A Non-Invasive Analysis of Seed Vigor by Infrared Thermography
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Materials
2.2. Seed Germination and Vigor Tests
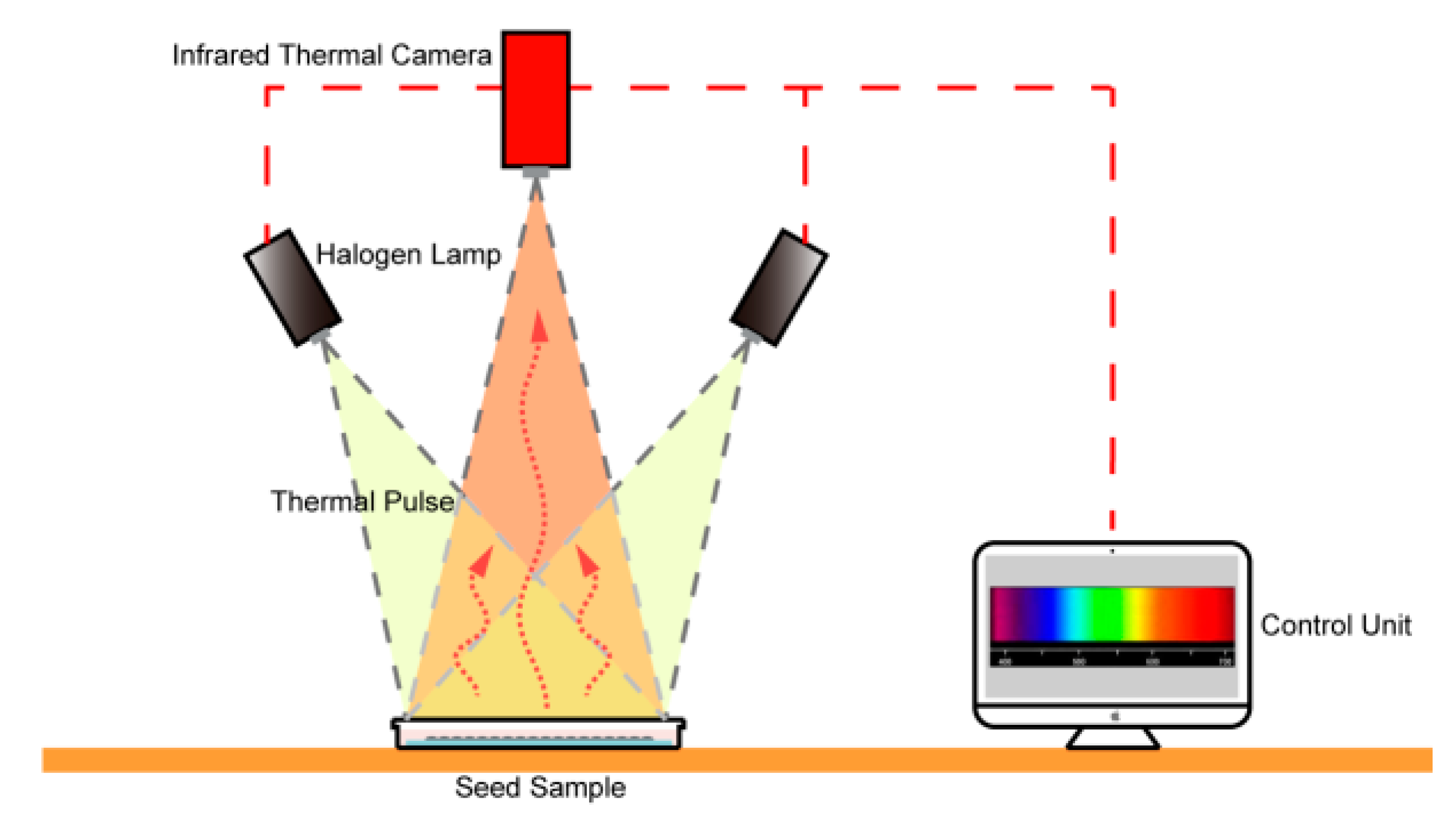
2.3. Setup of the Thermal Imaging System
2.4. Thermal Imaging of Seeds
2.5. Influence of Water Content on Seed Vigor Estimation
2.6. Validation of the Method Using O. sativa Seeds
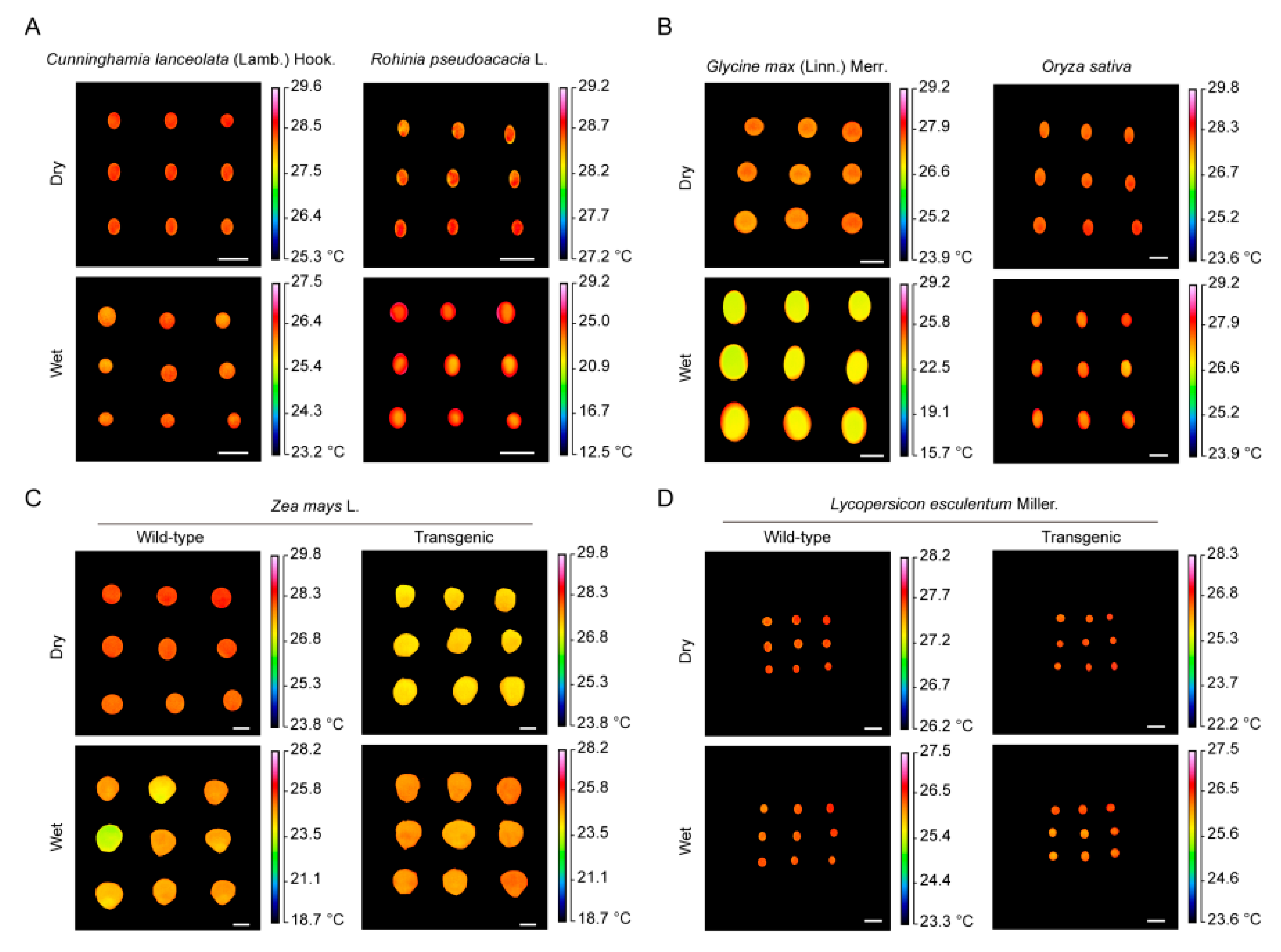
2.7. Thermal Imaging of Other Seeds
2.8. Data Analysis
3. Results
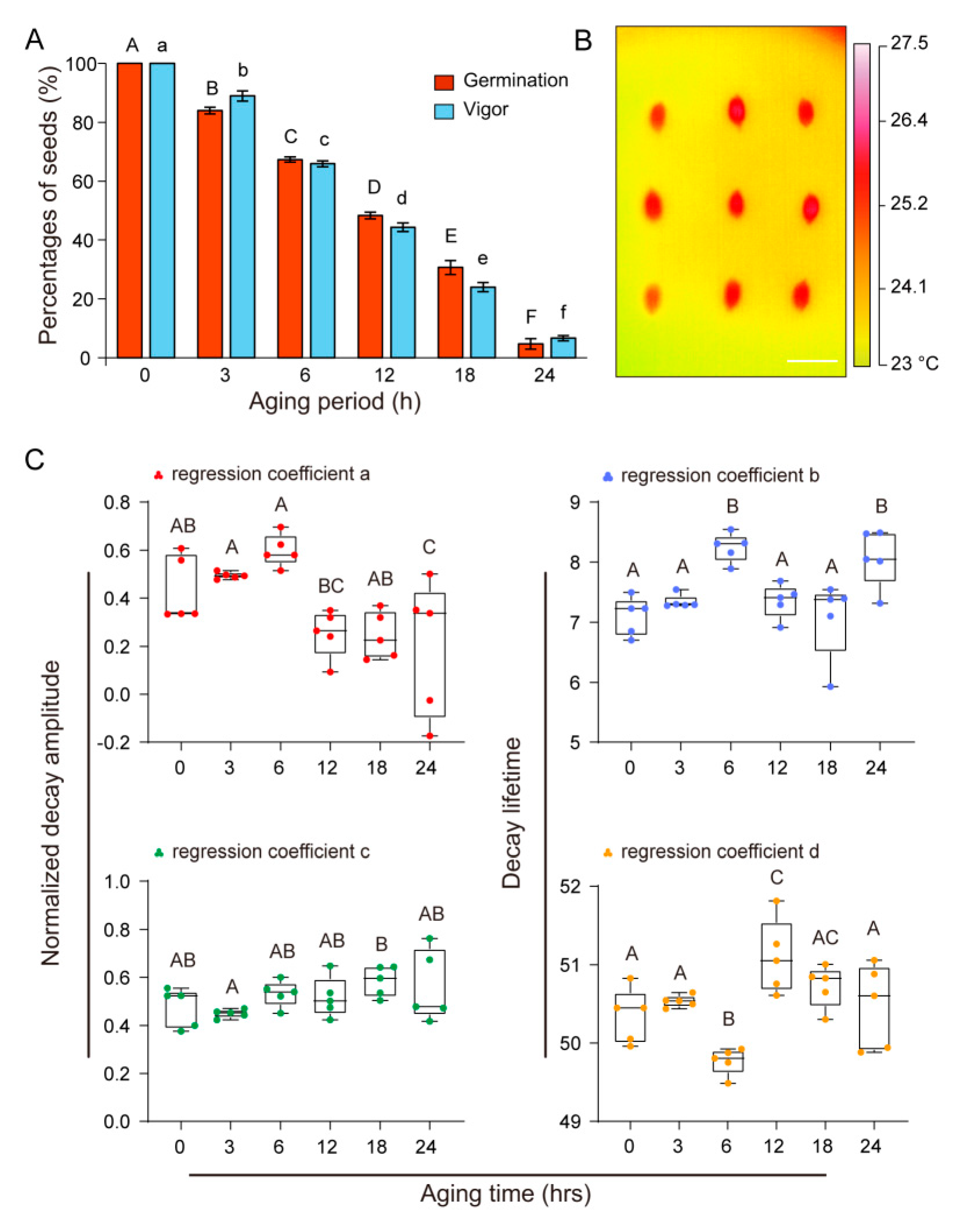
3.1. Germination and Vigor of U. pumila Seeds Decreased After Aging
3.2. Thermal Imaging of U. Pumila Seeds
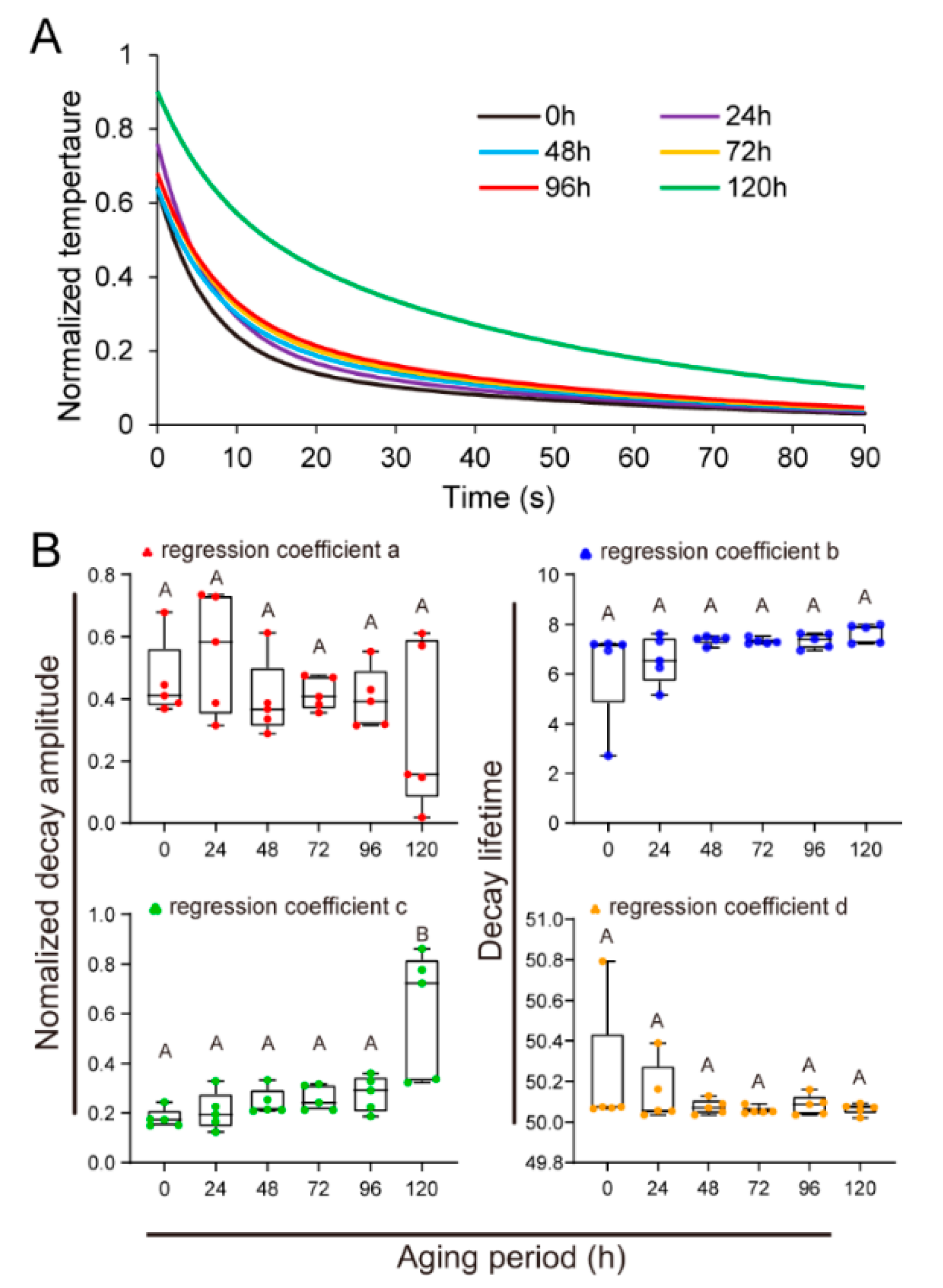
3.3. Time-Dependent Thermal Decay of U. Pumila Seeds
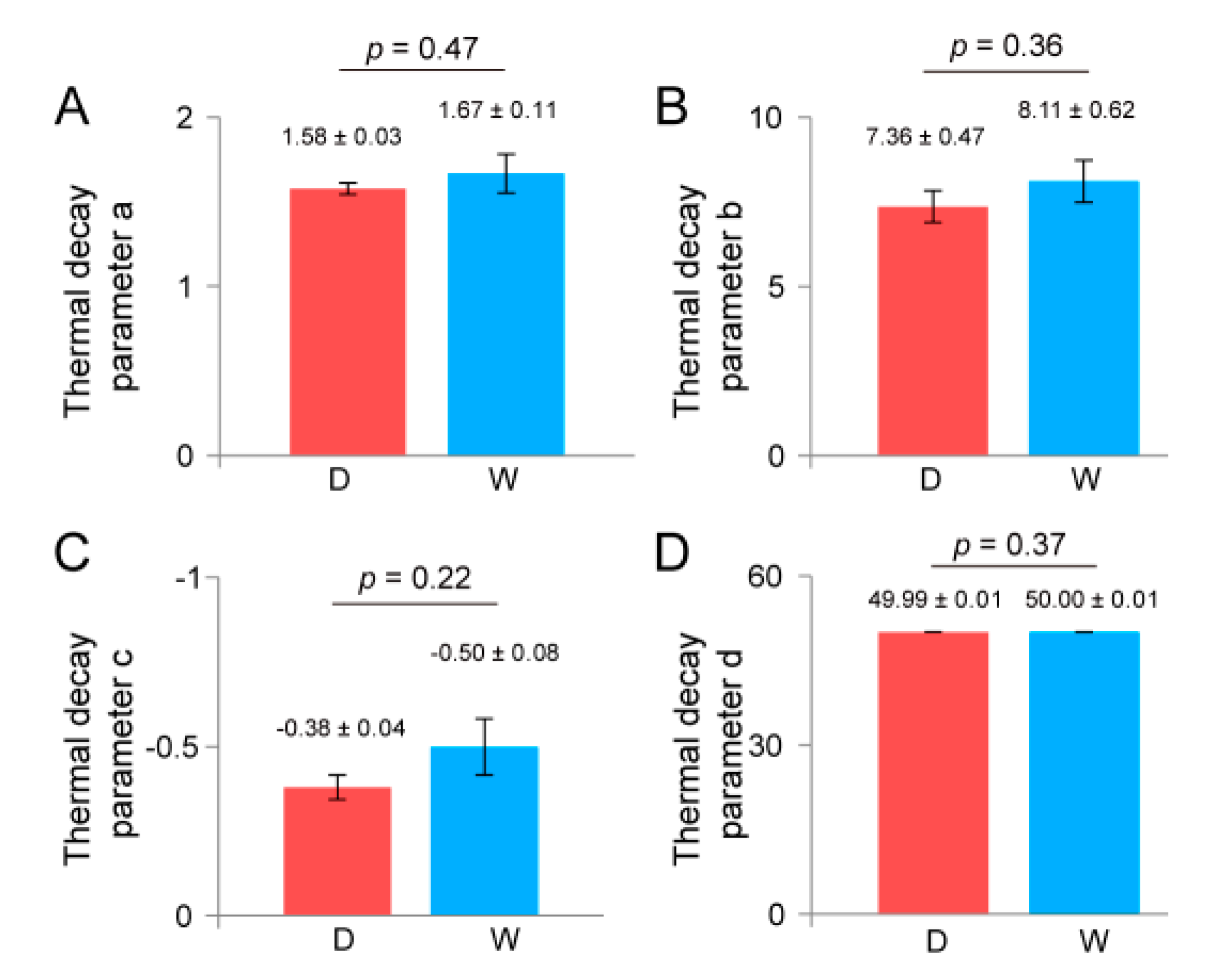
3.4. Influence of Water Content on Seed Vigor Estimation
3.5. Application of Seed Vigor Test by Infrared Thermography in O. sativa Seeds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDonald, M.B. Seed quality assessment. Seed Sci. Res. 1998, 8, 265–276. [Google Scholar] [CrossRef]
- Coolbear, P. Mechanisms of Seed Deterioration; Food Product Press: New York, NY, USA, 1995; pp. 223–277. [Google Scholar]
- Mcdonald, M.B. Seed deterioration: Physiology, repair and assessment. Seed Sci. Technol. 1999, 27, 177–237. [Google Scholar]
- Mondo, V.H.V.; Cicero, S.M.; Neto, D.D.; Pupim, T.L.; Dias, M.A.N. Seed vigor and initial growth of corn crop. J. Seed Sci. 2013, 35, 64–69. [Google Scholar] [CrossRef][Green Version]
- Yunbi, X.; Ping, L.; Cheng, Z.; Yanli, L.; Chuanxiao, X.; Xuecai, Z. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef]
- Yun, P.; Zhu, Y.; Wu, B.; Gao, G.; Sun, P.; Zhang, Q.; He, Y. Genetic mapping and confirmation of quantitative trait loci for grain chalkiness in rice. Mol. Breed. 2016, 36, 162. [Google Scholar] [CrossRef]
- Li, Y.; Colleoni, C.; Zhang, J.; Liang, Q.; Hu, Y.; Ruess, H.; Simon, R.; Liu, Y.; Liu, H.; Yu, G.; et al. Genomic analyses yield markers for identifying agronomically important genes in potato. Mol. Plant 2018, 11, 473–484. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Lu, Y.; Bhusal, S.J.; Song, Q.; Cregan, P.B.; Yen, Y.; Brown, M.; Jiang, G.L. Genome-wide scan for seed composition provides insights into soybean quality improvement and the impacts of domestication and breeding. Mol. Plant 2018, 3, 460–472. [Google Scholar] [CrossRef]
- Swinnen, G.; Goossens, A.; Pauwels, L. Lessons from domestication: Targeting cis-regulatory elements for crop improvement. Trends Plant Sci. 2016, 21, 506–515. [Google Scholar] [CrossRef]
- Schaart, J.G.; van de Wiel, C.C.M.; Lotz, L.A.P.; Smulders, M.J.M. Opportunities for products of new plant breeding techniques. Trends Plant Sci. 2016, 21, 438–449. [Google Scholar] [CrossRef]
- Tang, J.; Chu, C. MicroRNAs in crop improvement: Fine-tuners for complex traits. Nat. Plants 2017, 3, 17077. [Google Scholar] [CrossRef]
- Kaya, M.D. Conformity of vigor tests to determine the seed quality of safflower (Carthamus tinctorius L.) Cultivars. Aust. J. Crop Sci. 2014, 8, 455–459. [Google Scholar]
- Lombardi, T.; Fochetti, T.; Bertacchi, A.; Onnis, A. Germination requirements in a population of Typha latifolia. Aquat. Bot. 1997, 56, 1–10. [Google Scholar] [CrossRef]
- Valdes, P.A.; Ziobro, G.C.; Ferrera, R.S. Use of urease-bromothymol blue-agar method for large-scale testing of urine on grain and seeds. J. AOAC Int. 1996, 79, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Harris, D. Development and testing of “on-farm” seed priming. Adv. Agron. 2006, 90, 129–178. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, F.; He, Y.; Li, X. Application of hyperspectral imaging and chemometric calibrations for variety discrimination of maize seeds. Sensors 2012, 12, 17234–17246. [Google Scholar] [CrossRef]
- Calvi, G.P.; Aud, F.F.; Ferraz, I.D.K.; Pritchard, H.W.; Kranner, I. Analyses of several seed viability markers in individual recalcitrant seeds of Eugenia stipitata McVaugh with totipotent germination. Plant Biol. 2016, 19, 6–13. [Google Scholar] [CrossRef]
- Al-Turki, T.A.; Baskin, C.C. Determination of seed viability of eight wild Saudi Arabian species by germination and x-ray tests. Saudi J Biol Sci. 2016, 24, 822–829. [Google Scholar] [CrossRef][Green Version]
- Xin, X.; Wan, Y.; Wang, W.; Yin, G.; McLamore, E.S.; Lu, X. A real-time, non-invasive, micro-optrode technique for detecting seed viability by using oxygen influx. Sci. Rep. 2013, 3, 3057. [Google Scholar] [CrossRef]
- Kranner, I.; Kastbergerb, G.; Hartbauerb, M.; Pritcharda, H.W. Noninvasive diagnosis of seed viability using infrared thermography. Proc. Natl. Acad. Sci. USA 2010, 107, 3912–3917. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, R.; Bai, Y.; Hansen, L.D. Characterizing critical phases of germination in winter fat and malting barley with isothermal calorimetry. Seed Sci. Res. 2005, 15, 229–238. [Google Scholar] [CrossRef]
- Siddiqui, Z.S.; Cho, J.; Park, S.; Kwon, T.; Lee, G.; Jeong, M.; Kim, K.; Lee, S.; Park, S. Phenotyping of rice in salt stress environment using high-throughput infrared imaging. Acta Bot. Croat. 2014, 73, 312–321. [Google Scholar] [CrossRef]
- Mastrodimosa, N.; Lentzoub, D.; Templalexisb, C.; Tsitsigiannisa, D.I.; Xanthopoulos, G. Development of thermography methodology for early diagnosis of fungal infection in table grapes: The case of Aspergillus carbonarius. Comput. Electron. Agric. 2019, 165, 104972. [Google Scholar] [CrossRef]
- Belin, É.; Rousseau, D.; Rojas-Varela, J.; Demilly, D.; Wagner, M.H.; Cathala, M.H.; Dürr, C. Thermography as noninvasive functional imaging for monitoring seedling growth. Comput. Electron. Agric. 2011, 79, 236–240. [Google Scholar] [CrossRef]
- Wang, R.; Liu, X.; Mou, S.; Xu, S.; Zhang, Z. Temperature regulation of floral buds and floral thermogenicity in Magnolia denudata (Magnoliaceae). Trees-Struct Funct 2013, 27, 1755–1762. [Google Scholar] [CrossRef]
- Yuan, W.; Yu, Y.; Yue, Y.; Wang, J.; Zhang, F.; Dang, X. Use of infrared thermal imaging to diagnose health of Ammopiptanthus mongolicus in northwestern China. J. For. Res. 2015, 26, 605–612. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, R.H.; Zhang, Z.X.; Cheng, H.P. Progress in biological functions and regulation mechanisms of floral thermogenesis. Chin. Sci. Bull. 2015, 60, 3106–3113. [Google Scholar] [CrossRef][Green Version]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by ir and raman spectroscopy. Vib. Spectrosc. 2006, 43, 13–25. [Google Scholar] [CrossRef]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherland, 2011; p. 228. [Google Scholar] [CrossRef]
- Merlot, S.; Mustilli, A.C.; Genty, B.; North, H.; Lefebvre, V.; Sotta, B.; Vavasseur, A.; Giraudat, J. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 2002, 30, 601–609. [Google Scholar] [CrossRef]
- Ghiseok, K.; Hee, K.G.; Chi-Kook, A.; Yoonkyu, Y.; Byoung-Kwan, C. Mid-infrared lifetime imaging for viability evaluation of lettuce seeds based on time-dependent thermal decay characterization. Sensors 2013, 13, 2986–2996. [Google Scholar] [CrossRef]
- Barreto, L.C.; Garcia, Q.S. Accelerated ageing and subsequent imbibition affect seed viability and the efficiency of antioxidant system in macaw palm seeds. Acta Physiol. Plant 2017, 39, 72. [Google Scholar] [CrossRef]
- Hu, S.; Lübberstedt, T. Getting the ‘most’ out of crop improvement. Trends Plant. Sci. 2015, 20, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ma, G.; Wang, Q.; Yao, J.; Wang, Y.; Pritchard, H.W.; Wang, X. Spatial and temporal nature of reactive oxygen species production and programmed cell death in elm (Ulmus pumila L.) seeds during controlled deterioration. Plant Cell Environ. 2012, 35, 2045–2059. [Google Scholar] [CrossRef] [PubMed]
- Menezes, V.O.; Lopes, S.J.; Tedesco, S.B.; Henning, F.A.; Zen, H.D.; Mertz, L.M. Cytogenetic analysis of wheat seeds submitted to artificial aging stress. J. Seed Sci. 2014, 36, 71–78. [Google Scholar] [CrossRef][Green Version]
- de Tunes, L.M.; Pedroso, D.C.; Gadotti, G.I.; Muniz, M.F.B.; Barros, A.C.S.A.; Villela, F.A. Accelerated aging to assess parsley seed vigor. Hortic. Bras. 2013, 31, 457–460. [Google Scholar] [CrossRef][Green Version]
- Agelet, L.E.; Ellis, D.D.; Duvick, S.; Goggi, A.S.; Hurburgh, C.R.; Gardner, C.A. Feasibility of near infrared spectroscopy for analyzing corn kernel damage and viability of soybean and corn kernels. J. Cereal Sci. 2012, 55, 160–165. [Google Scholar] [CrossRef]
- Agelet, L.E.; Hurburgh, C.R. Limitations and current applications of near infrared spectroscopy for single seed analysis. Talanta 2014, 121, 288–299. [Google Scholar] [CrossRef]
- Kim, G.; Kim, G.; Lohumi, S.; Kang, J.; Cho, B. Viability estimation of pepper seeds using time-resolved photothermal signal characterization. Infrared Phys. Technol. 2014, 67, 214–221. [Google Scholar] [CrossRef]
- Sen, M.; Lei, Y.; Jiaxin, L.; Hua, Q.; Qinjuan, L. A classification method for seed viability assessment with infrared thermography. Sensors 2017, 17, 845. [Google Scholar] [CrossRef]
- Sun, X.M.; Han, Y.P.; Wang, H.H. Near-infrared light scattering by ice-water mixed clouds. Prog. Electromagn. Res. 2006, 61, 133–142. [Google Scholar] [CrossRef][Green Version]

| Seed Vigor of U. pumila | a | b | c | d |
|---|---|---|---|---|
| Correlation coefficients | 0.77 | −0.83 | −1.00 | 0.71 |
| p values | 0.07 | 0.04 | 0.00 | 0.11 |
| Seed Vigor of O. sativa | a | b | c | d |
|---|---|---|---|---|
| Correlation coefficients | 0.77 | −0.20 | −0.83 | −0.37 |
| p values | 0.07 | 0.70 | 0.04 | 0.47 |
| Regression Equation | Ulmus pumila | Oryza sativa |
|---|---|---|
| Polynomial * | y = 1292c2 − 1203c + 276 R2 = 0.94 | y = −624c2 + 10c + 220 R2 = 0.77 |
| Power | y = 9.67c−1.29 R2 = 0.84 | y = 0.24c−7.79 R2 = 0.57 |
| Exponential | y = 140.70e−3.35c R2 = 0.76 | y = 111519e−15.25c R2 = 0.58 |
| Linear | y = −152.71c + 104.86 R2 = 0.62 | y = −633.87c + 384.52 R2 = 0.77 |
| Logarithmic | y = −61.26ln(c) − 20.30 R2 = 0.75 | y = −324.50ln(c) − 158.62 R2 = 0.77 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, Z.; Li, J.; Zhang, X.; Wang, R. A Non-Invasive Analysis of Seed Vigor by Infrared Thermography. Plants 2020, 9, 768. https://doi.org/10.3390/plants9060768
Liu L, Wang Z, Li J, Zhang X, Wang R. A Non-Invasive Analysis of Seed Vigor by Infrared Thermography. Plants. 2020; 9(6):768. https://doi.org/10.3390/plants9060768
Chicago/Turabian StyleLiu, Liya, Zhongsi Wang, Jing Li, Xi Zhang, and Ruohan Wang. 2020. "A Non-Invasive Analysis of Seed Vigor by Infrared Thermography" Plants 9, no. 6: 768. https://doi.org/10.3390/plants9060768
APA StyleLiu, L., Wang, Z., Li, J., Zhang, X., & Wang, R. (2020). A Non-Invasive Analysis of Seed Vigor by Infrared Thermography. Plants, 9(6), 768. https://doi.org/10.3390/plants9060768






