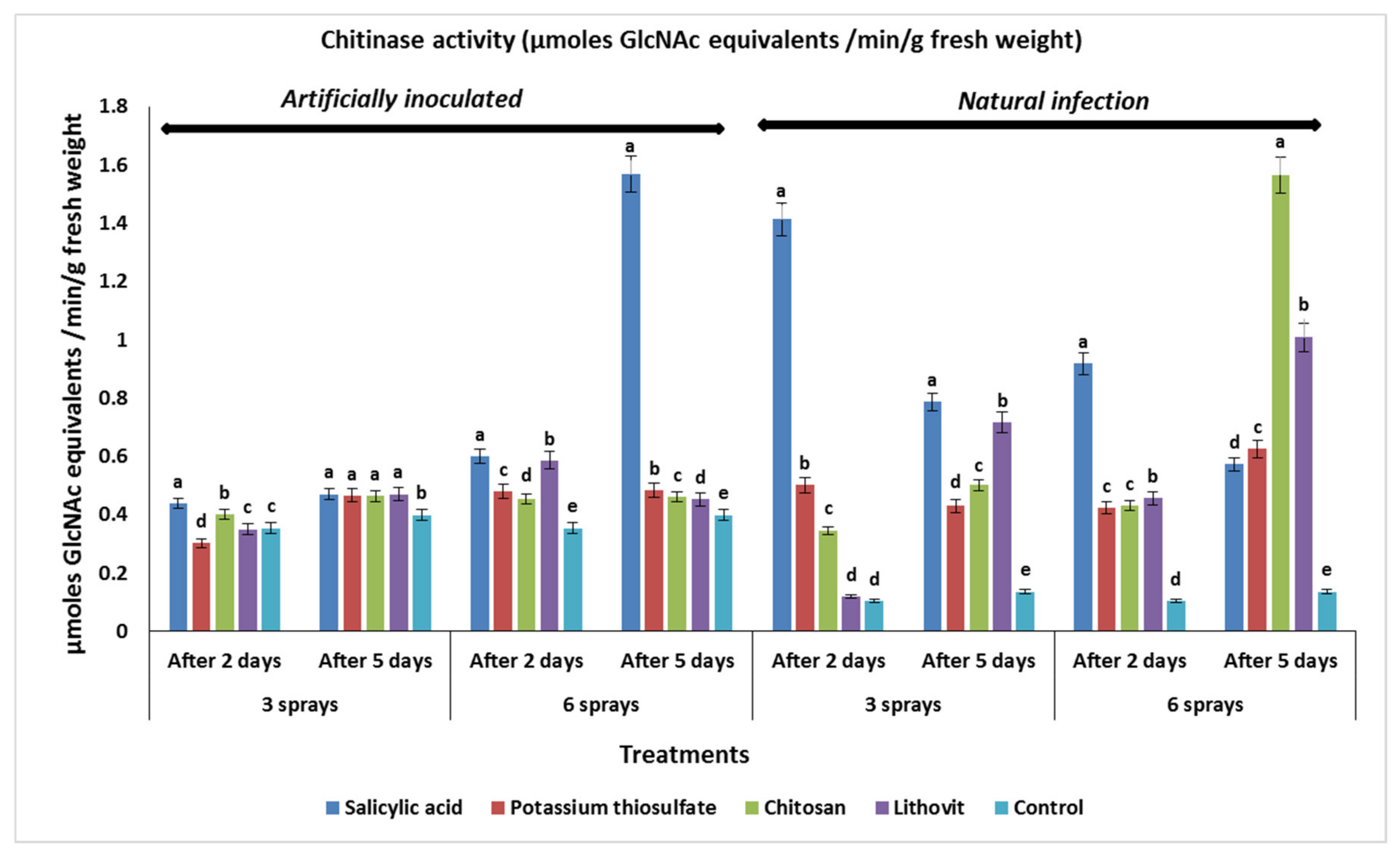
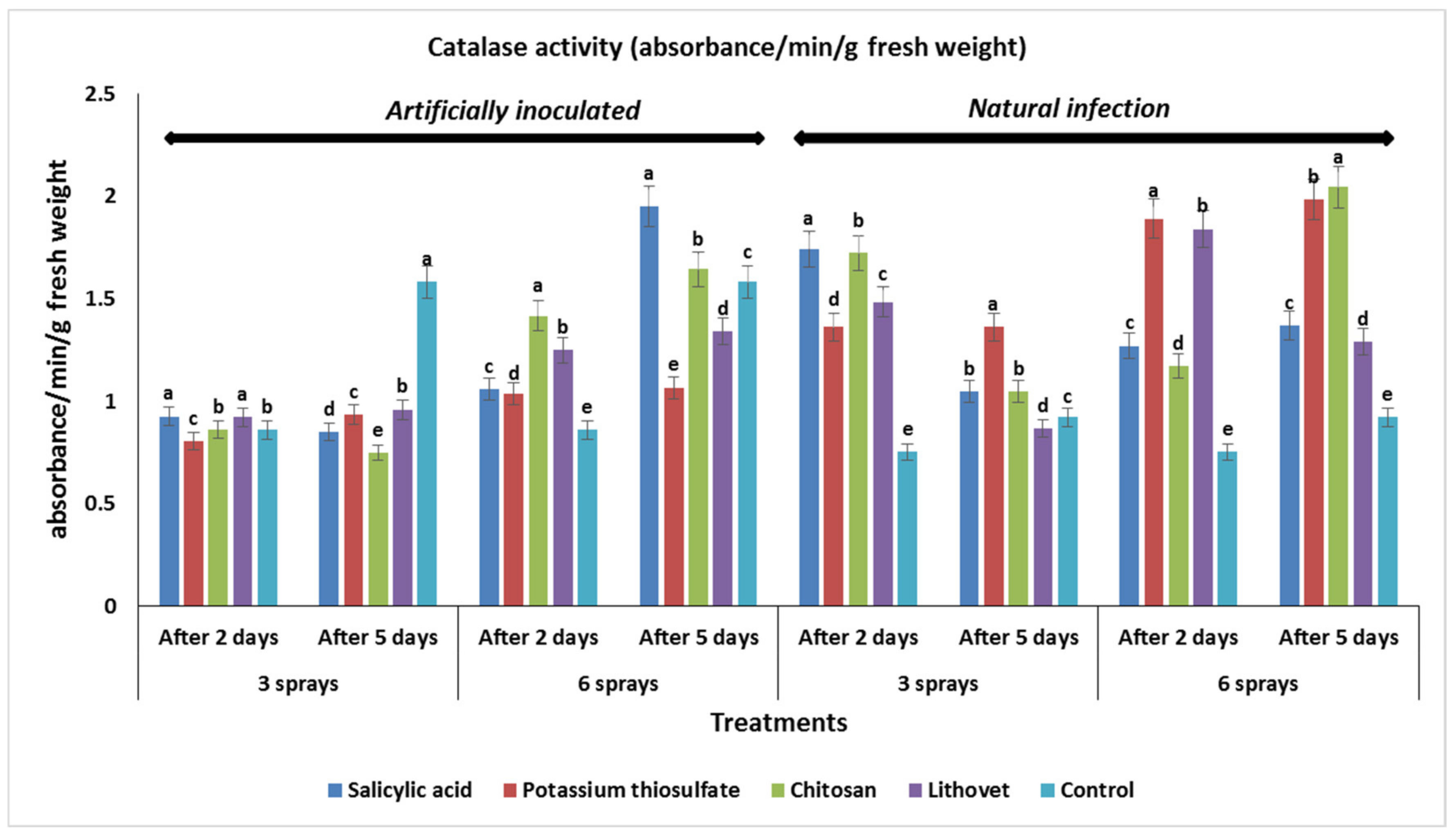
3.5. Effect of the Pre-Harvest Foliar Application of the Tested Chemicals on Activities of Chitinase and Catalase in Treated Tomato Fruits
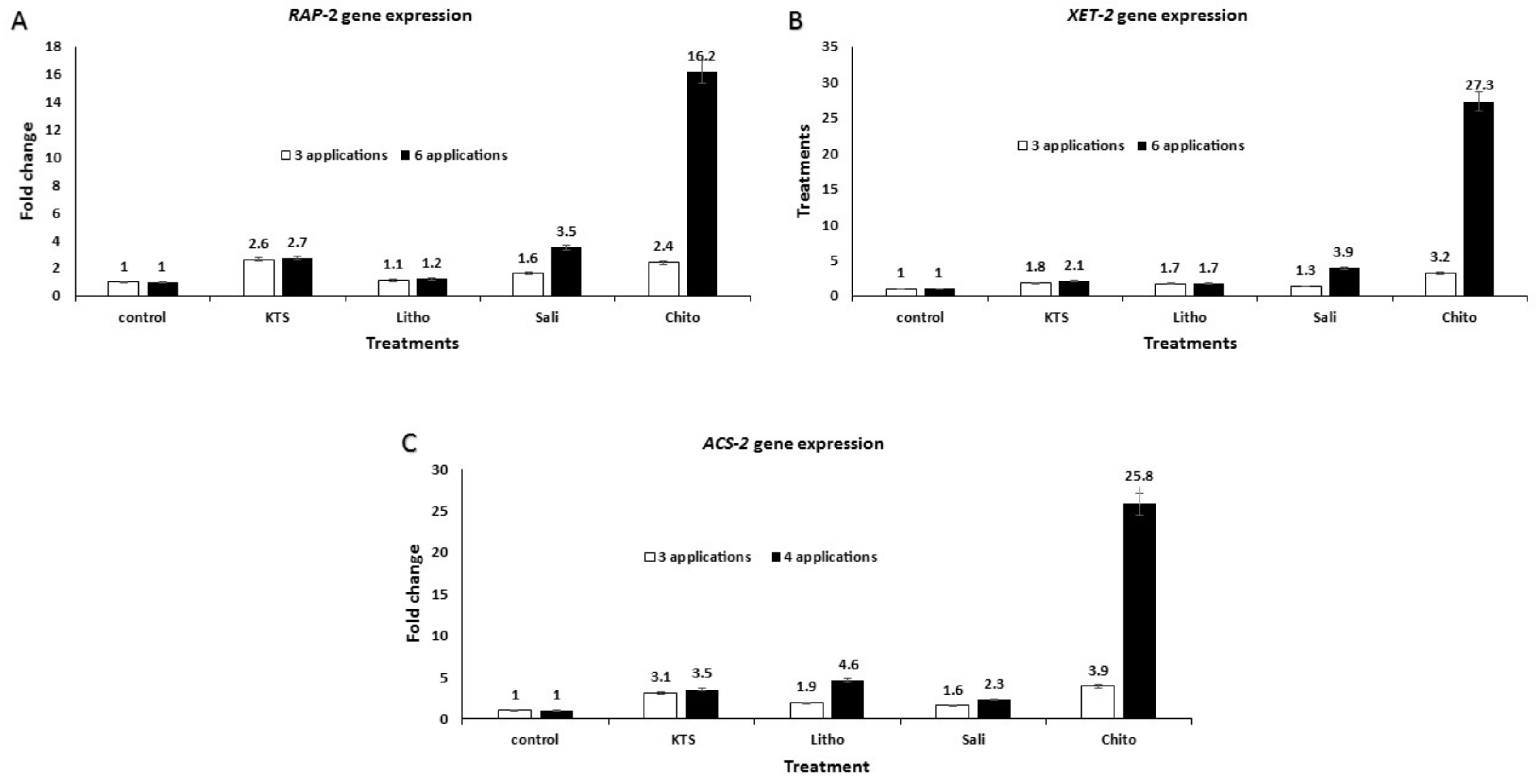
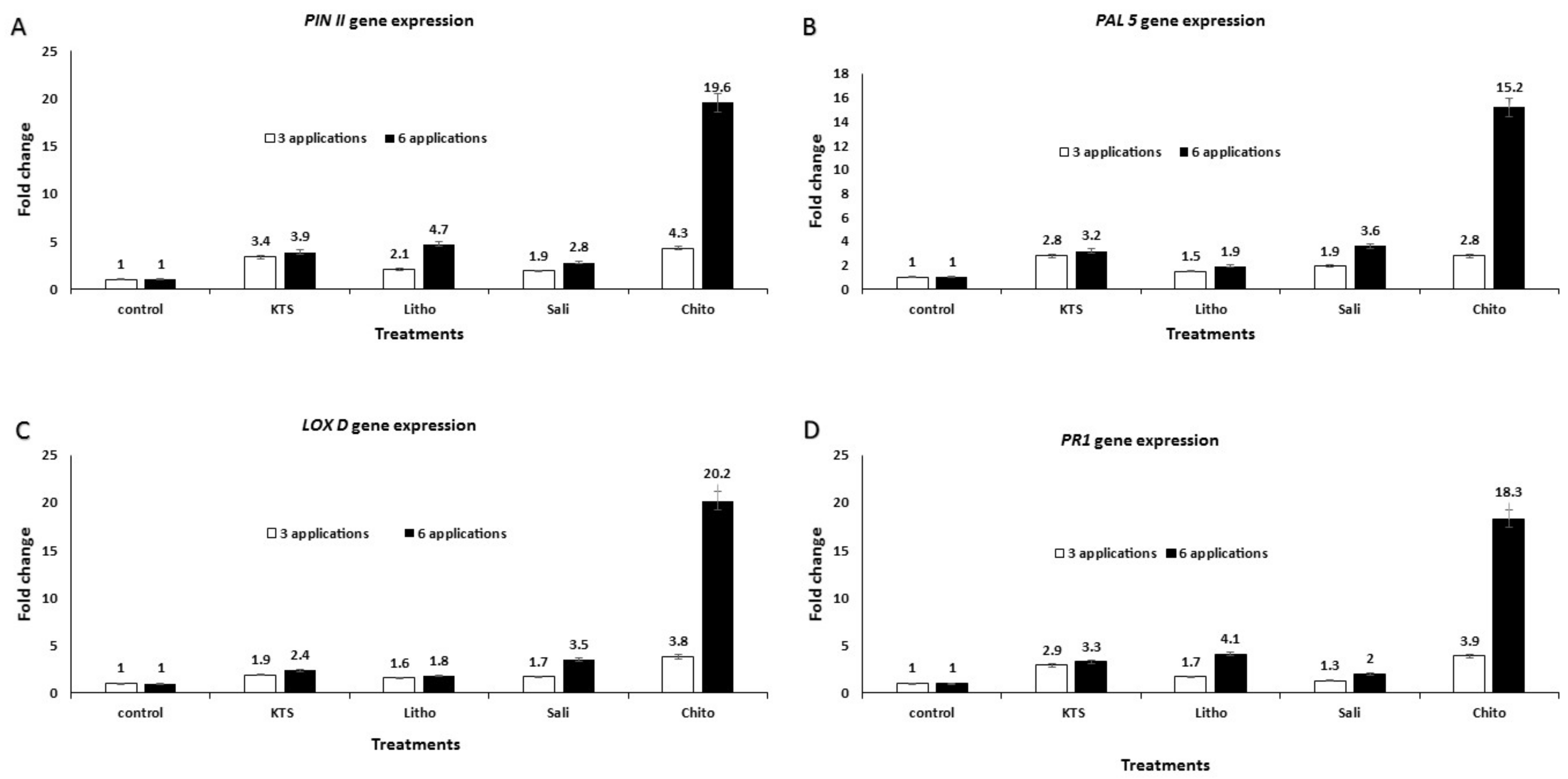
3.6. Effect of the Pre-Harvest Foliar Application of the Tested Chemicals on mRNA Expression of Defense-Related Genes in Treated Tomato Plants
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Availability of Data and Materials
References
- Sun, K.; Wolters, A.M.A.; Loonen, A.E.H.M.; Robin, P.; Huibers, R.P.; van der Vlugt, R.; Goverse, A.; Jacobsen, E.; Visser, R.G.F.; Bai, Y. Down-regulation of Arabidopsis DND1 orthologs in potato and tomato leads to broad-spectrum resistance to late blight and powdery mildew. Transgenic Res. 2016, 25, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Dimatira, J.B.U.; Dadios, E.P.; Culibrina, F. Application of fuzzy logic in recognition of tomato fruit maturity in smart farming. In Proceedings of the 2016 IEEE Region 10 Conference (TENCON), Singapore, 22–25 November 2016; pp. 2031–2035. [Google Scholar] [CrossRef]
- Wijnands, J. The international competitiveness of fresh tomatoes, peppers and cucumbers. Acta Hortic. 2003, 611, 79–90. [Google Scholar] [CrossRef]
- Asai, S.; Shirasu, K. Plant cells under siege: Plant immune system versus pathogen effectors. Curr. Opin. Plant Biol. 2015, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shams, A.S.; Morsy, N.M. Response of tomato plants to low plastic-ZnO nano-composite tunnels covering and chitosan-nanoparticles foliar spraying. J. Plant Prod. 2014, 5, 1535–1546. [Google Scholar] [CrossRef]
- Feliziani, E.; Santini, M.; Landi, L.; Romanazzi, G. Pre and postharvest treatment with alternatives to synthetic fungicides to control postharvest decay of sweet cherry. Postharvest Biol. Technol. 2013, 78, 133–138. [Google Scholar] [CrossRef]
- Hirano, S.; Nagao, N. Effect of chitosan, pectic acid, lysozyme and chitinase on the growth of several phytopathogens. Agric. Biol. Chem. 1989, 53, 3065–3066. [Google Scholar]
- Jiang, Y.; Li, Y. Effect of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2001, 73, 139–143. [Google Scholar] [CrossRef]
- Mehrabian, N.; Arvin, M.J.; Khajoie Nezhad, G.H.; Maghsodi, K. Effect of salisylic acid on growth, seed and forage yield of corn under field drought stress. Seed Plant Produc. J. 2011, 2, 41–55. (In Persian) [Google Scholar]
- Vernooij, B.; Uknes, S.; Ward, E.; Ryal, J. Salicylic acid as a signal molecule in plant–pathogen interactions. Curr. Opin. Cell. Biol. 1994, 6, 275–279. [Google Scholar] [CrossRef]
- Manochehrifar, P. Effect of Salicylic acid in plants. Paper Presented at 1st Symposium of New Findings in Chemistry and Engineering Chemistry, Iran, 2010. [Google Scholar]
- Baninaiem, E.; Mirzaaliandastjerdi, A.M.; Rastegar, S.; Abbaszade, K.H. Effect of pre- and postharvest salicylic acid treatment on quality characteristics of tomato during cold storage. Adv. Hortic. Sci. 2016, 30, 183–192. [Google Scholar]
- Byan, U.A.I. Influence of using some safety materials on water requirement and water use efficiency of snap bean plant. Arab Univ. J. Agric. Sci. 2014, 22, 381–394. [Google Scholar] [CrossRef]
- Artyszak, A.; Gozdowski, D.; Kucińska, K. The effect of foliar fertilization with marine calcite in sugar beet. Plant Soil Environ. 2014, 60, 413–417. [Google Scholar] [CrossRef]
- Abo-Sedera, F.A.; Shams, A.S.; Mohamed, M.H.M.; Hamoda, A.H.M. Effect of organic fertilizer and foliar spray with some safety compounds on growth and productivity of snap bean. Ann. Agric. Sci. Moshtohor 2016, 54, 105–118. [Google Scholar]
- Morsy, N.M.; Abdel-Salam, M.A.; Shams, A.S. Comparing Response of Melon (Cucumis melo) to Foliar Spray of Some Different Growth Stimulants under Two Nitrogen Fertilizer Forms. Egypt. J. Hortic. 2018, 45, 81–91. [Google Scholar]
- Raven, J.A. Cycling silicon–The role of accumulation in plants. New Phytol. 2003, 158, 419–421. [Google Scholar] [CrossRef]
- Carmen, B.; Şumặlan, R.; Gȃdea, Ş.; Vȃtcặ, S. Physiological Indicators Study Involved in Productivity Increasing in Tomato. ProEnvironment 2014, 7, 218–224. [Google Scholar]
- Cai, K.; Gao, D.; Chen, J.; Luo, S. Probing the mechanisms of silicon-mediated pathogen resistance. Plant Signal. Behav. 2009, 4, 1–3. [Google Scholar] [CrossRef]
- Díaz-Pérez, J.C.; Bautista, J.; Bateman, A.; Gunawati, G.; Riner, C. Reports—Soil Management, Fertilization, and Irrigation: Sweet Onion (Allium cepa) Plant Growth and Bulb Yield and Quality as Affected by Potassium and Sulfur Fertilization Rates. HortScience 2016, 51, 1592–1595. [Google Scholar] [CrossRef]
- Mengel, K. Principles of Plant Nutrition, 5th ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2001; pp. 481–509. [Google Scholar]
- El-Garhy, H.A.S.; Rashid, I.A.S.; Abou-Ali, R.M.; Moustafa, M.M.A. Field application of safe chemical elicitors induced the expression of some resistance genes against grey mold and cottony rot diseases during snap bean pods storage. Gene 2016, 576, 358–365. [Google Scholar] [CrossRef]
- Cui, Z.; Ito, J.; Dohi, H.; Amemiya, Y.; Nishida, Y. Molecular Design and Synthesis of Novel Salicyl Glycoconjugates as Elicitors against Plant Diseases. PLoS ONE 2014, 9, e108338. [Google Scholar] [CrossRef]
- Upadhyay, P.; Rai, A.; Kumar, R.; Singh, M.; Sinha, B. Differential Expression of Pathogenesis Related Protein Genes in Tomato during Inoculation with A. Solani. J. Plant Pathol. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef]
- Tolba, S.R.T.; Finetti Sialer, M.M.; Rosso, L.C.; Moustafa, M.M.A.; Ruggeri, C.; El-Shawaf, I.I.S.; Ciancio, A. Real-time assays for detection of Phytophthora spp. and identification of an avr3a gene variant. J. Plant Dis. Prot. 2018, 125, 331. [Google Scholar] [CrossRef]
- Rahman, M.Z.; Tsujimori, Y.; Maeda, M.; Hossain, M.A.; Ishimizu, T.; Kimura, Y. Molecular characterization of second tomato α1, 3/4-fucosidase (α-Fuc’ase Sl–2), a member of glycosyl hydrolase family 29 actives toward the core α1,3-fucosyl residue in plant N-glycan’s. J. Biochem. 2018, 164, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Jabnoun-Khiareddine, H.; El-Mohamedy, R.S.; Abdel-Kareem, F.; Ben Abdallah, R.A.; Gueddes-Chahed, M.; Daami-Remadi, M. Variation in Chitosan and Salicylic Acid Efficacy Toward Soil-borne and Air-borne Fungi and their Suppressive Effect of Tomato Wilt Severity. J. Plant Pathol. Microbiol. 2015, 6, 11. [Google Scholar] [CrossRef]
- Algam, S.A.E.; Xie, G.; Li, B.; Yu, S.; Su, T.; Lersen, J. Effect of Paenibacillus strains and chitosan on growth promotion and control of Ralstonia wilt in tomato. J. Plant Pathololgy 2010, 92, 593–600. [Google Scholar]
- Jackson, M.L. Soil Chemical Analysis-Advanced Course, 2nd ed.; Department of Soil Science, University of Wisconsin: Madison, WI, USA, 1969. [Google Scholar]
- Barnett, H.L.; Hunter, B.B. Illustrated Genera of Imperfect Fungi; Macmillan Publishing Co.: New York, NY, USA; Collier Macmillan: London, UK, 1972. [Google Scholar]
- Sirirat, S.; Rungprom, W.; Sawatdikarn, S. Antifungal activity of essential oils derived from some medicinal plants against grey mould (Botrytis cinerea). Food Ag-Ind 2009, 2, S229–S233. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; Methods 925.10, 65.17, 974.24, 992.16; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Romanazzi, G.; Nigro, F.; Ippolito, A. Effectiveness of pre and postharvest chitosan treatments on storage decay of strawberries. Frutticoltura 2000, 62, 71–75. [Google Scholar]
- Anand., T.; Raguchander, T.; Karthikeyan, G.; Prakasam, V.; Samiyappan, R. Chemically and biologically mediated systemic resistance in cucumber (Cucumis sativus L.) against Pseudoperonospora cubensis and Erysiphe cichoracearum. Phytopathol. Med. 2007, 46, 259–271. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Kato, M.; Shimizu, S. Chlorophyll metabolism in higher plants. VII. Chlorophyll degradation in senescing tobacco leaves; phenolic dependent peroxidative degradation. Can. J. Bot. 1987, 65, 729–735. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mageed, M.H.; Mohamed, F.G.; Hafez, M.S.; Soltan, H.H.; Abdel-Rahman, F.A. Controlling the grey and white rot diseases on bean pods under storage conditions using some safely chemical compounds. J. Biol. Chem. Environ. Sci. 2012, 7, 617–634. [Google Scholar]
- Saad, A.S.A.; Kadous, E.A.; Tayeb, E.H.; Massoud, M.A.; Ahmed, S.M.; Abou El-Ela, A.S.A. The inhibitory effect of some antioxidants and fungicides on the growth of Alternaria solani and Fusarium solani in vitro. Middle East J. Agric. Res. 2014, 3, 123–134. [Google Scholar]
- Vinai, K.; Gurdeep, B. Effect of salicylic acid on mycelial growth and conidial germination of two isolates of Fusarium mangiferae. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3704–3710. [Google Scholar]
- Leuba, J.L.; Stossel, P. Chitosan and other polyamines: Antifungal activity and intera ction with biologica l membranes. In Chitin in N ature and Technology; Muzzarelli, R., Jeauniaux, C., Gooday, G.W., Eds.; Plenum Press: New York, NY, USA, 1986; pp. 215–222. [Google Scholar]
- Al-Essawy, A.A.A. Integration of some Pre- and Post-Harvest Treatments for Management of Gray Mold of Table Grape. Master’s Thesis, Faculty of Agriculture Ain Shams University, Muḩāfaz̧at al Qalyūbīyah, Egypt, 2018; 196p. [Google Scholar]
- Khandaker, L.; Masum, A.A.; Oba, S. Foliar Application of Salicylic Acid Improved the Growth, Yield and Leaf’s Bioactive Compounds in Red Amaranth (Amaranthus tricolor L.). Veg. Crops Res. Bull. 2011, 74, 77–86. [Google Scholar] [CrossRef]
- Nassef, D.M.T.; Nabeel, A.H.M. Response of two broccoli cultivars to foliar application of Lithovit fertilizer under two planting methods. Assiut J. Agric. Sci. 2012, 43, 27–45. [Google Scholar]
- Rivas-San, V.M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Wang, L.J.; Li, S.-H. Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 2006, 170, 685–694. [Google Scholar] [CrossRef]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Bautista-Baños, S.; Hernández-Lauzardo, A.N.; Velázquez-del Valle, M.G.; Hernández-López, M.; Ait Barka, E.; Bosquez-Molina, E.; Wilson, C.L. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot. 2006, 25, 108–118. [Google Scholar] [CrossRef]
- Prisecaru, G.; Sala, F. Response model of vegetation parameters and yield in maize under the influence of Lithovit fertilizer. AIP Conf. Proc. 2017, 1863, 430005. [Google Scholar] [CrossRef]
- Wall, D.P.; O’Sullivan, L.; Creamer, R.; McLaughlin, M.J. Soil Fertility and Nutrient Cycling. In The Soils of Ireland; World Soils Book Series; Creamer, R., O’Sullivan, L., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Lester, G.E.; Jifon, J.L.; Makus, D.J. Impact of potassium nutrition on postharvest fruit quality: Melon (Cucumis melo L.) case study. Plant Soil 2010, 335, 117–131. [Google Scholar] [CrossRef]
- Jifo2n, J.; Lester, G.; Crosby, K.; Leskovar, D. Improving the quality attributes of melons through modified mineral nutrition. Acta Hortic. 2009, 841, 537–540. [Google Scholar] [CrossRef]
- Sharif, R.; Mujtaba, M.; Rahman, M.U.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. Review: The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef] [PubMed]
- Csiszár, J.; Brunner, S.; Horváth, E.; Bela, K.; Ködmön, P.; Riyazuddin, R.; Gallé, Á.; Hurton, Á.; Papdi, C.; Szabados, L.; et al. Exogenously applied salicylic acid maintains redox homeostasis in salt-stressed Arabidopsis gr1 mutants expressing cytosolic roGFP1. Plant Growth Regul. 2018, 86, 181–194. [Google Scholar] [CrossRef]
- Islam, M.Z.; Mele, M.A.; Choi, K.-Y.; Baek, J.P.; Kang, H.M. Salicylic acid in Nutrient Solution Influence the Fruit Quality and Shelf Life of Cherry Tomato Grown in Hydroponics. Sains Malays. 2018, 47, 537–542. [Google Scholar] [CrossRef]
- Sajyan, T.K.; Shaban, N.; Rizkallah, J.; Sassine, Y.N. Effects of Monopotassium-phosphate, Nano-calcium fertilizer, Acetyl salicylic acid and Glycine betaine application on growth and production of tomato (Solanum lycopersicum) crop under salt stress. Agron. Res. 2018, 16, 872–883. [Google Scholar] [CrossRef]
- Zakaria, M.A.T.; Sakimin, S.Z.; Baghdadi, A.; Jaafar, H.Z.E.; Din, S.N.M.; Ramlan, M.F. Effect of Different Water Regime and Plant Growth Regulators on Growth, Physiology and Yield of Banana (Musa acuminata cv. Berangan) in Tropical. Fundam. Appl. Agric. 2018, 3, 505–514. [Google Scholar] [CrossRef]
- Shafiee, M.; Taghavi, T.S.; Babalar, M. Addition of salicylic acid to nutrient solution combined with postharvest treatments (hot water, salicylic acid, and calcium dipping) improved postharvest fruit quality of strawberry. Sci. Hortic. 2010, 124, 40–45. [Google Scholar] [CrossRef]
- Jiankang, C.; Yan, J.; Zhao, Y.; Jiang, W. Effects of postharvest salicylic acid dipping on Alternaria rot and disease resistance of jujube fruit during storage. J. Sci. Food Agric. 2013, 93, 3252–3258. [Google Scholar]
- Mazaro, S.M.; Deschamps, C.; Mio, L.L.M.; Biase, L.A.; Gouvea, L.A.; Sautter, C.K. Post-harvest behavior of strawberry fruits after pre-harvest treatment with chitosan and acibenzolar-S-methyl. Rev. Bras. Frutic. 2008, 30, 185–190. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Zahedi, S.M.; Abadía, J.; Karimi, M. Effects of postharvest treatments with chitosan and putrescine to maintain quality and extend shelf-life of two banana cultivars. Food Sci. Nutr. 2018, 6, 1328–1337. [Google Scholar] [CrossRef]
- Long, L.T.; Tan, L.V.; Boi, V.N.; Trung, T.S. Antifungal activity of water-soluble chitosan against Colletotrichum capsici in postharvest chili pepper. J. Food Process. Preserv. 2018, 42, e13339. [Google Scholar] [CrossRef]
- Adss, I.A.A.; Hamza, H.A.; Hafez, E.E.; Heika, H.M. Enhancing Tomato Fruits Post-Harvest Resistance by Salicylic Acid and Hydrogen Peroxide Elicitors against Rot Caused by Alternaria solani. J. Agric. Chem. Biotechnol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.A. Safe Technologies for Controlling Post-Harvest Diseases of Green Bean Prepared for Exportation. Ph.D. Thesis, Fac. Agric., Benha University, Qalyubia, Egypt, 2015; 250p. [Google Scholar]
- Tosun, N.; Atkas, L.; Karabay-Yavasoglu, N.U.; Turkusay, H. Effects of salicylic acid, harpin and phosphorus acid in control of late blight. (Phytophthora infestans Mont. De Barry) disease and some physiological parameters of tomato. Turk. Phytopathol. Soc. 2006, 32, 1–10. [Google Scholar]
- Raut, S.A.; Borkar, S.G. PR-proteins Accumulation in Tomato Plant Due to Application of Resistance Inducing Chemicals during Period of Induced Resistance against Alternaria Leaf Blight. Sci. Int. 2014, 2, 72–75. [Google Scholar] [CrossRef][Green Version]
- Patricia, T.A.; Couderchet, M.; Vernet, G.; Aziz, A. Chitosan stimulates defense reactions in grapevine leaves and inhibits development of Botrytis cinerea. Eur. J. Plant Pathol. 2006, 114, 405–413. [Google Scholar]
- Romanazzi, G.; Lichter, A.; Mlikota, G.F.; Smilanick, J. Natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012, 63, 141–147. [Google Scholar] [CrossRef]
- Chan, Z.; Qin, G.; Xu, X.; Li, B.; Tian, S. Proteome approach to characterize proteins inducedby antagonist yeastandsalicylic acid in peach fruit. J. Proteome Res. 2007, 6, 1677–1688. [Google Scholar] [CrossRef]
- Xu, X.; Tian, S. Salicylic acid alleviated pathogen-induced oxidative stress in harvested sweet cherry fruit. Postharvest Biol. Technol. 2008, 49, 379–385. [Google Scholar] [CrossRef]
- Deenamo, N.; Kuyyogsuy, A.; Khompatara, K.; Chanwun, T.; Ekchaweng, K.; Churngchow, N. Salicylic Acid Induces Resistance in Rubber Tree against Phytophthora palmivora. Int. J. Mol. Sci. 2018, 19, 1883. [Google Scholar] [CrossRef] [PubMed]
- Hortensia, O.O.; Mendoza, A.B.; Villarreal, R.M.; Rodríguez, H.R.; Romenus, K.D. Enzymatic Activity in Tomato Fruits as a Response to Chemical Elicitors. J. Mex. Chem. Soc. 2007, 51, 141–144. [Google Scholar]
- Milena, P.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of Chitosan Coating on the Postharvest Quality and Antioxidant Enzyme System Response of Strawberry Fruit during Cold Storage. Foods 2015, 4, 501–523. [Google Scholar]
- Hashim, N.F.A.; Ahmad, A.; Bordoh, P.K. Effect of Chitosan Coating on Chilling Injury, Antioxidant Status and Postharvest Quality of Japanese Cucumber during Cold Storage. Sains Malays. 2018, 47, 287–294. [Google Scholar]
- Herman, M.A.B.; Restrepo, S.; Smart, C.D. Defense gene expression patterns of three SAR-induced tomato cultivars in the field. Physiol. Mol. Plant Pathol. 2007, 71, 192–200. [Google Scholar] [CrossRef]
- Phukan, U.J.; Jeena, G.S.; Tripathi, V.; Shukla, R.K. Regulation of Apetala2/Ethylene Response Factors in Plants. Front. Plant Sci. 2017, 8, 150. [Google Scholar] [CrossRef]
- Catala, C.; Rose, J.K.C.; York, W.S.; Albersheim, P.; Darvill, A.G.; Bennett, A.B. Characterization of a Tomato Xyloglucan Endotransglycosylase Gene That Is Down-Regulated by Auxin in Etiolated Hypocotyls. Plant Physiol. 2001, 127, 1180–1192. [Google Scholar] [CrossRef]
- Yang, L.; Hu, G.; Li, N.; Habib, S.; Huang, W.; Li, Z. Functional Characterization of SlSAHH2 in Tomato Fruit Ripening. Front. Plant Sci. 2017, 8, 1312. [Google Scholar] [CrossRef]
- Alexandersson, E.; Mulugeta, T.; Lankinen, Å.; Liljeroth, E.; Andreasson, E. Plant Resistance Inducers against Pathogens in Solanaceae Species—From Molecular Mechanisms to Field Application. Int. J. Mol. Sci. 2016, 17, 1673. [Google Scholar] [CrossRef]
- Turra, D.; Lorito, M. Potato type I and II proteinase inhibitors: Modulating plant physiology and host resistance. Curr. Protein Pept. Sci. 2011, 12, 374–385. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chun, S.C. Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato (Solanum lycopersicum) plants challenged with Erwinia carotovora subsp. Carotovora. Biosci. Biotechnol. Biochem. 2016, 80, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Safaie-Farahani, A.; Taghavi, S.M. Transcript analysis of some defense genes of tomato in response to host and non-host bacterial pathogens. Mol. Biol. Res. Commun. 2017, 6, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhai, Q.; Wei, J.; Li, S.; Wang, B.; Huang, T.; Du, M.; Sun, J.; Kang, L.; Li, C.; et al. Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores. PLoS Genet 2013, 9, e1003964. [Google Scholar] [CrossRef] [PubMed]
| Soil Texture | Soil Parameters | Soil Available Macronutrients (mg kg−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sand% | Silt% | Clay% | Texture * | PH | EC/dSm−1 | O.M/g kg−1 | CaCO/g kg−1 | N | K | P |
| 24.4 | 24.6 | 51 | Clay loam | 7.9 | 2.16 | 1.41 | 1.53 | 22.5 | 9.1 | 120 |
| Gene Name | Primer Forward | Primer Reverse | Reference |
|---|---|---|---|
| RAP | 5′-aaagaaccatctgtggcgtgtgag–3′ | 5′-cgaatcttgtaagcggcttggtca–3′ | Upadhyay et al. (2014) [24] |
| XET–2 | 5′-tggaggagattctgctggtgttgt–3′ | 5′-tctgtctcctttgcctcctgtgaa–3′ | Upadhyay et al. (2014) [24] |
| ACS–2 | 5′-ttccatcactgcagctttgcttcg–3′ | 5′-tttgtttgggccagcttctctctc–3′ | Upadhyay et al. (2014) [24] |
| PAL5 | 5′-gacagcaggaaggaatccaa–3′ | 5′-caaccaaatagggattcgaca–3′ | Tolba et al. (2018) [25] |
| LOXD | 5′-ttggcaccaagttcaggccc–3’ | 5′-tggacttaagctagtattag–3′ | Tolba et al. (2018) [25] |
| PR1 | 5′-tgccaagaccggtggtaatttc–3′ | 5′-tgcccgctagcacattggt–3′ | Tolba et al. (2018) [25] |
| PINII | 5′-ttgttgtgcaggcagtaagg–3′ | 5′-ttcaataattacgcgtgagcc–3′ | Tolba et al. (2018) [25] |
| Actin (reference gene) | 5′-ttgccgcatgccattct–3′ | 5′-tcggtgaggatattcatcaggtt–3′ | Upadhyay et al. (2014) [24] |
| Tested Chemicals | Conc. mg/mL | Linear Growth | |
|---|---|---|---|
| mm | Efficacy% | ||
| Salicylic acid | 0.75 | 0.0 f | 100.0 |
| 1.5 | 0.0 f | 100.0 | |
| Potassium thiosulfate | 2 | 55.2 cd | 38.7 |
| 4 | 45.7 e | 49.3 | |
| Chitosan | 2 | 57.3 c | 36.3 |
| 4 | 50.7 de | 43.7 | |
| Lithovit | 2 | 68.7 b | 23.7 |
| 4 | 56.3 cd | 37.4 | |
| Control | 90.00 a | ||
| Tested Chemicals | Six Application | |||||||
|---|---|---|---|---|---|---|---|---|
| Alternaria alternata | Natural Infection | |||||||
| 7 Days from Storage | 15 Days from Storage | 7 Days from Storage | 15 Days from Storage | |||||
| D.S | EF% | D.S | EF% | D.S | EF% | D.S | EF% | |
| Salicylic Acid | 0.00 b | 100.00 | 5.51 ef | 91.91 | 0.00b | 100.00 | 0.00 c | 100.00 |
| Potassium Thiosulfate | 1.33 b | 86.20 | 9.33 cde | 86.32 | 0.00 b | 100.00 | 1.03 bc | 95.99 |
| Chitosan | 0.00 b | 100.00 | 4.12 f | 93.96 | 0.00 b | 100.00 | 0.00 c | 100.00 |
| Lithovit | 1.24 b | 87.14 | 6.95 def | 89.81 | 0.00 b | 100.00 | 0.00 c | 100.00 |
| Three Applications | ||||||||
| Salicylic Acid | 0.00 b | 100.00 | 10.42 cd | 84.72 | 0.00 b | 100.00 | 2.08 bc | 91.89 |
| Potassium Thiosulfate | 1.77 b | 81.64 | 13.53 c | 80.16 | 0.00 b | 100.00 | 3.80 bc | 85.19 |
| Chitosan | 1.03 b | 89.32 | 7.64 def | 88.80 | 0.00 b | 100.00 | 2.73 bc | 89.36 |
| Lithovit | 1.65 b | 82.88 | 17.89 b | 73.76 | 0.00 b | 100.00 | 4.50 b | 82.46 |
| Control | 9.64 a | 68.19 a | 2.05 a | 25.66 a | ||||
| Tested Chemicals | Six Application | |||||||
|---|---|---|---|---|---|---|---|---|
| Alternaria alternata | Natural Infection | |||||||
| 7 Days from Storage | 15 Days from Storage | 7 Days from Storage | 15 Days from Storage | |||||
| D.S | EF% | D.S | EF% | D.S | EF% | D.S | EF% | |
| Salicylic Acid | 0.00 b | 100.00 | 6.19 cd | 90.52 | 0.00 a | 100.00 | 0.00 b | 100.00 |
| Potassium Thiosulfate | 1.53 b | 85.99 | 10.63 bcd | 83.72 | 0.00 a | 100.00 | 1.39 b | 94.76 |
| Chitosan | 0.00 b | 100.00 | 3.81 d | 94.17 | 0.00 a | 100.00 | 0.00 | 100.00 |
| Lithovit | 1.39 b | 87.27 | 7.25 cd | 88.90 | 0.00 a | 100.00 | 0.00 | 100.00 |
| Three Application | ||||||||
| Salicylic Acid | 0.00 b | 100.00 | 9.20 bcd | 85.91 | 0.00 a | 100.00 | 1.93 b | 92.73 |
| Potassium Thiosulfate | 1.81 b | 83.42 | 12.29 bc | 81.18 | 0.00 a | 100.00 | 3.33 b | 87.45 |
| Chitosan | 1.16 b | 89.38 | 7.16 cd | 89.04 | 0.00 a | 100.00 | 2.42 b | 90.88 |
| Lithovit | 2.58 b | 80.95 | 15.03 b | 76.99 | 0.00 a | 100.00 | 4.52 b | 82.96 |
| Control | 10.92 a | 65.31 a | 2.08 a | 26.53 a | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Garhy, H.A.S.; Abdel-Rahman, F.A.; Shams, A.S.; Osman, G.H.; Moustafa, M.M.A. Comparative Analyses of Four Chemicals Used to Control Black Mold Disease in Tomato and Its Effects on Defense Signaling Pathways, Productivity and Quality Traits. Plants 2020, 9, 808. https://doi.org/10.3390/plants9070808
El-Garhy HAS, Abdel-Rahman FA, Shams AS, Osman GH, Moustafa MMA. Comparative Analyses of Four Chemicals Used to Control Black Mold Disease in Tomato and Its Effects on Defense Signaling Pathways, Productivity and Quality Traits. Plants. 2020; 9(7):808. https://doi.org/10.3390/plants9070808
Chicago/Turabian StyleEl-Garhy, Hoda A. S., Fayz A. Abdel-Rahman, Abdelhakeem S. Shams, Gamal H. Osman, and Mahmoud M. A. Moustafa. 2020. "Comparative Analyses of Four Chemicals Used to Control Black Mold Disease in Tomato and Its Effects on Defense Signaling Pathways, Productivity and Quality Traits" Plants 9, no. 7: 808. https://doi.org/10.3390/plants9070808
APA StyleEl-Garhy, H. A. S., Abdel-Rahman, F. A., Shams, A. S., Osman, G. H., & Moustafa, M. M. A. (2020). Comparative Analyses of Four Chemicals Used to Control Black Mold Disease in Tomato and Its Effects on Defense Signaling Pathways, Productivity and Quality Traits. Plants, 9(7), 808. https://doi.org/10.3390/plants9070808






