Rpv Mediated Defense Responses in Grapevine Offspring Resistant to Plasmopara viticola
Abstract
1. Introduction
2. Results
2.1. Segregating Populations and MAS
2.2. Phenotyping
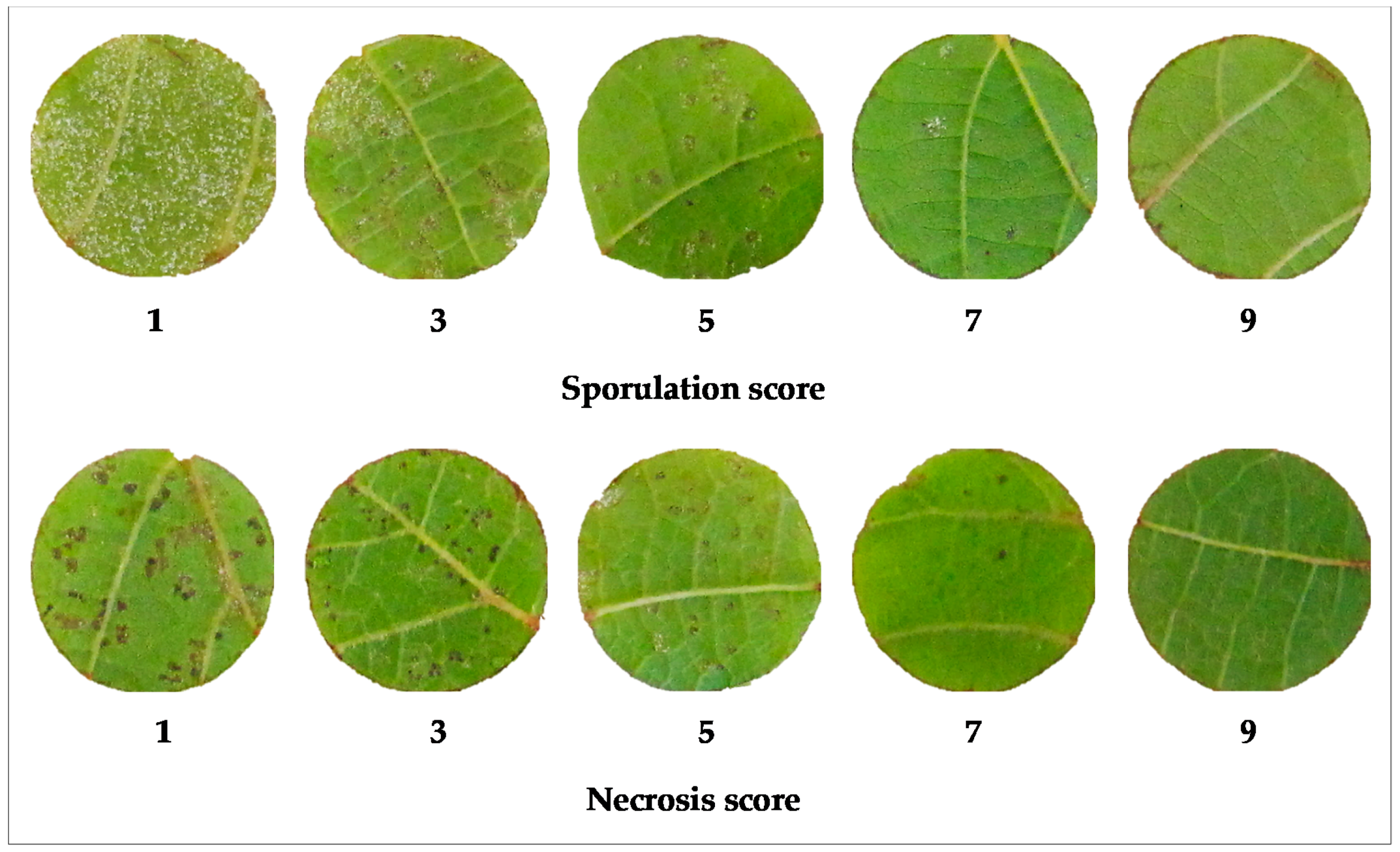
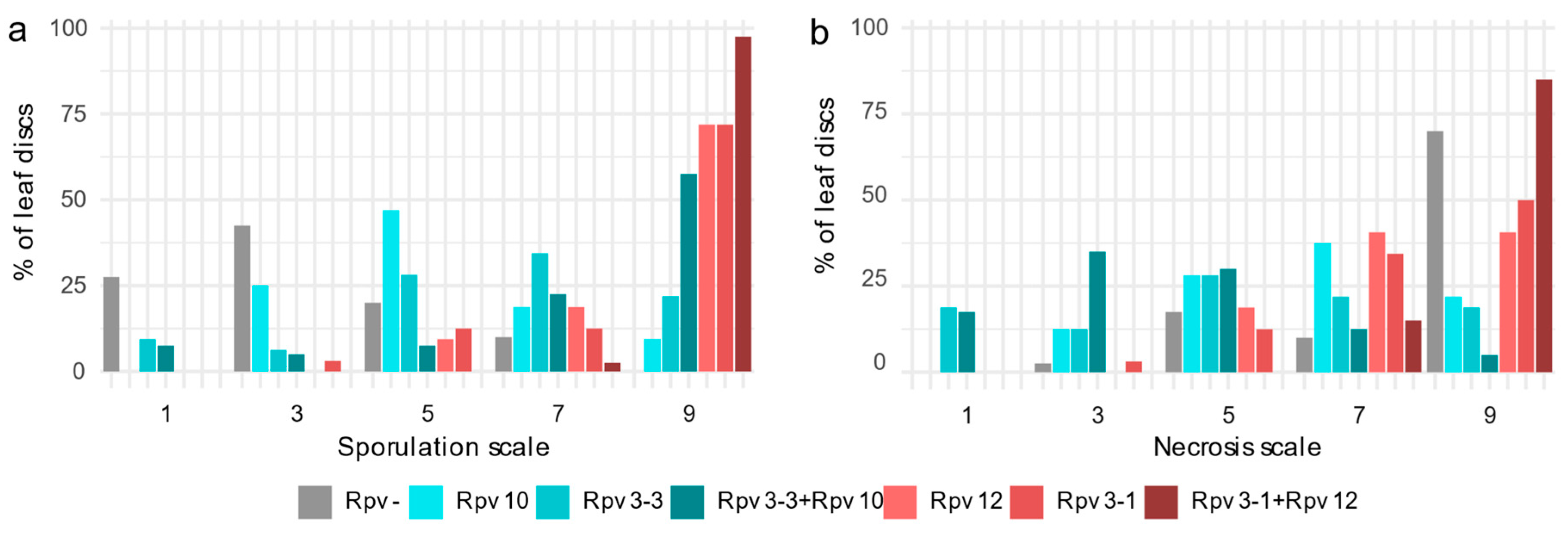
2.2.1. Discs Scores
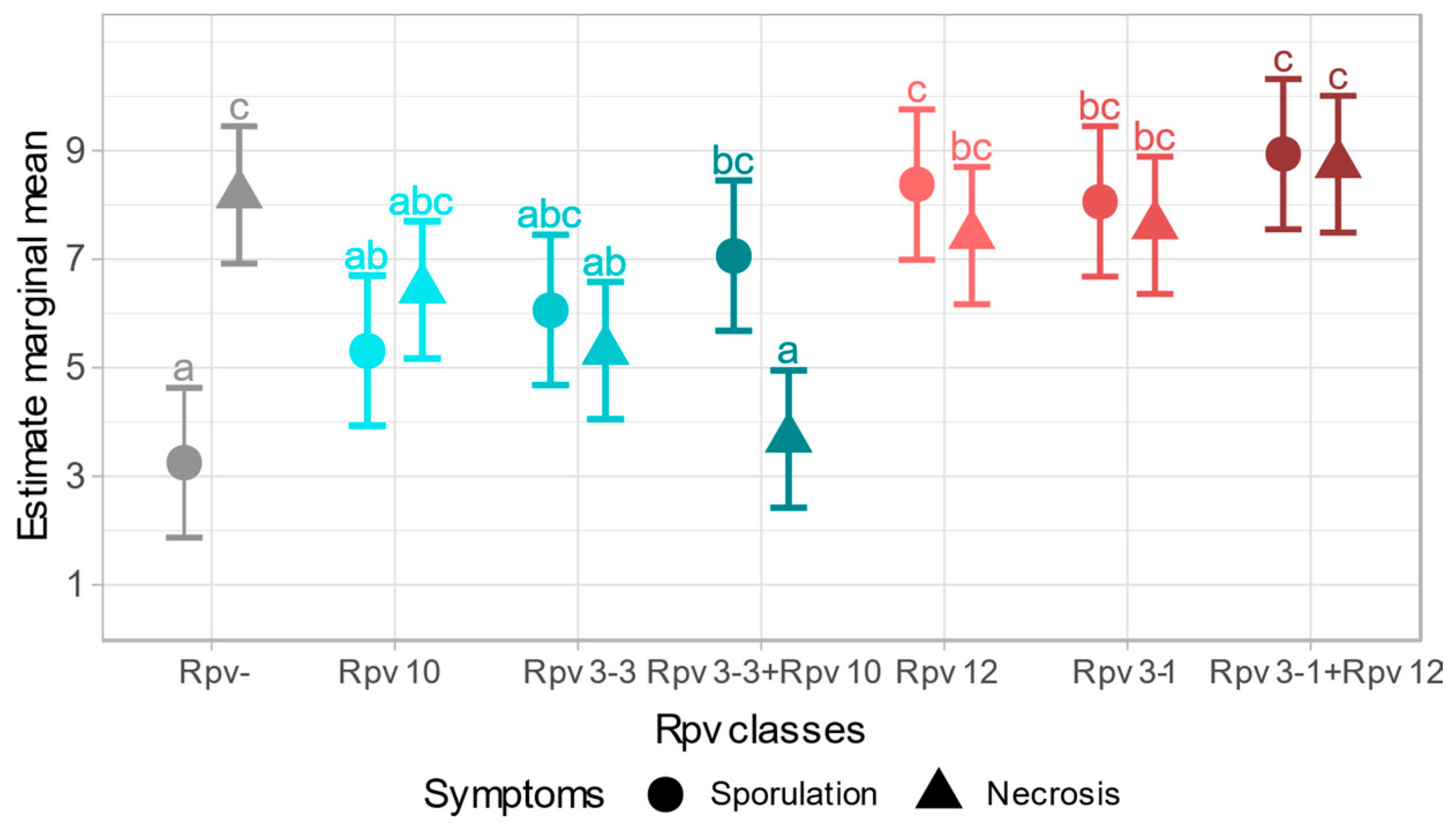
2.2.2. Sporulation and Necrosis Correlation
2.2.3. Symptomatic Profile of Different Rpv Classes
3. Discussion
4. Materials and Methods
4.1. Segregating Populations and Varieties under Study
4.2. Genotyping MAS
4.3. Phenotyping: Leaf Discs Infections
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Díez-Navajas, A.M.; Wiedemann-Merdinoglu, S.; Greif, C.; Merdinoglu, D. Nonhost Versus Host Resistance to the Grapevine Downy Mildew, Plasmopara viticola, Studied at the Tissue Level. Phytopathology 2008, 98, 776–780. [Google Scholar] [CrossRef]
- Cadle-Davidson, L. Variation within and between Vitis spp. for Foliar Resistance to the Downy Mildew Pathogen Plasmopara viticola. Plant Dis. 2008, 92, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Maul, E.; Sudharma, K.N.; Ganesh, A.; Hundemer, M.; Walk, M.; vom Weg, S.; Mahler-Ries, A.; Brühl, U.; Töpfer, R. Vitis International Variety Catalogue. Available online: www.vivc.de (accessed on 22 April 2020).
- Di Gaspero, G.; Copetti, D.; Coleman, C.; Castellarin, S.D.; Eibach, R.; Kozma, P.; Lacombe, T.; Gambetta, G.; Zvyagin, A.; Cindrić, P.; et al. Selective Sweep at the Rpv3 Locus during Grapevine Breeding for Downy Mildew Resistance. Theor. Appl. Genet. 2012, 124, 277–286. [Google Scholar] [CrossRef]
- Bellin, D.; Peressotti, E.; Merdinoglu, D.; Wiedemann-Merdinoglu, S.; Adam-Blondon, A.F.; Cipriani, G.; Morgante, M.; Testolin, R.; Di Gaspero, G. Resistance to Plasmopara viticola in Grapevine ‘Bianca’ Is Controlled by a Major Dominant Gene Causing Localised Necrosis at the Infection Site. Theor. Appl. Genet. 2009, 120, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Venuti, S.; Copetti, D.; Foria, S.; Falginella, L.; Hoffmann, S.; Bellin, D.; Cindrić, P.; Kozma, P.; Scalabrin, S.; Morgante, M.; et al. Historical Introgression of the Downy Mildew Resistance Gene Rpv12 from the Asian Species Vitis amurensis into Grapevine Varieties. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.M.; Salakhutdinov, I.; Akkurt, M.; Eibach, R.; Edwards, K.J.; Töpfer, R.; Zyprian, E.M. Quantitative Trait Locus Analysis of Fungal Disease Resistance Factors on a Molecular Map of Grapevine. Theor. Appl. Genet. 2004, 108, 501–515. [Google Scholar] [CrossRef] [PubMed]
- van Heerden, C.J.; Burger, P.; Vermeulen, A.; Prins, R. Detection of Downy and Powdery Mildew Resistance QTL in a ‘Regent’ × ‘RedGlobe’ Population. Euphytica 2014, 200, 281–295. [Google Scholar] [CrossRef]
- Vezzulli, S.; Malacarne, G.; Masuero, D.; Vecchione, A.; Dolzani, C.; Goremykin, V.; Mehari, Z.H.; Banchi, E.; Velasco, R.; Stefanini, M.; et al. The Rpv3-3 Haplotype and Stilbenoid Induction Mediate Downy Mildew Resistance in a Grapevine Interspecific Population. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Schwander, F.; Eibach, R.; Fechter, I.; Hausmann, L.; Zyprian, E.; Töpfer, R. Rpv10: A New Locus from the Asian Vitis Gene Pool for Pyramiding Downy Mildew Resistance Loci in Grapevine. Theor. Appl. Genet. 2012, 124, 163–176. [Google Scholar] [CrossRef]
- Eibach, R.; Zyprian, E.; Welter, L.; Töpfer, R. The Use of Molecular Markers for Pyramiding Resistance Genes in Grapevine Breeding. Vitis 2007, 46, 120–124. [Google Scholar]
- OIV. Descriptor List for Grape Varieties and Vitis Species, 2nd ed.; Office International de la Vigne et du Vin: Paris, France, 2009. [Google Scholar]
- Gómez-Zeledón, J.; Kaiser, M.; Spring, O. An Extended Leaf Disc Test for Virulence Assessment in Plasmopara viticola and Detection of Downy Mildew Resistance in Vitis. J. Plant Pathol. Microbiol. 2016, 7, 5. [Google Scholar] [CrossRef]
- Blasi, P.; Blanc, S.; Wiedemann-Merdinoglu, S.; Prado, E.; Rühl, E.H.; Mestre, P.; Merdinoglu, D. Construction of a Reference Linkage Map of Vitis amurensis and Genetic Mapping of Rpv8, a Locus Conferring Resistance to Grapevine Downy Mildew. Theor. Appl. Genet. 2011, 123, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Divilov, K.; Barba, P.; Cadle-Davidson, L.; Reisch, B.I. Single and Multiple Phenotype QTL Analyses of Downy Mildew Resistance in Interspecific Grapevines. Theor. Appl. Genet. 2018, 131, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Matasci, C.L.; Jermini, M.; Gobbin, D.; Gessler, C. Microsatellite Based Population Structure of Plasmopara viticola at Single Vine Scale. Eur. J. Plant Pathol. 2010, 127, 501–508. [Google Scholar] [CrossRef]
- Delmotte, F.; Mestre, P.; Schneider, C.; Kassemeyer, H.H.; Kozma, P.; Richart-Cervera, S.; Rouxel, M.; Delière, L. Rapid and Multiregional Adaptation to Host Partial Resistance in a Plant Pathogenic Oomycete: Evidence from European Populations of Plasmopara viticola, the Causal Agent of Grapevine Downy Mildew. Infect. Genet. Evol. 2014, 27, 500–508. [Google Scholar] [CrossRef]
- Welter, L.J.; Göktürk-Baydar, N.; Akkurt, M.; Maul, E.; Eibach, R.; Töpfer, R.; Zyprian, E.M. Genetic Mapping and Localization of Quantitative Trait Loci Affecting Fungal Disease Resistance and Leaf Morphology in Grapevine (Vitis vinifera L). Mol. Breed. 2007, 20, 359–374. [Google Scholar] [CrossRef]
- Foria, S.; Magris, G.; Morgante, M.; Di Gaspero, G. The Genetic Background Modulates the Intensity of Rpv3-Dependent Downy Mildew Resistance in Grapevine. Plant Breed. 2018, 137, 220–228. [Google Scholar] [CrossRef]
- Boso, S.; Kassemeyer, H.H. Different Susceptibility of European Grapevine Cultivars for Downy Mildew. Vitis 2008, 47, 39–49. [Google Scholar]
- Gindro, K.; Pezet, R.; Viret, O. Histological Study of the Responses of Two Vitis vinifera Cultivars (Resistant and Susceptible) to Plasmopara viticola Infections. Plant Physiol. Biochem. 2003, 41, 846–853. [Google Scholar] [CrossRef]
- Pezet, R.; Gindro, K.; Viret, O.; Spring, J.L. Glycosylation and Oxidative Dimerization of Resveratrol Are Respectively Associated to Sensitivity and Resistance of Grapevine Cultivars to Downy Mildew. Physiol. Mol. Plant Pathol. 2004, 65, 297–303. [Google Scholar] [CrossRef]
- Oerke, E.C.; Herzog, K.; Toepfer, R. Hyperspectral Phenotyping of the Reaction of Grapevine Genotypes to Plasmopara viticola. J. Exp. Bot. 2016, 67, 5529–5543. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, S.; Vecchione, A.; Stefanini, M.; Zulini, L. Downy Mildew Resistance Evaluation in 28 Grapevine Hybrids Promising for Breeding Programs in Trentino Region (Italy). Eur. J. Plant Pathol. 2018, 150, 485–495. [Google Scholar] [CrossRef]
- De Nardi, B.; Santellani, F.; Possamai, T.; Velasco, R. Breeding for Mildew Resistance in Grapevine to Improve Environmental and Socio-Economic Sustainability in Hotspot Areas of Veneto. Acta Hortic. 2019, 1248, 313–318. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M.; Walker, Z.I.; Smith, S. Mixed Effects Modelling for Nested Data. In Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 101–142. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org (accessed on 22 April 2020).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Christensen, R.H.B.; Singmann, H.; Dai, B.; Eigen, C. Lme4: Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Russell, L. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. R Package Version 1.3.2. 2019. Available online: https://cran.r-project.org/web/packagies/emmeans (accessed on 22 April 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Possamai, T.; Migliaro, D.; Gardiman, M.; Velasco, R.; De Nardi, B. Rpv Mediated Defense Responses in Grapevine Offspring Resistant to Plasmopara viticola. Plants 2020, 9, 781. https://doi.org/10.3390/plants9060781
Possamai T, Migliaro D, Gardiman M, Velasco R, De Nardi B. Rpv Mediated Defense Responses in Grapevine Offspring Resistant to Plasmopara viticola. Plants. 2020; 9(6):781. https://doi.org/10.3390/plants9060781
Chicago/Turabian StylePossamai, Tyrone, Daniele Migliaro, Massimo Gardiman, Riccardo Velasco, and Barbara De Nardi. 2020. "Rpv Mediated Defense Responses in Grapevine Offspring Resistant to Plasmopara viticola" Plants 9, no. 6: 781. https://doi.org/10.3390/plants9060781
APA StylePossamai, T., Migliaro, D., Gardiman, M., Velasco, R., & De Nardi, B. (2020). Rpv Mediated Defense Responses in Grapevine Offspring Resistant to Plasmopara viticola. Plants, 9(6), 781. https://doi.org/10.3390/plants9060781







