Short-Term Pre-Harvest UV-B Supplement Enhances the Polyphenol Content and Antioxidant Capacity of Ocimum basilicum Leaves during Storage
Abstract
1. Introduction
2. Results
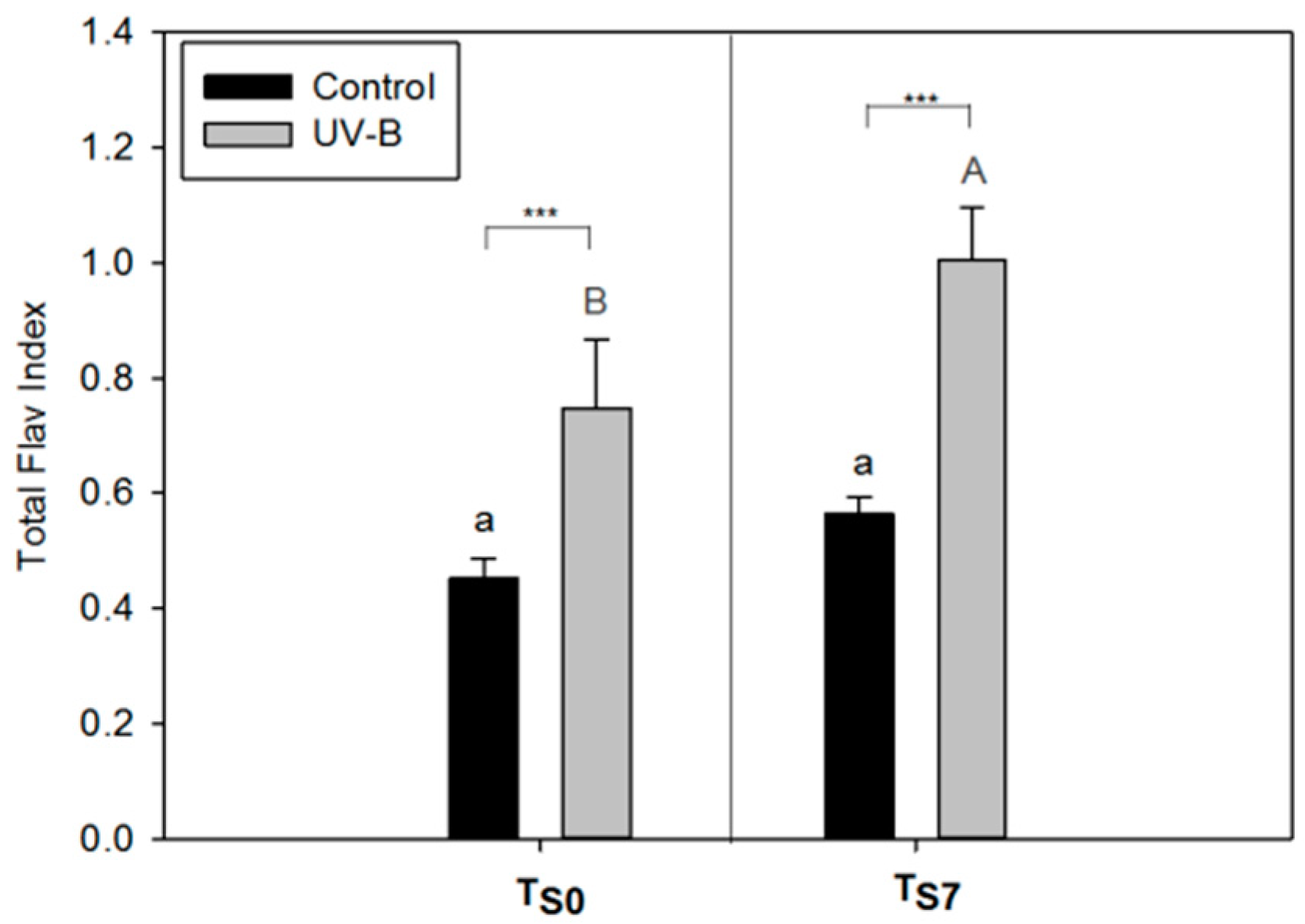
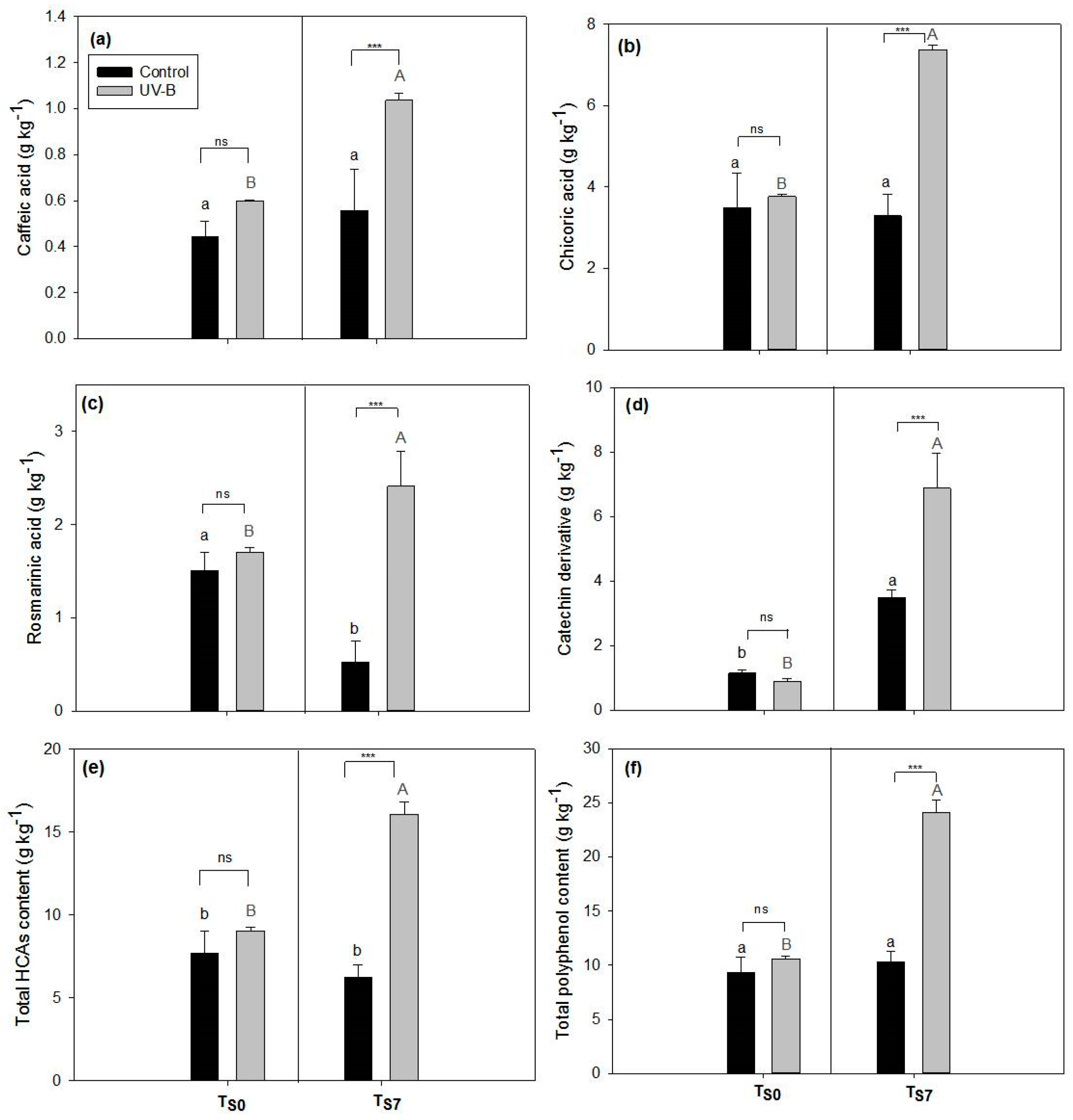
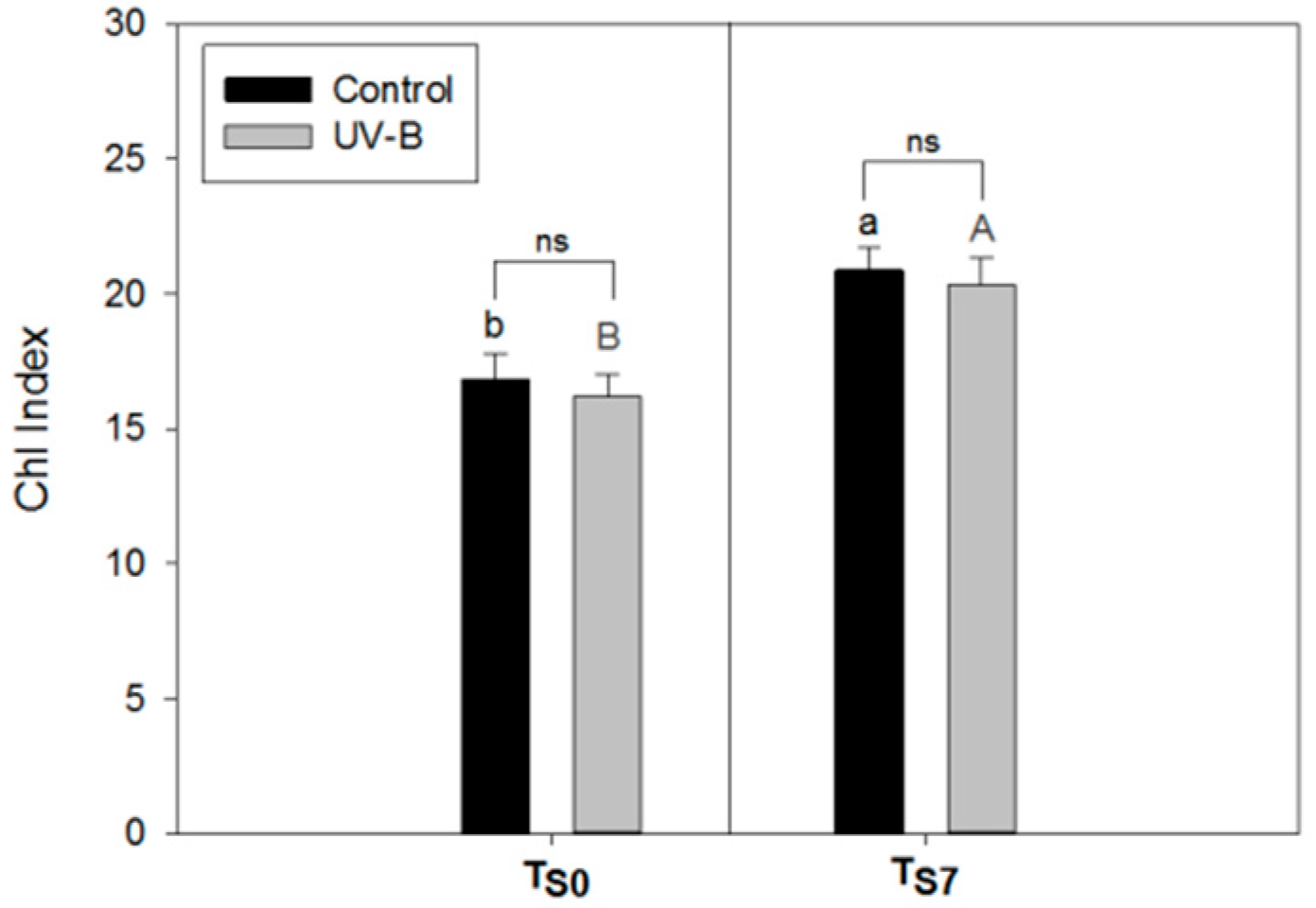
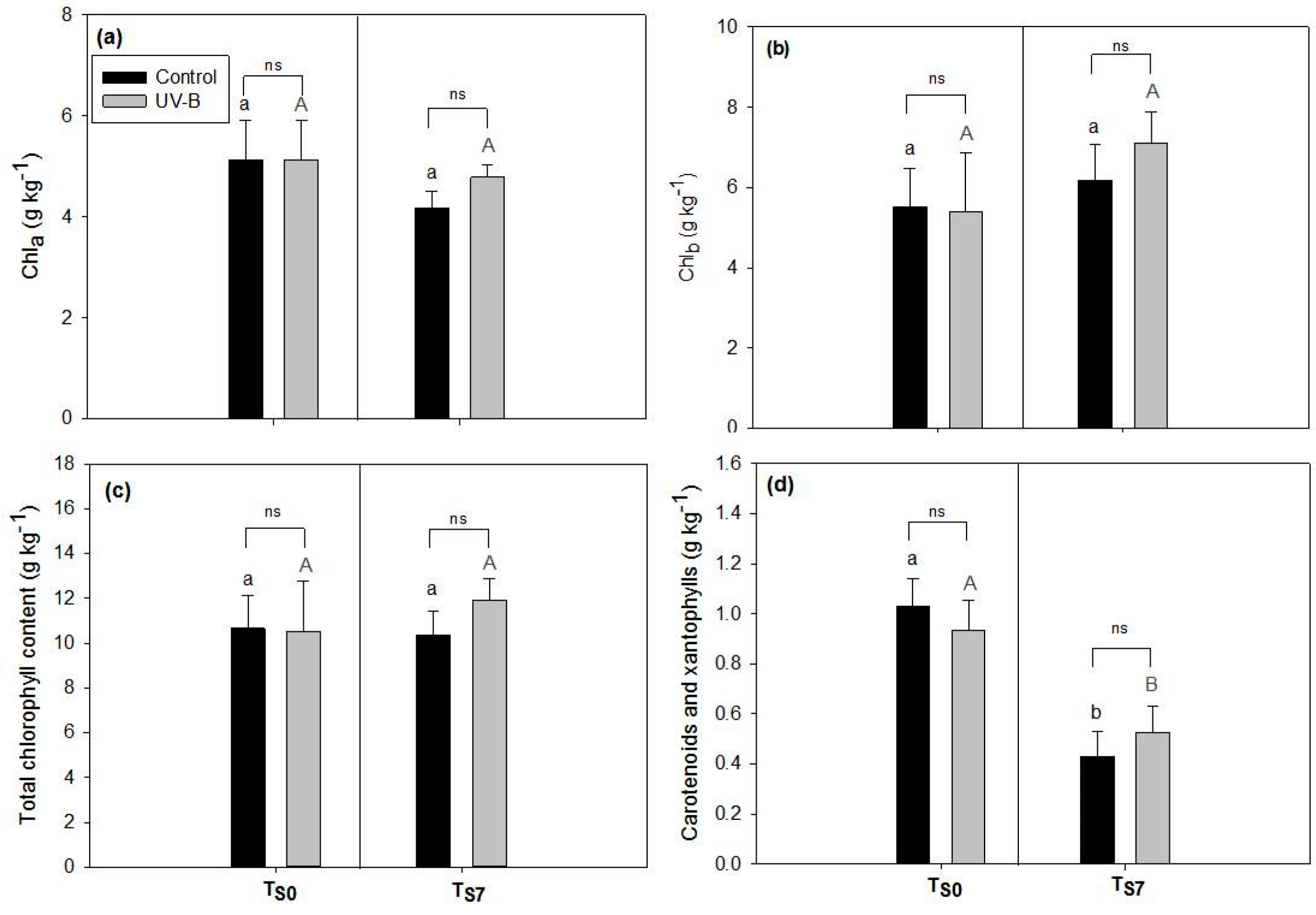
2.1. Phytochemical Quality of O. basilicum Leaves: Non-Destructive and Destructive Analyses
2.2. Antioxidant Capacity of Leaf Extracts: Effects of Light Treatments and Storage
2.3. Visual Quality of O. basilicum Leaves: % FWLR and Colorimetric Analysis
2.4. Applicability of the Non-Destructive Dualex® Device
3. Discussion
3.1. Effect of UV-B Treatment on Post-Harvest Phytochemical Quality of O. basilicum Leaves
3.2. Visual Quality of O. basilicum Leaves
3.3. Applicability of Dualex® Instrument as a Non-Destructive Tool to Monitor the Phytochemical Quality of Detached Leaves of O. basilicum
4. Materials and Methods
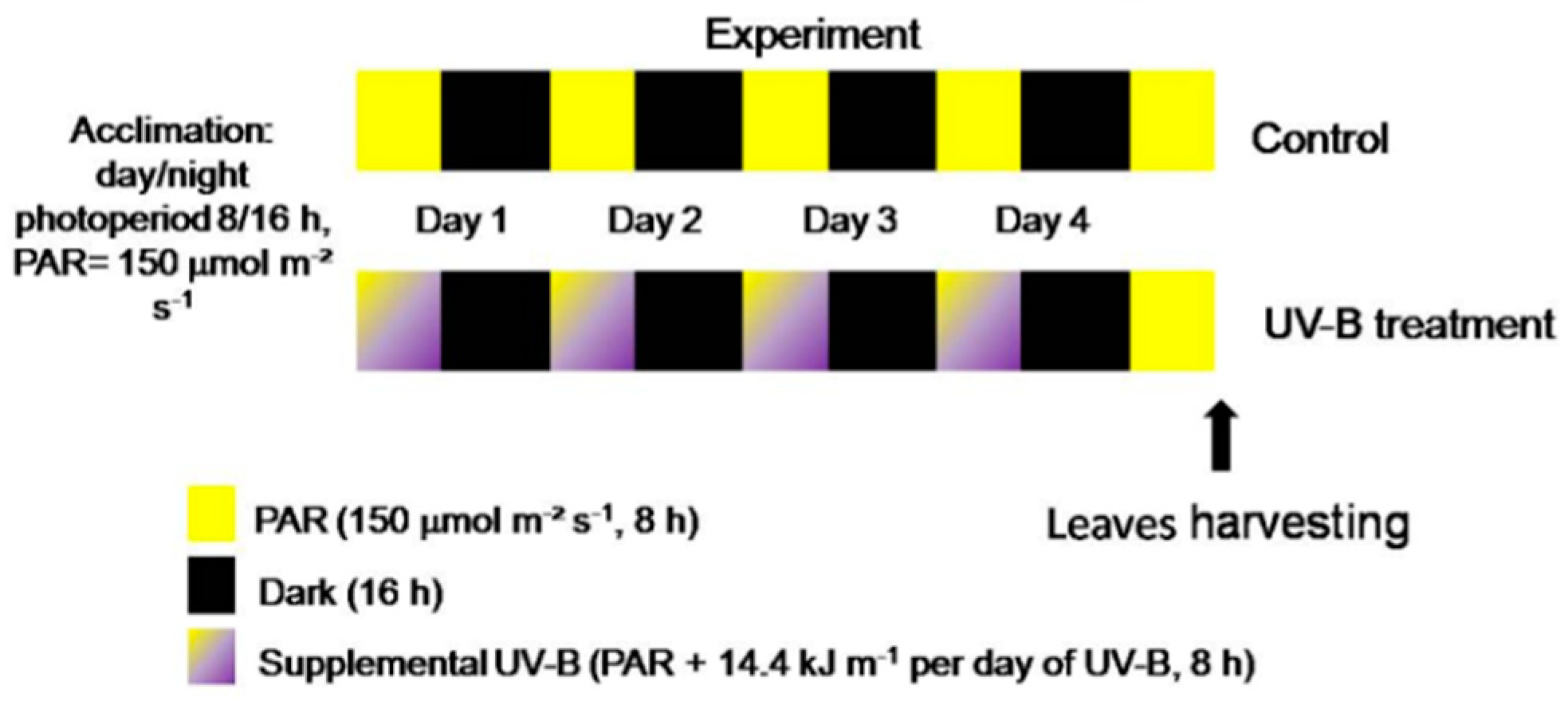
4.1. Plant Material
4.2. Light Treatments, Storage Conditions and Sampling Details
4.3. Non-Destructive Analysis of Epidermal Flavonol Content (Flav Index) and Chlorophyll Content (Chl Index) by Dualex®
4.4. Polyphenolic Analysis by HPLC-DAD
4.5. Spectrophotometric Quantification of Photosynthetic Pigments
4.6. Antioxidant Capacity of the Leaf Extracts
4.7. Percentage of Fresh Weight Loss Rate (% FWLR)
4.8. Colorimetric Analysis
4.9. Applicability of the Non-Destructive Dualex® Device
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tohge, T.; Fernie, A.R. Leveraging Natural Variance towards Enhanced Understanding of Phytochemical Sunscreens. Trends Plant Sci. 2017, 22, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Neugart, S.; Schreiner, M. UVB and UVA as eustressors in horticultural and agricultural crops. Sci. Hortic. 2018, 234, 370–381. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum Basilicum) Plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends Food Sci. Technol. 2019, 92, 71–80. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Ferreira, I.C.F.R. In vivo antioxidant activity of phenolic compounds: Facts and gaps. Trends Food Sci. Technol. 2016, 48, 1–12. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Vaštakaitė-Kairienė, V.; Rasiukevičiūtė, N.; Valiuškaitė, A. UV LEDs in Postharvest Preservation and Produce Storage. In Ultraviolet LED Technology for Food Applications; Academic Press: Cambridge, MA, USA, 2019; pp. 67–90. [Google Scholar] [CrossRef]
- Toscano, S.; Trivellini, A.; Cocetta, G.; Bulgari, R.; Francini, A.; Romano, D.; Ferrante, A. Effect of Preharvest Abiotic Stresses on the Accumulation of Bioactive Compounds in Horticultural Produce. Front. Plant Sci. 2019, 10, 1212. [Google Scholar] [CrossRef]
- Filip, S. Basil (Ocimum basilicum L.) a Source of Valuable Phytonutrients. Int. J. Clin. Nutr. Diet. 2017, 3, 118. [Google Scholar] [CrossRef]
- Sharma, R.; Bhatia, S.; Kaur, P. Influence of packaging and storage conditions on biochemical quality and enzymatic activity in relation to shelf life enhancement of fresh basil leaf. J. Food Sci. Technol. 2018, 55, 3199–3211. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.E.; Rahmat, A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef]
- Mosadegh, H.; Trivellini, A.; Ferrante, A.; Lucchesini, M.; Vernieri, P.; Mensuali, A. Applications of UV-B lighting to enhance phenolic accumulation of sweet basil. Sci. Hortic. 2018, 229, 107–116. [Google Scholar] [CrossRef]
- Costa, L.; Montano, Y.M.; Carrion, C.; Rolny, N.; Guiamet, J.J. Application of low intensity light pulses to delay postharvest senescence of Ocimum basilicum leaves. Postharvest Biol. Technol. 2013, 86, 181–191. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R.; Nenadis, N.; Neugart, S.; Robson, M.; Agati, G.; Vepsalainen, J.; Zipoli, G.; Nybakken, L.; Winkler, B.; Jansen, M.A.K. Assessing the response of plant flavonoids to UV radiation: An overview of appropriate techniques. Phytochem. Rev. 2014, 14, 273–297. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef]
- Li, Y.F.; Liu, Y.; Wang, X.; Li, Y.; Han, R. Lower levels of UV-B light trigger the adaptive responses by inducing plant antioxidant metabolism and flavonoid biosynthesis in Medicago sativa seedlings. Funct. Plant Biol. 2019, 46, 896. [Google Scholar] [CrossRef]
- Barnes, P.W.; Flint, S.D.; Tobler, M.A.; Ryel, R.J. Diurnal adjustment in ultraviolet sunscreen protection is widespread among higher plants. Oecologia 2016, 181, 55–63. [Google Scholar] [CrossRef]
- Bidel, L.P.; Chomicki, G.; Bonini, F.; Mondolot, L.; Soulé, J.; Coumans, M.; La Fisca, P.; Baissac, Y.; Petit, V.; Loiseau, A.; et al. Dynamics of flavonol accumulation in leaf tissues under different UV-B regimes in Centella asiatica (Apiaceae). Planta 2015, 242, 545–559. [Google Scholar] [CrossRef]
- Morales, L.O.; Tegelberg, R.; Brosche, M.; Keinänen, M.; Lindfors, A.; Aphalo, P.J. Effects of solar UV-A and UV-B radiation on gene expression and phenolic accumulation in Betula pendula leaves. Tree Physiol. 2010, 30, 923–934. [Google Scholar] [CrossRef]
- Bernula, P.; Crocco, C.D.; Arongaus, A.B.; Ulm, R.; Nagy, F.; Viczián, A. Expression of the UVR8 photoreceptor in different tissues reveals tissue-autonomous features of UV-B signalling. Plant Cell Environ. 2017, 40, 1104–1114. [Google Scholar] [CrossRef]
- Day, T.A.; Martin, G.; Vogelmann, T.C. Penetration of UV-B radiation in foliage: Evidence that the epidermis behaves as a non-uniform filter. Plant Cell Environ. 1993, 16, 735–741. [Google Scholar] [CrossRef]
- Rodríguez-Calzada, T.; Qian, M.; Strid, Å.; Neugart, S.; Schreiner, M.; Torres-Pacheco, I.; Guevara-González, R.G. Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 134, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Comont, D.; Winters, A.; Gwynn-Jones, D. Acclimation and interaction between drought and elevated UV-B inA. thaliana: Differences in response over treatment, recovery and reproduction. Ecol. Evol. 2012, 2, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Robson, T.M.; Hartikainen, S.; Aphalo, P.J. How does solar ultraviolet-B radiation improve drought tolerance of silver birch (B etula pendula Roth.) seedlings? Plant Cell Environ. 2014, 38, 953–967. [Google Scholar] [CrossRef] [PubMed]
- Tossi, V.E.; Regalado, J.J.; Iannicelli, J.; Laino, L.E.; Burrieza, H.P.; Escandón, A.S.; Pitta-Álvarez, S.I. Beyond Arabidopsis: Differential UV-B Response Mediated by UVR8 in Diverse Species. Front. Plant Sci. 2019, 10, 780. [Google Scholar] [CrossRef]
- Kaplan, F.; Kopka, J.; Sung, D.Y.; Zhao, W.; Popp, M.; Porat, R.; Guy, C.L. Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Bilger, W.; Rolland, M.; Nybakken, L. UV screening in higher plants induced by low temperature in the absence of UV-B radiation. Photochem. Photobiol. Sci. 2007, 6, 190. [Google Scholar] [CrossRef]
- Olsen, K.M.; Lea, U.S.; Slimestad, R.; Verheul, M.; Lillo, C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. J. Plant Physiol. 2008, 165, 1491–1499. [Google Scholar] [CrossRef]
- Gul, S.; Ahmad, M.; Zafar, M.; Bahadur, S.; Sultana, S.; Ashfaq, S.; Ullah, F.; Kilic, O.; Hassan, F.U.; Siddiq, Z. Foliar epidermal anatomy of Lamiaceae with special emphasis on their trichomes diversity using scanning electron microscopy. Microsc. Res. Tech. 2019, 82, 206–223. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. J. For. Res. 2019, 31, 1–12. [Google Scholar] [CrossRef]
- Jensen, N.B.; Clausen, M.R.; Kjaer, K.H. Spectral quality of supplemental LED grow light permanently alters stomatal functioning and chilling tolerance in basil (Ocimum basilicum L.). Sci. Hortic. 2018, 227, 38–47. [Google Scholar] [CrossRef]
- Misra, B.B.; Acharya, B.R.; Granot, D.; Assmann, S.M.; Chen, S. The guard cell metabolome: Functions in stomatal movement and global food security. Front. Plant Sci. 2015, 6, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.; Hechler, P.J.; Muday, G.K. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol. 2014, 164, 1707–1717. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics Composition and Antioxidant Activity of Sweet Basil (Ocimum basilicumL.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Hectors, K.; O’Brien, N.M.; Guisez, Y.; Potters, G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci. 2008, 175, 449–458. [Google Scholar] [CrossRef]
- Cantarello, C.; Volpe, V.; Azzolin, C.; Bertea, C. Modulation of enzyme activities and expression of genes related to primary and secondary metabolism in response to UV-B stress in cucumber (Cucumis sativus L.). J. Plant Interact. 2005, 1, 151–161. [Google Scholar] [CrossRef]
- Liu, L.; McClure, J.W. Effects of UV-B on activities of enzymes of secondary phenolic metabolism in barley primary leaves. Physiol. Plant. 1995, 93, 734–739. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Weng, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of UV-B radiation on phenolic accumulation, antioxidant activity and physiological changes in wheat (Triticum aestivum L.) seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Harbaum-Piayda, B.; Palani, K.; Schwarz, K. Influence of postharvest UV-B treatment and fermentation on secondary plant compounds in white cabbage leaves. Food Chem. 2016, 197, 47–56. [Google Scholar] [CrossRef]
- Interdonato, R.; Rosa, M.; Nieva, C.B.; González, J.A.; Hilal, M.; Prado, F.E. Effects of low UV-B doses on the accumulation of UV-B absorbing compounds and total phenolics and carbohydrate metabolism in the peel of harvested lemons. Environ. Exp. Bot. 2011, 70, 204–211. [Google Scholar] [CrossRef]
- Hectors, K.; Van Oevelen, S.; Geuns, J.; Guisez, Y.; Jansen, M.A.K.; Prinsen, E. Dynamic changes in plant secondary metabolites during UV acclimation in Arabidopsis thaliana. Physiol. Plant. 2014, 152, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free. Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Khan, F.A.; Naushin, F. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance. Plant Signal. Mol. 2019, 24, 157–168. [Google Scholar] [CrossRef]
- Gunia-Krzyżak, A.; Słoczyńska, K.; Popiół, J.; Koczurkiewicz-Adamczyk, P.; Marona, H.; Pękala, E. Cinnamic acid derivatives in cosmetics: Current use and future prospects. Int. J. Cosmet. Sci. 2018, 229, 752–760. [Google Scholar] [CrossRef]
- Amoah, S.K.S.; Sandjo, L.P.; Kratz, J.M.; Biavatti, M.W. Rosmarinic Acid—Pharmaceutical and Clinical Aspects. Planta Med. 2016, 82, 388–406. [Google Scholar] [CrossRef]
- Li, Z.; Feng, H.; Han, L.; Ding, L.; Shen, B.; Tian, Y.; Zhao, L.; Jin, M.; Wang, Q.; Qin, H.; et al. Chicoric acid ameliorate inflammation and oxidative stress in Lipopolysaccharide and d-galactosamine induced acute liver injury. J. Cell. Mol. Med. 2020, 24, 3022–3033. [Google Scholar] [CrossRef]
- Castronuovo, D.; Russo, D.; Libonati, R.; Faraone, I.; Candido, V.; Picuno, P.; Andrade, P.B.; Valentão, P.; Milella, L. Influence of shading treatment on yield, morphological traits and phenolic profile of sweet basil (Ocimum basilicum L.). Sci. Hortic. 2019, 254, 91–98. [Google Scholar] [CrossRef]
- Fratianni, F.; Cefola, M.; Pace, B.; Cozzolino, R.; De Giulio, B.; Cozzolino, A.; D’Acierno, A.; Coppola, R.; Logrieco, A.; Nazzaro, F. Changes in visual quality, physiological and biochemical parameters assessed during the postharvest storage at chilling or non-chilling temperatures of three sweet basil (Ocimum basilicum L.) cultivars. Food Chem. 2017, 229, 752–760. [Google Scholar] [CrossRef]
- Kaewsuksaeng, S.; Urano, Y.; Aiamla-Or, S.; Shigyo, M.; Yamauchi, N. Effect of UV-B irradiation on chlorophyll-degrading enzyme activities and postharvest quality in stored lime (Citrus latifolia Tan.) fruit. Postharvest Biol. Technol. 2011, 61, 124–130. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Lozano-Pastor, P.; Artés-Hernández, F.; Artés, F.; Aguayo, E. Preharvest UV-C treatment improves the quality of spinach primary production and postharvest storage. Postharvest Biol. Technol. 2019, 155, 130–139. [Google Scholar] [CrossRef]
- Srilaong, V.; Aiamla-Or, S.; Soontornwat, A.; Shigyo, M.; Yamauchi, N. UV-B irradiation retards chlorophyll degradation in lime (Citrus latifolia Tan.) fruit. Postharvest Biol. Technol. 2011, 59, 110–112. [Google Scholar] [CrossRef]
- Aiamla-Or, S.; Kaewsuksaeng, S.; Shigyo, M.; Yamauchi, N. Impact of UV-B irradiation on chlorophyll degradation and chlorophyll-degrading enzyme activities in stored broccoli (Brassica oleracea L. Italica Group) florets. Food Chem. 2010, 120, 645–651. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Changes in carotenoids during processing and storage of foods. Arch. Latinoam. Nutr. 1999, 49, 38–47. [Google Scholar]
- Hideg, É.; Jansen, M.A.K.; Strid, Å. UV-B exposure, ROS, and stress: Inseparable companions or loosely linked associates? Trends Plant Sci. 2013, 18, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pathare, P.; Opara, U.L.; Al-Said, F.A.-J. Colour Measurement and Analysis in Fresh and Processed Foods: A Review. Food Bioprocess Technol. 2012, 6, 36–60. [Google Scholar] [CrossRef]
- Kasım, M.U.; Kasım, R. Yellowing of fresh-cut spinach (Spinacia oleracea L.) leaves delayed by UV-B applications. Inf. Process. Agric. 2017, 4, 214–219. [Google Scholar] [CrossRef]
- Gnanasekharan, V.; Shewfelt, R.; Chinnan, M. Detection of Color Changes in Green Vegetables. J. Food Sci. 1992, 57, 149–154. [Google Scholar] [CrossRef]
- Koukounaras, A.; Siomos, A.S.; Sfakiotakis, E. Postharvest CO2 and ethylene production and quality of rocket (Eruca sativa Mill.) leaves as affected by leaf age and storage temperature. Postharvest Biol. Technol. 2007, 46, 167–173. [Google Scholar] [CrossRef]
- Kowalczyk, D.; Kordowska-Wiater, M.; Kałwa, K.; Skrzypek, T.; Sikora, M.; Łupina, K. Physiological, qualitative, and microbiological changes of minimally processed Brussels sprouts in response to coating with carboxymethyl cellulose/candelilla wax emulsion. J. Food Process. Preserv. 2019, 43, e14004. [Google Scholar] [CrossRef]
- Abdipour, M.; Hosseinifarahi, M.; Naseri, N. Combination method of UV-B and UV-C prevents post-harvest decay and improves organoleptic quality of peach fruit. Sci. Hortic. 2019, 256, 27–31. [Google Scholar] [CrossRef]
- Scattino, C.; Castagna, A.; Neugart, S.; Chan, H.M.; Schreiner, M.; Crisosto, C.H.; Tonutti, P.; Ranieri, A. Post-harvest UV-B irradiation induces changes of phenol contents and corresponding biosynthetic gene expression in peaches and nectarines. Food Chem. 2014, 163, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Masdoumier, G.; Ben Ghozlen, N.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Cerovic, Z.G.; Marta, A.D.; Di Stefano, V.; Pinelli, P.; Traversi, M.L.; Orlandini, S. Optically-assessed preformed flavonoids and susceptibility of grapevine to Plasmopara viticola under different light regimes. Funct. Plant Biol. 2008, 35, 77–84. [Google Scholar] [CrossRef]
- Agati, G.; Tuccio, L.; Kusznierewicz, B.; Chmiel, T.; Bartoszek, A.; Kowalski, A.; Grzegorzewska, M.; Kosson, R.; Kaniszewski, S. Nondestructive Optical Sensing of Flavonols and Chlorophyll in White Head Cabbage (Brassica oleraceaL. var.capitatasubvar.alba) Grown under Different Nitrogen Regimens. J. Agric. Food Chem. 2015, 64, 85–94. [Google Scholar] [CrossRef]
- Stelzner, J.; Roemhild, R.; Garibay-Hernández, A.; Harbaum-Piayda, B.; Mock, H.P.; Bilger, W. Hydroxycinnamic acids in sunflower leaves serve as UV-A screening pigments. Photochem. Photobiol. Sci. 2019, 18, 1649–1659. [Google Scholar] [CrossRef]
- Gang, D.R.; Simon, J.; Lewinsohn, E.; Pichersky, E. Peltate Glandular Trichomes of Ocimum basilicum L. (Sweet Basil) Contain High Levels of Enzymes Involved in the Biosynthesis of Phenylpropenes. J. Herbs Spices Med. Plants 2002, 9, 189–195. [Google Scholar] [CrossRef]
- Berim, A.; Hyatt, D.C.; Gang, D.R. A set of regioselective O-methyltransferases gives rise to the complex pattern of methoxylated flavones in sweet basil. Plant Physiol. 2012, 160, 1052–1069. [Google Scholar] [CrossRef]
- Grayer, R.; Veitch, N.C.; Kite, G.C.; Price, A.M.; Kokubun, T. Distribution of 8-oxygenated leaf-surface flavones in the genus Ocimum. Phytochemistry 2001, 56, 559–567. [Google Scholar] [CrossRef]
- Berim, A.; Park, J.-J.; Gang, D.R. Unexpected roles for ancient proteins: Flavone 8-hydroxylase in sweet basil trichomes is a Rieske-type, PAO-family oxygenase. Plant J. 2014, 80, 385–395. [Google Scholar] [CrossRef]
- Chang, X.; Alderson, P.G.; Wright, C.J. Enhanced UV-B radiation alters basil (Ocimum basilicum L.) growth and stimulates the synthesis of volatile oils. J. Hortic. For. 2009, 12, 27–31. [Google Scholar]
- Ioannidis, D.; Bonner, L.; Johnson, C.B. UV-B is Required for Normal Development of Oil Glands in Ocimum basilicum L. (Sweet Basil). Ann. Bot. 2002, 90, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Kirby, J.; Naxakis, G.; Pearson, S. Substantial UV-B-mediated induction of essential oils in sweet basil (Ocimum basilicum L.). Phytochemistry 1999, 51, 507–510. [Google Scholar] [CrossRef]
- Al-Fartosy, A.; Al-Rikaby, A.K.J. The Antioxidative Action of Monoterpene from Loranthus europaeus L.seeds. Basrah J. Agric. Sci. 2007, 20, 322–336. [Google Scholar] [CrossRef]
- Dong, T.; Shang, J.; Chen, J.M.; Liu, J.; Qian, B.; Ma, B.L.; Morrison, M.J.; Zhang, C.; Liu, Y.; Shi, Y.; et al. Assessment of Portable Chlorophyll Meters for Measuring Crop Leaf Chlorophyll Concentration. Remote. Sens. 2019, 11, 2706. [Google Scholar] [CrossRef]
- Vogelmann, T.C. Plant Tissue Optics. Annu. Rev. Plant Biol. 1993, 44, 231–251. [Google Scholar] [CrossRef]
- Martinez, D.E.; Guiamét, J.J. Distortion of the SPAD 502 chlorophyll meter readings by changes in irradiance and leaf water status. Agronomie 2004, 24, 41–46. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1. [Google Scholar] [CrossRef]
- Kandi, S.; Charles, A.L.; Sridhar, K. Statistical comparative study between the conventional DPPH spectrophotometric and dropping DPPH analytical method without spectrophotometer: Evaluation for the advancement of antioxidant activity analysis. Food Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef]

| Light Treatments | Moment of Harvesting | |
|---|---|---|
| TS0 | TS7 | |
| Control | 0.13 ± 0.07 b | 0.24 ± 0.04 a |
| UV-B | 0.16 ± 0.02 a, ns | 0.05 ± 0.009 b,*** |
| Polyphenolic Content | Pearson Coefficient—r (EC50 Values) | p Value |
|---|---|---|
| Total HCAs | −0.74 | 0.006 ** |
| Total polyphenols | −0.65 | 0.02 * |
| Caffeic acid | −0.49 | 0.10 ns |
| Catechin derivative | −0.41 | 0.19 ns |
| Chicoric acid | −0.67 | 0.02 * |
| Rosmarinic acid | −0.80 | 0.0003 *** |
| Light Treatments | Storage Time | |
|---|---|---|
| TS4 | TS7 | |
| Control | 20.1 ± 7.0 b, ns | 32.7 ± 6.8 a, ns |
| UV-B | 21.5 ± 9.2 b, ns | 35. 5 ± 8.5 a, ns |
| Samples | Color Parameters | ||
|---|---|---|---|
| L | a | ∆E | |
| Control TS0 | 65.2± 0.6 b | −4.7 ± 0.5 b | - |
| UV-B TS0 | 64.3 ± 0.8 b,* | −4.4 ± 0.5 b, ns | - |
| Control TS7 | 66.1 ± 0.2 a | −3.0 ± 0.1 a | 3.4 ± 0.9 |
| UV-B TS7 | 65.4 ± 0.4 a,* | −2.9 ± 0.2 a, ns | 2.9 ± 0.9 ns |
| Polyphenolic Content | Pearson Coefficient—r (Total Flav Index) | p Value |
|---|---|---|
| Total HCAs | 0.85 | 0.0004 *** |
| Total polyphenols | 0.86 | 0.0004 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos S. Nascimento, L.B.; Brunetti, C.; Agati, G.; Lo Iacono, C.; Detti, C.; Giordani, E.; Ferrini, F.; Gori, A. Short-Term Pre-Harvest UV-B Supplement Enhances the Polyphenol Content and Antioxidant Capacity of Ocimum basilicum Leaves during Storage. Plants 2020, 9, 797. https://doi.org/10.3390/plants9060797
dos S. Nascimento LB, Brunetti C, Agati G, Lo Iacono C, Detti C, Giordani E, Ferrini F, Gori A. Short-Term Pre-Harvest UV-B Supplement Enhances the Polyphenol Content and Antioxidant Capacity of Ocimum basilicum Leaves during Storage. Plants. 2020; 9(6):797. https://doi.org/10.3390/plants9060797
Chicago/Turabian Styledos S. Nascimento, Luana Beatriz, Cecilia Brunetti, Giovanni Agati, Clara Lo Iacono, Cassandra Detti, Edgardo Giordani, Francesco Ferrini, and Antonella Gori. 2020. "Short-Term Pre-Harvest UV-B Supplement Enhances the Polyphenol Content and Antioxidant Capacity of Ocimum basilicum Leaves during Storage" Plants 9, no. 6: 797. https://doi.org/10.3390/plants9060797
APA Styledos S. Nascimento, L. B., Brunetti, C., Agati, G., Lo Iacono, C., Detti, C., Giordani, E., Ferrini, F., & Gori, A. (2020). Short-Term Pre-Harvest UV-B Supplement Enhances the Polyphenol Content and Antioxidant Capacity of Ocimum basilicum Leaves during Storage. Plants, 9(6), 797. https://doi.org/10.3390/plants9060797










