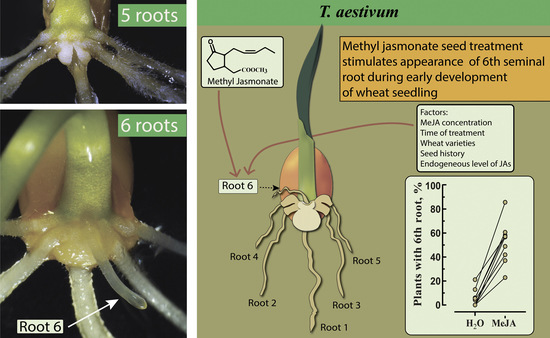
Regulation of Sixth Seminal Root Formation by Jasmonate in Triticum aestivum L.
Abstract
1. Introduction
2. Results
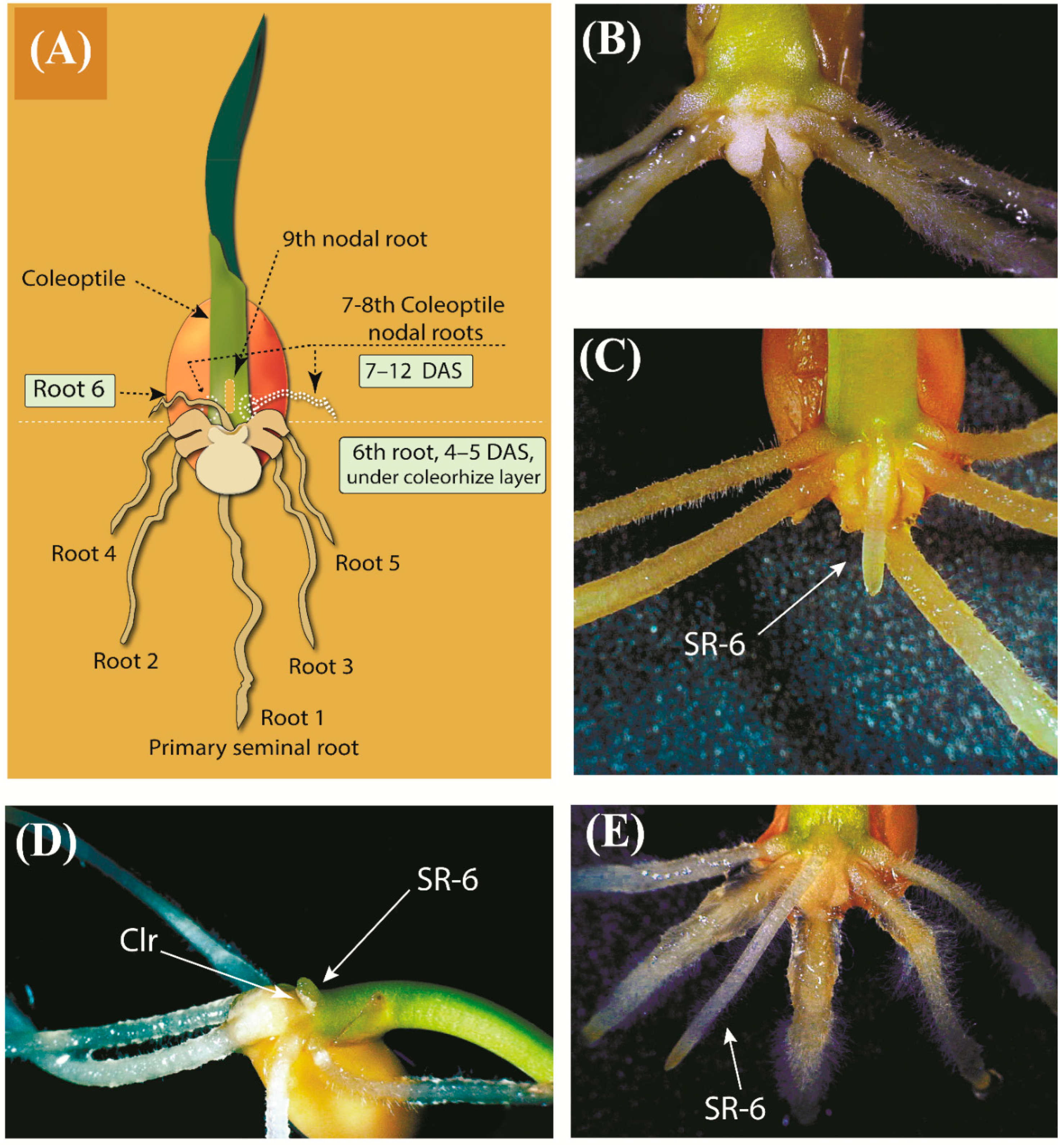
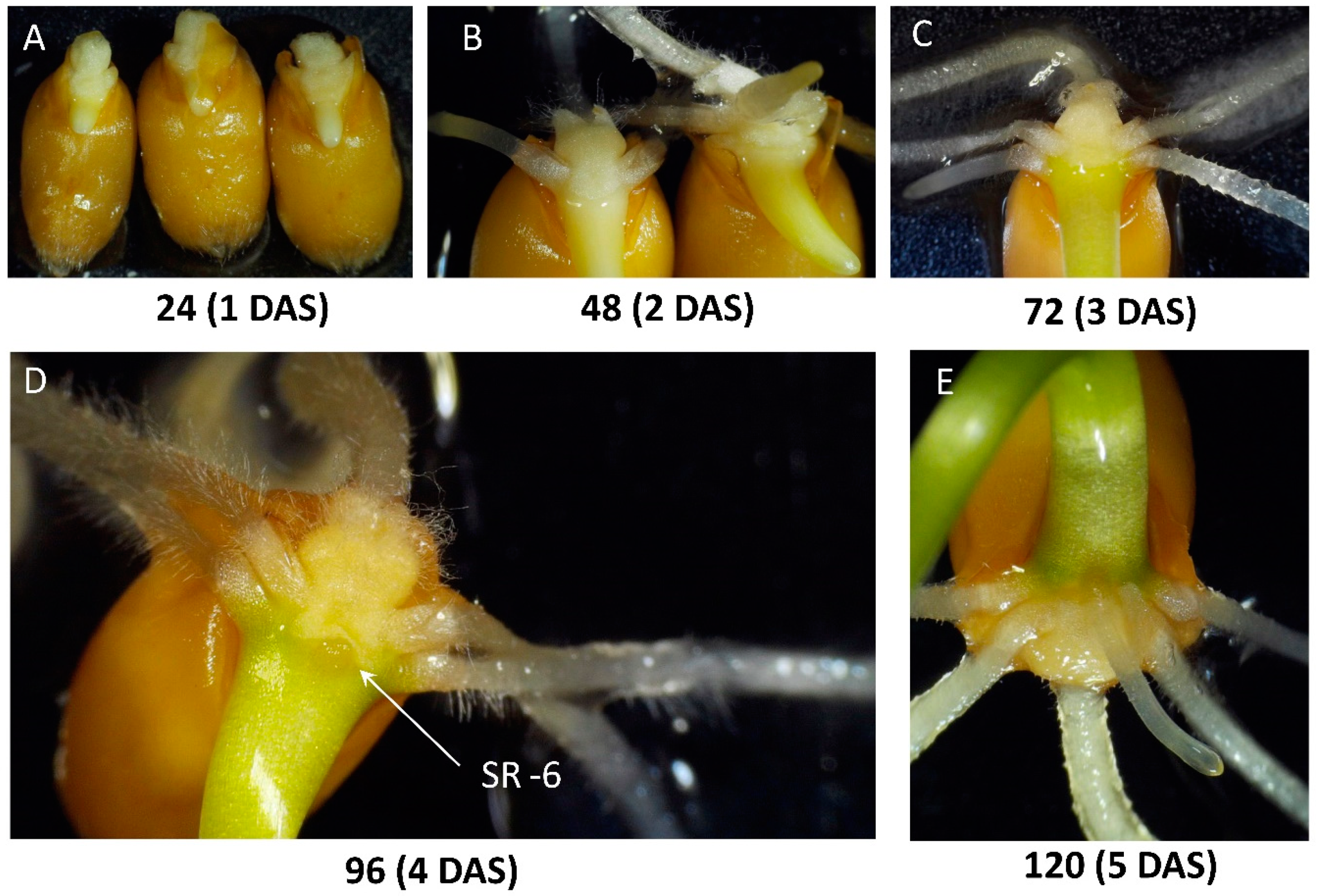
2.1. Observations on the Early Development of the Root System
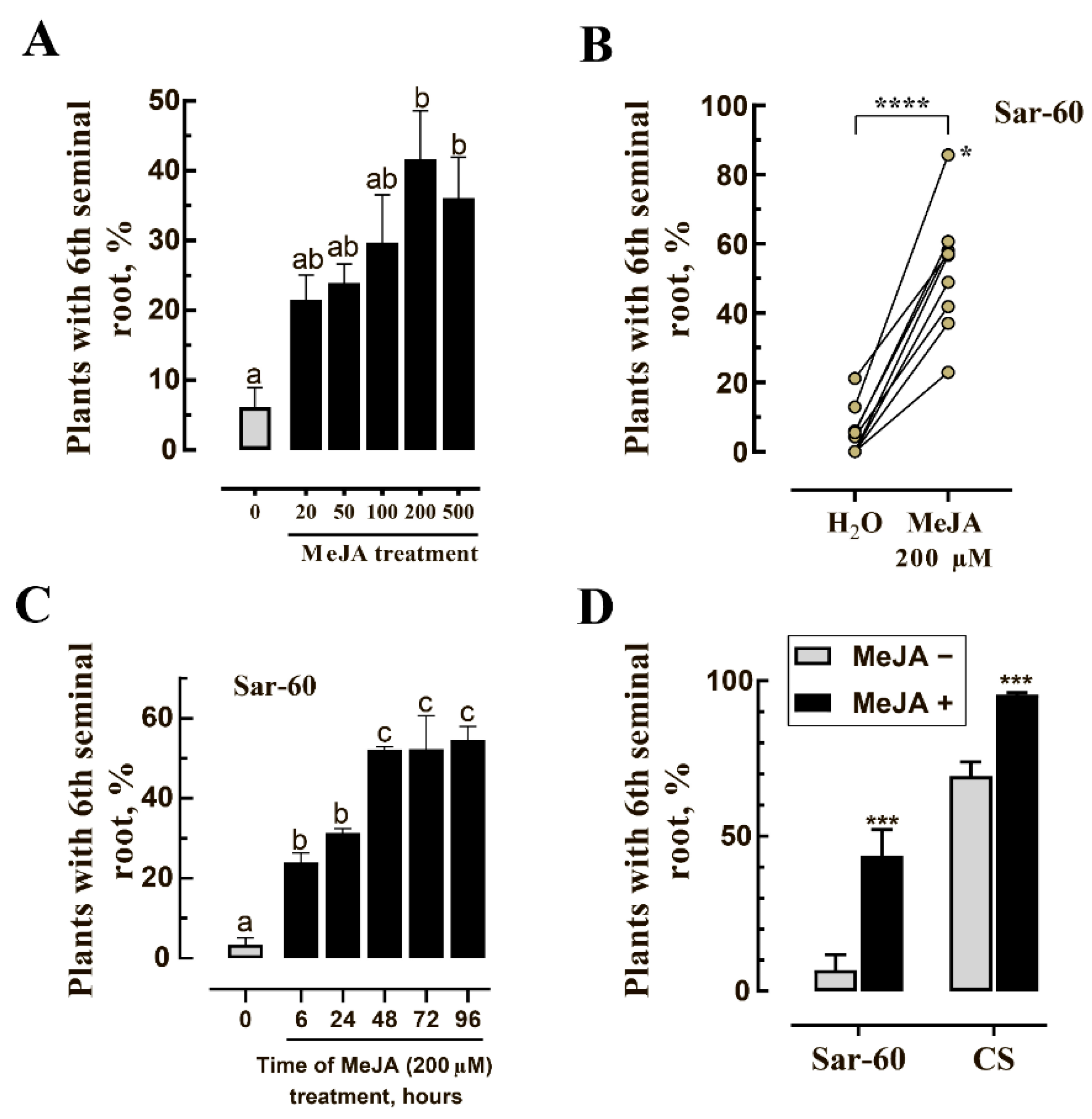
2.2. Exogenous Treatment of Non-Transgenic Seeds with MeJA
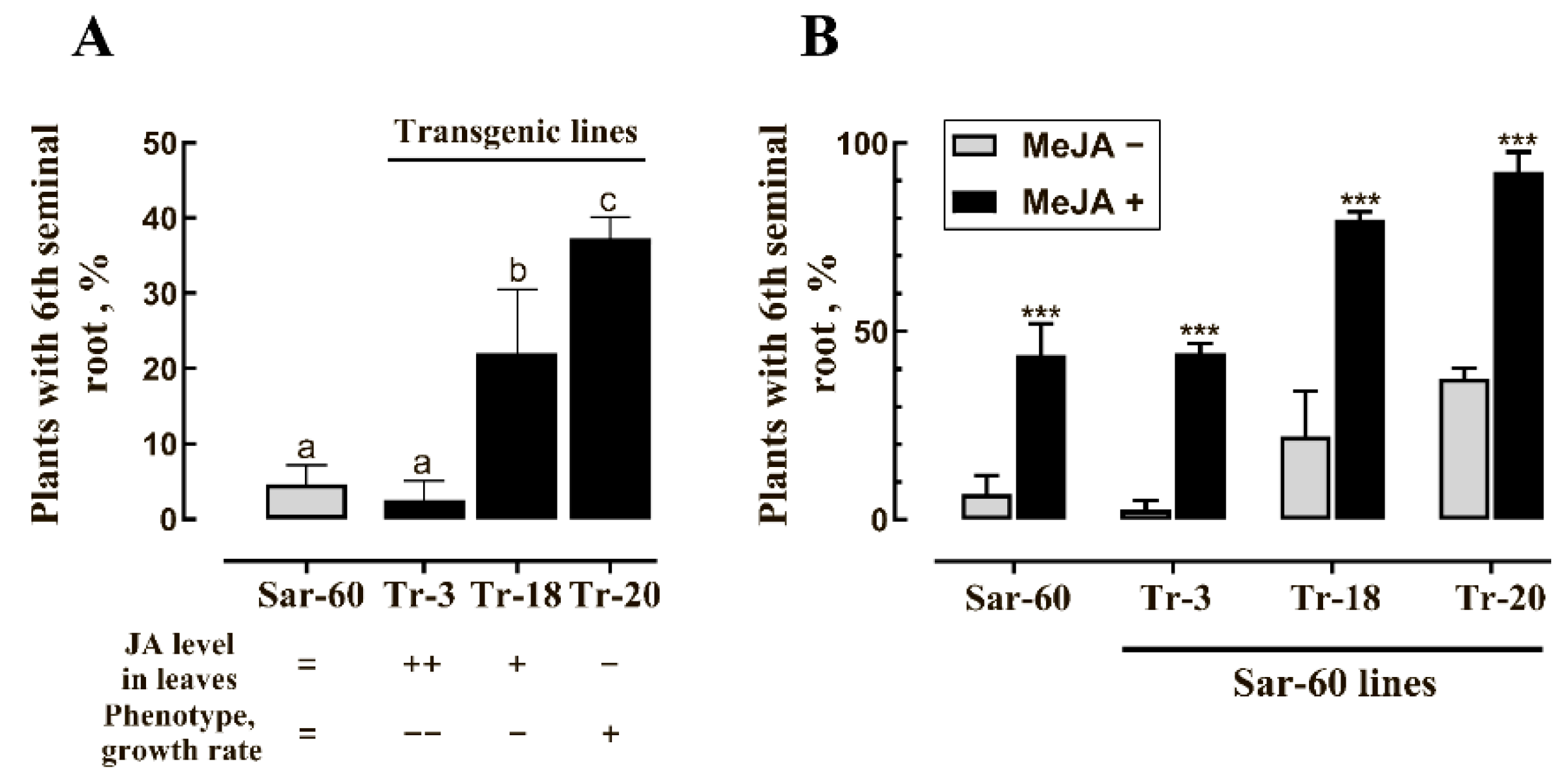
2.3. Analysis of Transgenic Wheat Plants Overexpressing AtOPR3
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robertson, B.M.; Waines, J.G.; Gill, B.S. Genetic variability for seedling root numbers in wild and domesticated wheats. Crop Sci. 1979, 19, 843–847. [Google Scholar] [CrossRef]
- Golan, G.; Hendel, E.; Mendez Espitia, G.E.; Schwartz, N.; Peleg, Z. Activation of seminal root primordia during wheat domestication reveals underlying mechanisms of plant resilience. Plant Cell Environ. 2018, 41, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Hoshikawa, K. Studies on the ripening of wheat grain: 7. Development of embryo with special reference to the differentiation of seminal roots. Jpn. J. Crop Sci. 1964, 33, 119–124. [Google Scholar] [CrossRef][Green Version]
- Meyer, W.S. Seminal Roots of Wheat: Manipulation of Their Geometry to Increase the Availability of Soil Water and to Improve the Efficiency of Water Use. Ph.D. Thesis, University of Adelaide, Adelaide, Australia, 1976. Available online: https://digital.library.adelaide.edu.au/dspace/handle/2440/21044 (accessed on 15 January 2021).
- Kirby, E.J.M. Botany of the wheat plant. In Bread Wheat. Improvement and Production; Curtis, B.C., Rajaram, S., Gomez Macpherson, H., Eds.; Food and Agriculture Organisation: Rome, Italy, 2002; pp. 30–52. [Google Scholar]
- Adeleke, E.; Millas, R.; McNeal, W.; Faris, J.; Taheri, A. Variation analysis of root system development in wheat seedlings using root phenotyping system. Agronomy 2020, 10, 206. [Google Scholar] [CrossRef]
- Hamada, A.; Nitta, M.; Nasuda, S.; Kato, K.; Fujita, M.; Matsunaka, H.; Okumoto, Y. Novel QTLs for growth angle of seminal roots in wheat (Triticum aestivum L.). Plant Soil 2012, 354, 395–405. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; de Voil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- O’Brien, L. Genetic variability of root growth in wheat (Triticum aestivum L.). Aust. J. Agric. Res. 1979, 30, 587–595. [Google Scholar] [CrossRef]
- Shorinola, O.; Kaye, R.; Golan, G.; Peleg, Z.; Kepinski, S.; Uauy, C. Genetic screening for mutants with altered seminal root numbers in hexaploid wheat using a high-throughput root phenotyping platform. G3 (Bethesda) 2019, 9, 2799–2809. [Google Scholar] [CrossRef]
- Canè, M.A.; Maccaferri, M.; Nazemi, G.; Salvi, S.; Francia, R.; Colalongo, C.; Tuberosa, R. Association mapping for root architectural traits in durum wheat seedlings as related to agronomic performance. Mol. Breed. 2014, 34, 1629–1645. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Weiner, J.; Yu, M.-X.; Li, F.-M. Evolutionary agroecology: Trends in root architecture during wheat breeding. Evol. Appl. 2018, 12, 733–743. [Google Scholar] [CrossRef]
- McCall, M.A. Developmental anatomy and homologies in wheat. J. Agric. Res. 1934, 48, 283–321. [Google Scholar]
- Kuhlmann, H.; Barraclough, P.B. Comparison between the seminal and nodal root systems of winter wheat in their activity for N and K uptake. Pflanzenernaehr. Bodenk. 1987, 150, 24–30. [Google Scholar] [CrossRef]
- Slack, S.; York, L.M.; Roghazai, Y.; Lynch, J.; Bennett, M.; Foulkes, J. Wheat shovelomics ii: Revealing relationships between root crown traits and crop growth. bioRxiv 2018, 280917. [Google Scholar] [CrossRef]
- Gregory, P.; McGowan, M.; Biscoe, P.; Hunter, B. Water relations of winter wheat: 1. Growth of the root system. J. Agric. Sci. 1978, 91, 91–102. [Google Scholar] [CrossRef]
- Pinthus, M.G.; Eshel, Y. Observation on the development of the root system of some wheat varieties. Israel J. Agric. Res. 1969, 12, 13–20. [Google Scholar]
- Sallans, B.J. The importance of various roots to the wheat plant. Sci. Agric. 1942, 23, 17–26. [Google Scholar]
- Manske, G.; Vlek, P. Root architecture—Wheat as a model plant. In Plant Roots: The Hidden Half; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 249–259. [Google Scholar]
- Kirkegaard, J.A.; Lilley, J.M. Root penetration rate a benchmark to identify soil and plant limitations to rooting depth in wheat. Aust. J. Exp. Agric. 2007, 47, 590. [Google Scholar] [CrossRef]
- Morozov, P.V. Deposition of the embryonic roots in the embryo of hybrid seed of spring wheat. Selec. Semenovod 1950, 17, 28–35. [Google Scholar]
- Troughton, A.; Whittington, W.J.; Troughton, A.; Whittington, W.J. The Significance of Genetic Variation in Root System; Butterworths: London, UK, 1970; pp. 296–313. [Google Scholar]
- Reynolds, M.; Tuberosa, R. Translational research impacting on crop productivity in drought-prone environments. Curr. Opin. Plant Biol. 2008, 11, 171–179. [Google Scholar] [CrossRef]
- Richards, R.A. Genetic opportunities to improve cereal root systems for dryland agriculture. Plant Prod. Sci. 2008, 11, 12–16. [Google Scholar] [CrossRef]
- El Hassouni, K.; Alahmad, S.; Belkadi, B.; Filali-Maltouf, A.; Hickey, L.T.; Bassi, F.M. Root system architecture and its association with yield under different water regimes in durum wheat. Crop Sci. 2018, 58, 2331–2346. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Chang, X.; Jing, R. Mapping QTLs for seedling root traits in a doubled haploid wheat population under different water regimes. Euphytica 2013, 189, 51–66. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Wingen, L.U.; Griffiths, M.; Pound, M.P.; Gaju, O.; Foulkes, M.J.; Le Gouis, J.; Griffiths, S.; Bennett, M.J.; King, J.; et al. Phenotyping pipeline reveals major seedling root growth QTL in hexaploid wheat. J. Exp. Bot. 2015, 66, 2283–2292. [Google Scholar] [CrossRef]
- Iannucci, A.; Marone, D.; Russo, M.A.; De Vita, P.; Miullo, V.; Ferragonio, P.; Blanco, A.; Gadaleta, A.; Mastrangelo, A.M. Mapping QTL for root and shoot morphological traits in a durum wheat × T. dicoccum segregating population at seedling stage. Int. J. Genom. 2017, 6876393. [Google Scholar] [CrossRef]
- Ma, J.; Luo, W.; Zhang, H.; Zhou, X.-H.; Qin, N.-N.; Wei, Y.-M.; Liu, Y.-X.; Jiang, Q.-T.; Chen, G.-Y.; Zheng, Y.-L.; et al. Identification of quantitative trait loci for seedling root traits from Tibetan semi-wild wheat (Triticum aestivum subsp. tibetanum). Genome 2017, 60, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; He, X.; Liu, D.; Li, J.; Zhao, X.; Li, B.; Tong, Y.; Zhang, A.; Li, Z. Major quantitative trait loci for seminal root morphology of wheat seedlings. Mol. Breed. 2012, 30, 139–148. [Google Scholar] [CrossRef]
- Sanguineti, M.C.; Li, S.; Maccaferri, M.; Corneti, S.; Rotondo, F.; Chiari, T.; Tuberosa, R. Genetic dissection of seminal root architecture in elite durum wheat germplasm. Ann. Appl. Biol. 2007, 151, 291–305. [Google Scholar] [CrossRef]
- Maccaferri, M.; El-Feki, W.; Nazemi, G.; Salvi, S.; Canè, M.A.; Colalongo, M.C.; Stefanelli, S.; Tuberosa, R. Prioritizing quantitative trait loci for root system architecture in tetraploid wheat. J. Exp. Bot. 2016, 67, 1161–1178. [Google Scholar] [CrossRef]
- Manner, R. The number of seminal roots in certain species of wheat. Plant Breed. Abstr. 1965, 35, 3. [Google Scholar]
- Williams, R. The physiology of growth in the wheat plant I. Seedling growth and the pattern of growth at the shoot apex. Aust. J. Biol. Sci. 1960, 13, 401–428. [Google Scholar] [CrossRef][Green Version]
- Meyer, W.; Alston, A. Wheat responses to seminal root geometry and subsoil water. Agron. J. 1978, 70, 981–986. [Google Scholar] [CrossRef]
- Huang, B.R.; Taylor, H.M.; McMichael, B.L. Growth and development of seminal and crown roots of wheat seedlings as affected by temperature. Environ. Exp. Bot. 1991, 31, 471–477. [Google Scholar] [CrossRef]
- Wasternack, C.; Strnad, M. Jasmonates: News on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int. J. Mol. Sci. 2018, 19, 2539. [Google Scholar] [CrossRef] [PubMed]
- Feussner, I.; Wasternack, C. The lipoxygenase pathway. Annu. Rev. Plant Biol. 2002, 53, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Schaller, A.; Stintzi, A. Enzymes in jasmonate biosynthesis—Structure, function, regulation. Phytochemistry 2009, 70, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
- Stintzi, A.; Browse, J. The Arabidopsis male-sterile mutant, opr3, lacks the 12-oxophytodienoic acid reductase required for jasmonate synthesis. Proc. Natl. Acad. Sci. USA 2000, 97, 10625–10630. [Google Scholar] [CrossRef]
- Miersch, O.; Wasternack, C. Octadecanoid and jasmonate signaling in tomato (Lycopersicon esculentum mill.) leaves: Endogenous jasmonates do not induce jasmonate biosynthesis. Biol. Chem. 2000, 381, 715–722. [Google Scholar] [CrossRef]
- Seo, H.S.; Song, J.T.; Cheong, J.J.; Lee, Y.H.; Lee, Y.W.; Hwang, I.; Lee, J.S.; Choi, Y.D. Jasmonic acid carboxyl methyltransferase: A key enzyme for jasmonate-regulated plant responses. Proc. Natl. Acad. Sci. USA 2001, 98, 4788–4793. [Google Scholar] [CrossRef]
- Fonseca, S.; Chini, A.; Hamberg, M.; Adie, B.; Porzel, A.; Kramell, R.; Miersch, O.; Wasternack, C.; Solano, R. (+)-7-iso-jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat. Chem. Biol. 2009, 5, 344–350. [Google Scholar] [CrossRef]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Hickman, R.; Van Verk, M.C.; Van Dijken, A.J.H.; Mendes, M.P.; Vroegop-Vos, I.A.; Caarls, L.; Steenbergen, M.; Van der Nagel, I.; Wesselink, G.J.; Jironkin, A.; et al. Architecture and dynamics of the jasmonic acid gene regulatory network. Plant Cell 2017, 29, 2086–2105. [Google Scholar] [CrossRef] [PubMed]
- Woldemariam, M.G.; Onkokesung, N.; Baldwin, I.T.; Galis, I. Jasmonoyl-l-isoleucine hydrolase 1 (JIH1) regulates jasmonoyl-l-isoleucine levels and attenuates plant defenses against herbivores. Plant J. 2012, 72, 758–767. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Suzumura, N.; Mitoma, M.; Matsuo, S.; Ikeuchi, T.; Mori, M.; Murakami, K.; Ozaki, Y.; Matsumoto, M.; Uragami, A.; et al. Comparative de novo transcriptome profiles in Asparagus officinalis and A. kiusianus during the early stage of Phomopsis asparagi infection. Sci. Rep. 2017, 7, 2608. [Google Scholar] [CrossRef] [PubMed]
- Lyons, R.; Manners, J.M.; Kazan, K. Jasmonate biosynthesis and signaling in monocots: A comparative overview. Plant Cell Rep. 2013, 32, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Falasca, G.; Kevers, C.; Rocca, L.M.; Zadra, C.; Altamura, M.M. Adventitious rooting is enhanced by methyl jasmonate in tobacco thin cell layers. Planta 2009, 231, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Raya-González, J.; Pelagio-Flores, R.; López-Bucio, J. The jasmonate receptor COI1 plays a role in jasmonate-induced lateral root formation and lateral root positioning in Arabidopsis thaliana. J. Plant Physiol. 2012, 169, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, D.; Chetelat, A.; Acosta, I.F.; Goossens, J.; Pauwels, L.; Goossens, A.; Dreos, R.; Alfonso, E.; Farmer, E.E. Multilayered organization of jasmonate signalling in the regulation of root growth. PLoS Genet. 2015, 11, e1005300. [Google Scholar] [CrossRef]
- Tung, P.; Hooker, T.S.; Tampe, P.A.; Reid, D.M.; Thorpe, T.A. Jasmonic acid: Effects on growth and development of isolated tomato roots cultured in vitro. Int. J. Plant Sci. 1996, 157, 713–721. [Google Scholar] [CrossRef]
- Gutjahr, C.; Riemann, M.; Müller, A.; Düchting, P.; Weiler, E.; Nick, P. Cholodny-Went revisited: A role for jasmonate in gravitropism of rice coleoptiles. Planta 2005, 222, 575–585. [Google Scholar] [CrossRef]
- Rufo, R.; Salvi, S.; Royo, C.; Soriano, J.M. Exploring the genetic architecture of root-related traits in mediterranean bread wheat landraces by genome-wide association analysis. Agronomy 2020, 10, 613. [Google Scholar] [CrossRef]
- Xu, F.; Chen, S.; Yang, X.; Zhou, S.; Chen, X.; Wang, J.; Zhang, Z.; Huang, Y.; Song, M.; Han, S.; et al. Genome-wide association study on root traits under different cultivation patterns in wheat. PREPRINT (Version 1). Res. Sq. 2020. [Google Scholar] [CrossRef]
- Pigolev, A.V.; Miroshnichenko, D.N.; Pushin, A.S.; Terentyev, V.V.; Boutanayev, A.M.; Dolgov, S.V.; Savchenko, T.V. Overexpression of Arabidopsis OPR3 in hexaploid wheat (Triticum aestivum L.) alters plant development and freezing tolerance. Int. J. Mol. Sci. 2018, 19, 3989. [Google Scholar] [CrossRef] [PubMed]
- Chehab, E.W.; Kim, S.; Savchenko, T.; Kliebenstein, D.; Dehesh, K.; Braam, J. Intronic T-DNA insertion renders Arabidopsis OPR3 a conditional jasmonic acid-producing mutant. Plant Physiol. 2011, 156, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.V.; Zastrijnaja, O.M.; Klimov, V.V. Oxylipins and plant abiotic stress resistance. Biochemistry 2014, 79, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. A bypass in jasmonate biosynthesis—The OPR3-independent formation. Trends Plant Sci. 2018, 23, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, B.; Geng, S.; Arellano, C.; Chen, S.; Qu, R. Effects of overexpression of jasmonic acid biosynthesis genes on nicotine accumulation in tobacco. Plant Direct 2018, 2, e00036. [Google Scholar] [CrossRef] [PubMed]
- Laudert, D.; Schaller, F.; Weiler, E.W. Transgenic Nicotiana tabacum and Arabidopsis thaliana plants overexpressing allene oxide synthase. Planta 2000, 211, 163–165. [Google Scholar] [CrossRef]
- Park, J.H.; Halitschke, R.; Kim, H.B.; Baldwin, I.T.; Feldmann, K.A.; Feyereisen, R. A knock-out mutation in allene oxide synthase results in male sterility and defective wound signal transduction in Arabidopsis due to a block in jasmonic acid biosynthesis. Plant J. 2002, 31, 1–12. [Google Scholar] [CrossRef]
- Breithaupt, C.; Kurzbauer, R.; Lilie, H.; Schaller, A.; Strassner, J.; Huber, R.; Macheroux, P.; Clausen, T. Crystal structure of 12-Oxophytodienoate reductase 3 from tomato: Self-Inhibition by dimerization. Proc. Natl. Acad. Sci. USA 2006, 103, 14337–14342. [Google Scholar] [CrossRef]
- Tillich, H.-J. Seedling diversity and the homologies of seedling organs in the order Poales (monocotyledons). Ann. Bot. 2007, 100, 1413–1429. [Google Scholar] [CrossRef]
- Kumakov, V.A. Physiology of Spring Wheat; Kolos: Moscow, Russia, 1980; p. 207. [Google Scholar]
- Lakehal, A.; Dob, A.; Novak, O.; Bellini, C. A DAO1-mediated circuit controls auxin and jasmonate crosstalk robustness during adventitious root initiation in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 4428. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.; Karafyllidis, I.; Wasternack, C.; Turner, J.G. The Arabidopsis mutant cev1 links cell wall signaling to jasmonate and ethylene responses. Plant Cell 2002, 14, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Monte, I.; Hamberg, M.; Chini, A.; Gimenez-Ibanez, S.; García-Casado, G.; Porzel, A.; Pazos, F.; Boter, M.; Solano, R. Rational design of a ligand-based antagonist of jasmonate perception. Nat. Chem. Biol. 2014, 10, 671–676. [Google Scholar] [CrossRef]
- Shyu, C.; Figueroa, P.; DePew, C.L.; Cooke, T.F.; Sheard, L.B.; Moreno, J.E.; Katsir, L.; Zheng, N.; Browse, J.; Howe, G.A. JAZ8 lacks a canonical degron and has an ear motif that mediates transcriptional repression of jasmonate responses in Arabidopsis. Plant Cell 2012, 24, 536–550. [Google Scholar] [CrossRef]
- Staswick, P.E.; Su, W.; Howell, S.H. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proc. Natl. Acad. Sci. USA 1992, 89, 6837–6840. [Google Scholar] [CrossRef]
- Thatcher, L.F.; Cevik, V.; Grant, M.; Zhai, B.; Jones, J.D.G.; Manners, J.M.; Kazan, K. Characterization of a JAZ7 activation-tagged Arabidopsis mutant with increased susceptibility to the fungal pathogen Fusarium oxysporum. J. Exp. Bot. 2016, 67, 2367–2386. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Howe, G.A. A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell 2009, 21, 131–145. [Google Scholar] [CrossRef]
- Mosblech, A.; Thurow, C.; Gatz, C.; Feussner, I.; Heilmann, I. Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. Plant J. 2011, 65, 949–957. [Google Scholar] [CrossRef]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef]
- Corti Monzón, G.; Pinedo, M.; Lamattina, L.; de la Canal, L. Sunflower root growth regulation: The role of jasmonic acid and its relation with auxins. Plant Growth Regul. 2012, 66, 129–136. [Google Scholar] [CrossRef]
- Lischweski, S.; Muchow, A.; Guthorl, D.; Hause, B. Jasmonates act positively in adventitious root formation in petunia cuttings. BMC Plant Biol. 2015, 15, 229. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.; Mongelard, G.; Flokova, K.; Pacurar, D.I.; Novak, O.; Staswick, P.; Kowalczyk, M.; Pacurar, M.; Demailly, H.; Geiss, G.; et al. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell 2012, 24, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Fattorini, L.; Veloccia, A.; Della Rovere, F.; D’Angeli, S.; Falasca, G.; Altamura, M.M. Indole-3-butyric acid promotes adventitious rooting in Arabidopsis thaliana thin cell layers by conversion into indole-3-acetic acid and stimulation of anthranilate synthase activity. BMC Plant Biol. 2017, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-R.; Chen, Y.-J.; Lee, C.-Y.; Lin, T.-Y. MeJA-induced transcriptional changes in adventitious roots of Bupleurum kaoi. Plant Sci. 2007, 173, 12–24. [Google Scholar] [CrossRef]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef]
- Cai, X.T.; Xu, P.; Zhao, P.X.; Liu, R.; Yu, L.H.; Xiang, C.B. Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat. Commun. 2014, 5, 5833. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Y.; Ye, S.; Jiang, H.; Chen, Q.; Liu, F.; Zhou, W.; Chen, R.; Li, X.; Tietz, O.; et al. Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell 2009, 21, 1495–1511. [Google Scholar] [CrossRef]
- De Smet, I. Lateral root initiation: One step at a time. New Phytol. 2012, 193, 867–873. [Google Scholar] [CrossRef]
- McSteen, P. Auxin and monocot development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001479. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, a001537. [Google Scholar] [CrossRef]
- Lakehal, A.; Ranjan, A.; Bellini, C. Multiple roles of jasmonates in shaping rhizotaxis: Emerging integrators. Methods Mol. Biol. 2020, 2085, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, F.; Lechner, E.; Genschik, P.; Crosby, W.L.; Ma, H.; Peng, W.; Huang, D.; Xie, D. The SCF(COI1) ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 2002, 14, 1919–1935. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in annals of botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Oka, M.; Ueda, J. Update on the possible mode of action of the jasmonates: Focus on the metabolism of cell wall polysaccharides in relation to growth and development. Physiol. Plant. 1997, 100, 631–638. [Google Scholar] [CrossRef]
- Irving, H.R.; Dyson, G.; McConchie, R.; Parish, R.W.; Gehring, C.A. Effects of exogenously applied jasmonates on growth and intracellular pH in maize coleoptile segments. J. Plant Growth Regul. 1999, 18, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Miyamoto, K.; Kamisaka, S. Inhibition of the synthesis of cell wall polysaccharides in oat coleoptile segments by jasmonic acid: Relevance to its growth inhibition. J. Plant Growth Regul. 2004, 14, 69–76. [Google Scholar] [CrossRef]
- Brummer, B.; Bertl, A.; Potrykus, I.; Felle, H.; Parish, R.W. Evidence that fusicoccin and indole-3-acetic acid induce cytosolic acidification of Zea mays cells. FEBS Lett. 1985, 189, 109–114. [Google Scholar] [CrossRef]
- Kutschera, U.; Briggs, W.R. Rapid auxin-induced stimulation of cell wall synthesis in pea internodes. Proc. Natl. Acad. Sci. USA 1987, 84, 2747–2751. [Google Scholar] [CrossRef]
- Evans, N.H. Modulation of guard cell plasma membrane potassium currents by methyl jasmonate. Plant Physiol. 2003, 131, 8–11. [Google Scholar] [CrossRef]
- Philippar, K.; Fuchs, I.; Lüthen, H.; Hoth, S.; Bauer, C.S.; Haga, K.; Thiel, G.; Ljung, K.; Sandberg, G.; Böttger, M.; et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci. USA 1999, 96, 12186–12191. [Google Scholar] [CrossRef]
- Schwechheimer, C.; Serino, G.; Callis, J.; Crosby, W.L.; Lyapina, S.; Deshaies, R.J.; Gray, W.M.; Estelle, M.; Deng, X.W. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 2001, 292, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Xu, Y.; Xiong, B.; Dai, L.; Huang, S.; Dong, T.; Sun, G.; Liao, L.; Deng, Q.; Wang, X.; et al. Effects of exogenous methyl jasmonate on the synthesis of endogenous jasmonates and the regulation of photosynthesis in citrus. Physiol. Plant. 2020, 170, 398–414. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigolev, A.; Miroshnichenko, D.; Dolgov, S.; Savchenko, T. Regulation of Sixth Seminal Root Formation by Jasmonate in Triticum aestivum L. Plants 2021, 10, 219. https://doi.org/10.3390/plants10020219
Pigolev A, Miroshnichenko D, Dolgov S, Savchenko T. Regulation of Sixth Seminal Root Formation by Jasmonate in Triticum aestivum L. Plants. 2021; 10(2):219. https://doi.org/10.3390/plants10020219
Chicago/Turabian StylePigolev, Alexey, Dmitry Miroshnichenko, Sergey Dolgov, and Tatyana Savchenko. 2021. "Regulation of Sixth Seminal Root Formation by Jasmonate in Triticum aestivum L." Plants 10, no. 2: 219. https://doi.org/10.3390/plants10020219
APA StylePigolev, A., Miroshnichenko, D., Dolgov, S., & Savchenko, T. (2021). Regulation of Sixth Seminal Root Formation by Jasmonate in Triticum aestivum L. Plants, 10(2), 219. https://doi.org/10.3390/plants10020219