Phytochemical Profile and Pharmacological Activities of Water and Hydroethanolic Dry Extracts of Calluna vulgaris (L.) Hull. Herb
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Analysis
2.2. The Pharmacological Activity of the DECV
2.2.1. Determination of Acute Toxicity
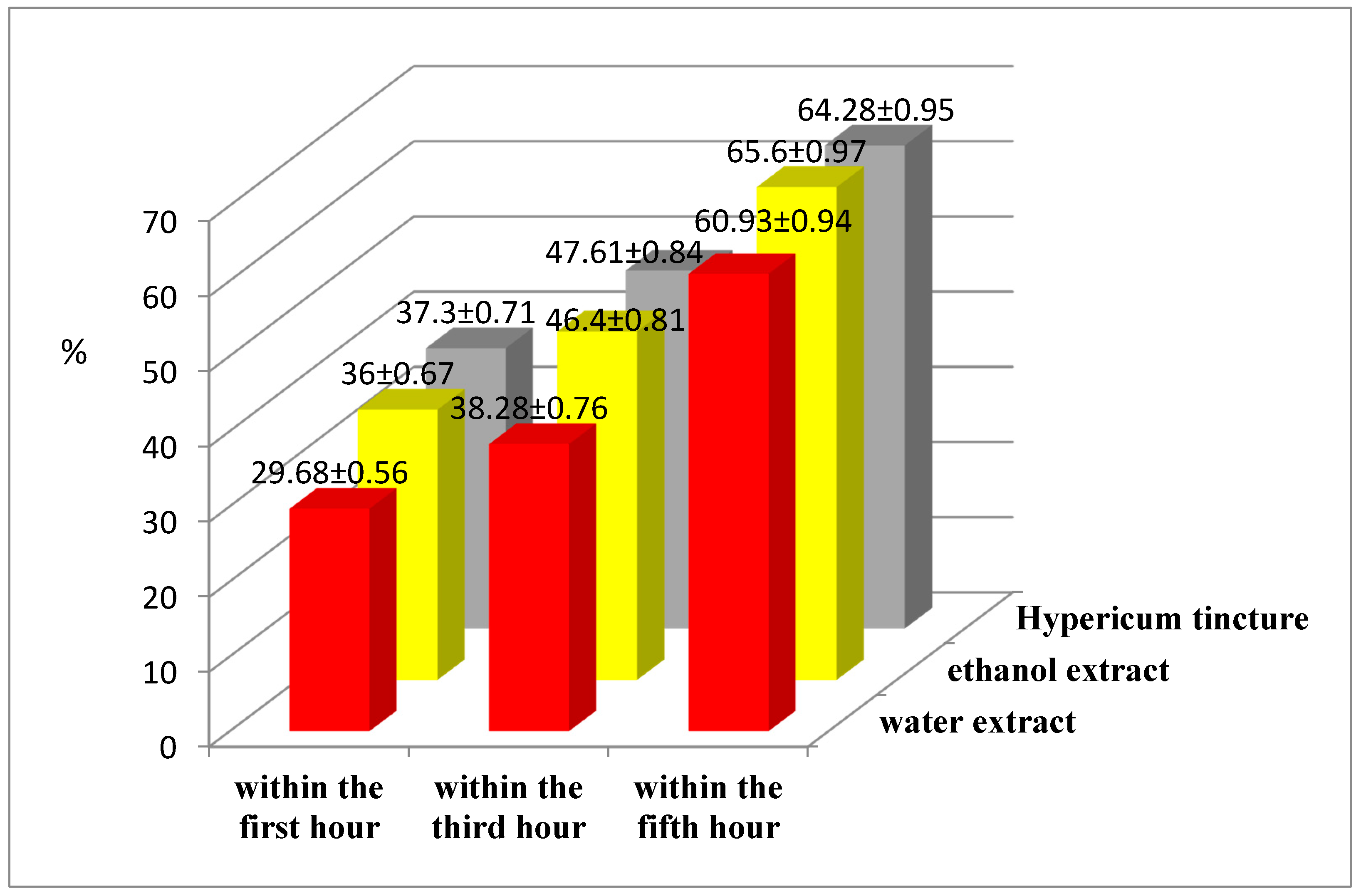
2.2.2. Determination of Anti-Inflammatory Activity
2.2.3. Determination of Antimicrobial Activity
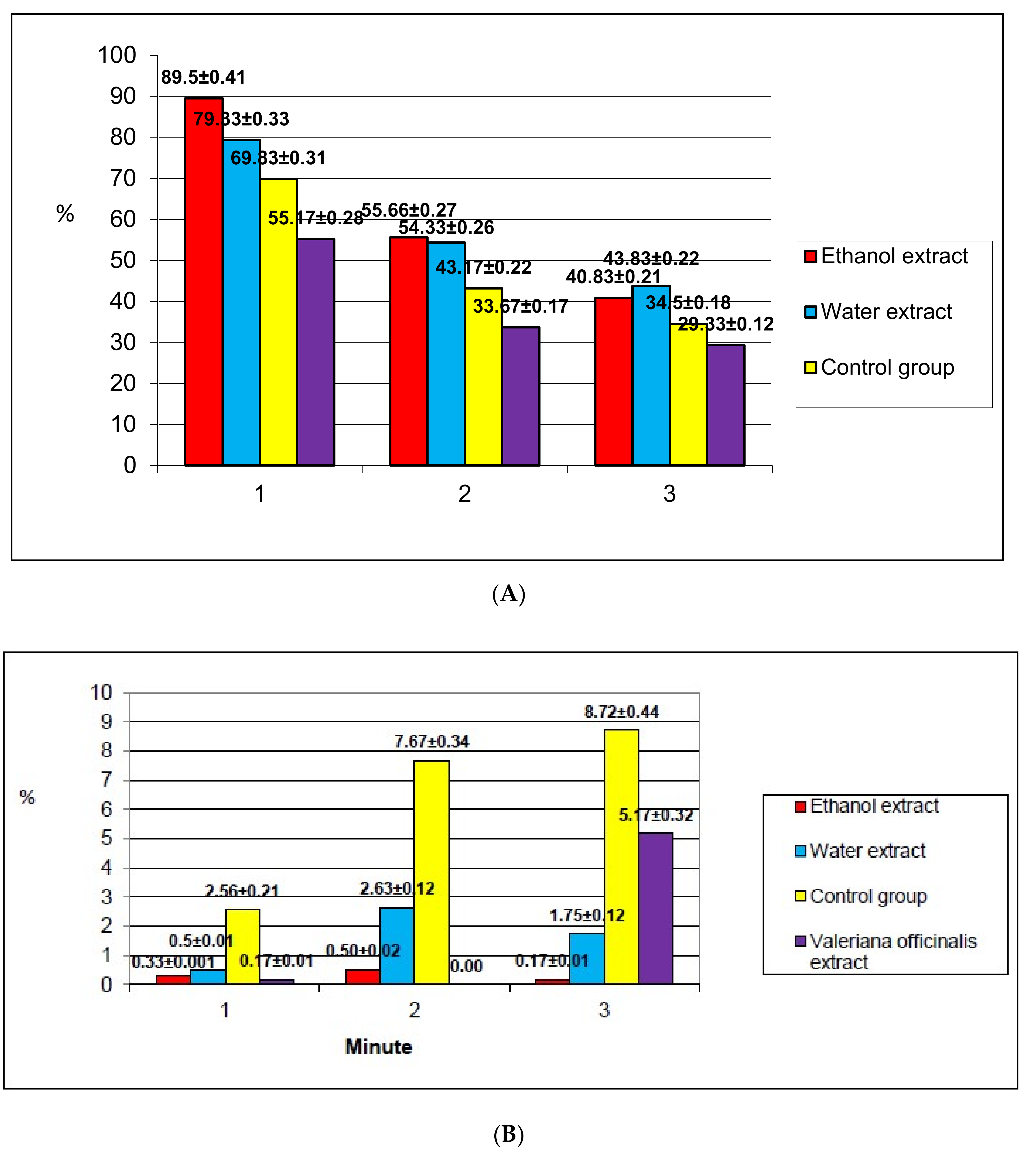
2.2.4. Determination of Neurotropic Activity
2.2.5. Determination of Anti-Anxiety and Antidepressant Activities
2.2.6. Discussion of the Results
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Extracts
3.3. Analysis of the Chemical Composition of the DECV
3.3.1. Analysis of Hydroxycinnamic Acids, Flavonoids and Tannins
3.3.2. Determination of the Quantitative Content of Different Groups of BAS
3.3.3. Quantitative Analysis of Extracts
3.4. Study of the Pharmacological Activities of DECV
3.4.1. Determination of Acute Toxicity
3.4.2. Determination of Anti-Inflammatory Activity
3.4.3. Determination of Antimicrobial Activity
3.4.4. Determination of the Neurotropic Activity
3.4.5. Determination of Anti-Anxiety and Antidepressant Effects
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whorld Health Organization (WHO). Depression and Other Common Mental Disorders. Global Health Estimates WHO/MSD/MER/2017.2. Available online: https://apps.who.int/ (accessed on 21 May 2020).
- Covarrubias-Pinto, A.; Acuña, A.I.; Beltrán, F.A.; Torres-Díaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef] [PubMed]
- Rovnaia, M. Calluna vulgaris. Diabetic 2007, 2, 44–46. [Google Scholar]
- Kovalenko, V.N. Compendium 2014—Medicines; Morion: Kiev, Ukraine, 2014; p. 2700. [Google Scholar]
- Plant Resources of the USSR. Flowering Plants, their Chemical Composition, Use; Families Paeoniaceae—Thymelaeceae. L.; Science: Leningrad, U.S.S.R., 1985. [Google Scholar]
- Zabokritskii, N.A.; Cherepanova, O.E.; Dudukina, N.N. Seasonal dynamics of the accumulation of biologically active substances in the shoots of Calluna vulgaris L. Agrar. Bull. Ural 2017, 3, 31–34. [Google Scholar]
- Kuritsyn, A.V.; Belonogova, V.D.; Vlasov, A.S. Raw material potential of wild medicinal plants of the Perm region. Med. Alm. 2011, 5, 292–294. [Google Scholar]
- Onegin, S.V. Pharmacognostic Study of Common Heather (Calluna vulgaris (L.) Hull). Ph.D. Thesis, Yaroslav State Medical Academy, Perm, Russia, 2008. [Google Scholar]
- Jurica, K.; Gobin, I.; Kremer, D.; Čepo, D.V.; Grubešić, R.J.; Karačonji, I.B.; Kosalec, I. Arbutin and its metabolite hydroquinone as the main factors in the antimicrobial effect of strawberry tree (Arbutus unedo L.) leaves. J. Herb. Med. 2017, 8, 17–23. [Google Scholar] [CrossRef]
- Ma, C.; He, N.; Zhao, Y.; Xia, D.; Wei, J.; Kang, W. Antimicrobial Mechanism of Hydroquinone. Appl. Biochem. Biotechnol. 2019, 189, 1291–1303. [Google Scholar] [CrossRef]
- Pavlović, R.D.; Lakusić, B.; Doslov-Kokorus, Z.; Kovacević, N. Arbutin content and antioxidant activity of some Ericaceae species. Die Pharm. 2009, 64, 656–659. [Google Scholar]
- Dróżdż, P.; Sentkowska, A.; Pyrzynska, K. Flavonoid Content and Antioxidant Properties in Different Extracts of Calluna vulgaris (L.) Flowers. J. Agric. Sci. Technol. A 2017, 7, 39–44. [Google Scholar] [CrossRef][Green Version]
- Mandim, F.; Barros, L.; Calhelha, R.C.; Abreu, R.; Pinela, J.; Alves, M.J.; Heleno, S.A.; Santos, P.F.; Ferreira, I.C.F.R. Calluna vulgaris (L.) Hull: Chemical characterization, evaluation of its bioactive properties and effect on the vaginal microbiota. Food Funct. 2019, 10, 78–89. [Google Scholar] [CrossRef]
- Mandim, F.; Barros, L.; Heleno, S.A.; Pires, T.C.S.P.; Dias, M.I.; Alves, M.J.; Santos, P.F.; Ferreira, I.C.F.R. Phenolic profile and effects of acetone fractions obtained from the inflorescences of Calluna vulgaris (L.) Hull on vaginal pathogenic and non-pathogenic bacteria. Food Funct. 2019, 10, 2399–2407. [Google Scholar] [CrossRef]
- Rodrigues, F.; Moreira, T.; Pinto, D.; Pimentel, F.B.; Costa, H.S.; Nunes, M.A.; Albuquerque, T.G.; Costaa, H.S.; Palmeira-de-Oliveira, A.; Sut, S.; et al. The phytochemical and bioactivity profiles of wild Calluna vulgaris L. flowers. Food Res. Int. 2018, 111, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Kutsyk, R.V.; Kurovets, L.M. Synergism of the antimicrobial action of extracts of plants of the Ericaceae Juss. family and cefazolin: Features of the effect against S. haemolyticus with different mechanisms of resistance to β-lactam antibiotics. Biomed. Andanthropol. 2010, 15, 167–172. [Google Scholar]
- Dróżdż, P.; Sentkowska, A.; Pyrzyńska, K. Biophenols and antioxidant activity in wild and cultivated heather. Nat. Prod. Res. 2016, 31, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Frych, N.I.; Vivcharuk, L.M.; Miziuk, R.M. Study of the antimicrobial activity of plants of the Ericaceae Juss. family. Pharm. J. 2005, 2, 97–104. [Google Scholar]
- Villanueva-Bermejo, D.; Vázquez, E.; Villalva, M.; Santoyo, S.; Fornari, T.; Reglero, G.; Garcia-Risco, M.R. Simultaneous Supercritical Fluid Extraction of Heather (Calluna vulgaris L.) and Marigold (Calendula officinalis L.) and Anti-Inflammatory Activity of the Extracts. Appl. Sci. 2019, 9, 2245. [Google Scholar] [CrossRef]
- Kakouri, E.; Daferera, D.; Paramithiotis, S.; Astraka, K.; Drosinos, E.H.; Polissiou, M.G. Crocus sativus L. tepals: The natural source of antioxidant and antimicrobial factors. J. Appl. Res. Med. Aromat. Plants 2017, 4, 66–74. [Google Scholar] [CrossRef]
- Bessalova, E. Methods of researching the behavior of rats under conditions of “Open Field” test. Neurosci. Theor. Clin. Asp. 2011, 7, 106–109. [Google Scholar]
- Mamylina, N.V.; Pavlova, V.I. Physiological Aspects of the Behavioral Activity of Animals Under Conditions of Emotional Stress; Publishing House of “Cicero”: Chelyabinsk, Russia, 2013. [Google Scholar]
- Soukand, R.; Raal, A. How the name Arnica was borrowed into Estonian. Trames. J. Humanit. Soc. Sci. 2008, 12, 29. [Google Scholar] [CrossRef]
- Sak, K.; Jürisoo, K.; Raal, A. Estonian folk traditional experiences on natural anticancer remedies: From past to the future. Pharm. Boil. 2014, 52, 855–866. [Google Scholar] [CrossRef]
- Raal, A.; Sarv, M.; Vilbaste, K. The Medicinal Plants of Estonia; Varrak: Tallinn, Estonia, 2018; Volume 1, pp. 80–83. [Google Scholar]
- Arief, Z.M.; Shawl, A.S.; Munshi, A.H. Altitudinal variation in pharmacologically active compounds of wild and cultivated populations of Epimedium elatum. J. Appl. Res. Med. Aromat. Plants 2016, 3, 48–51. [Google Scholar] [CrossRef]
- Shekari, A.; Nazeri, V.; Shokrpour, M. Pollen viability and storage life in Leonurus cardiaca L. J. Appl. Res. Med. Aromat. Plants 2016, 3, 101–104. [Google Scholar] [CrossRef]
- Segone, R.T.; Tankeu, S.Y.; Chen, W.; Combrinck, S.; Schmidt, M.; Viljoen, A.M. Rapid differentiation of Piper methysticum (kava) plant parts using single point and imaging vibrational spectroscopy. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100235. [Google Scholar] [CrossRef]
- Dhiman, B.; Sharma, P.; Shivani; Pal, P.K. Biology, chemical diversity, agronomy, conservation and industrial importance of Valeriana jatamansi: A natural sedative. J. Appl. Res. Med. Aromat. Plants 2020, 16, 100243. [Google Scholar] [CrossRef]
- Romanenko, Y.A.; Koshovyi, O.M.; Kireiev, I.V.; Tryshchuk, N.M.; Ilina, T.V.; Borodina, N.V. Investigation of the interconnection of the content of the basic groups of BAS in the Leonurus cardiaca herb infusion and its psychotropic activity. Ukr. Biopharm. J. 2018, 4, 69–74. [Google Scholar] [CrossRef]
- Koshovyi, O.; Romanenko, Y.; Komissarenko, A. The study of the phenolic composition of the dry extract of motherwort herb and its psychotropic activity. Am. J. Sci. Technol. 2016, 1, 1055–1059. [Google Scholar]
- Koshovyi, O.M.; Zagayko, A.L.; Kolychev, I.O.; Akhmedov, E.Y.; Komissarenko, A.N. Phytochemical study of the dry extract from bilberry leaves. Azerb. Pharm. Pharmacother. J. 2016, 16, 18–23. [Google Scholar]
- Kalenichenko, H.S.; Maloshtan, L.M.; Shatalova, O.M.; Dorovskyi, O.V. Investigation of the mechanism of anti-inflammatory effect of the thick extract from the Corylus avellana leaves. Ukr. Biopharm. J. 2015, 6, 30–33. [Google Scholar]
- Dobrochaeva, D.N.; Kotov, M.I.; Prokudin, Y.N.; Barbarich, A.I. Key to Higher Plants of Ukraine, 2nd ed.; Science Dumka: Kiev, Ukraine, 1999. [Google Scholar]
- State Pharmacopoeia of Ukraine, 2nd ed.; SO Ukrainian Scientific Pharmacopoeial Center of Drugs Quality: Kharkiv, Ukraine, 2015.
- Zabolotnyi, O.; Zabolotnyi, V.; Koshevoi, M. Conditionality examination of the new testing algorithms for coal-water slurries moisture measurement. Nauk. Visnyk Nat. Hirnychoho Univ. 2018, 1, 51–59. [Google Scholar] [CrossRef]
- Borodina, N.V.; Kovalyov, V.V.; Koshovyi, O.M.; Akhmedov, E.Y. The chromatography-mass spectrometry study of Salix rosmarinifolia L. Azerb. Pharm. Pharmacother. J. 2016, 16, 15–20. [Google Scholar]
- Krivoruchko, E.; Markin, A.; Samoilova, V.A.; Ilina, T.; Koshovyi, O. Research in the chemical composition of the bark of Sorbus aucuparia. Ceska Sl. Farmac. 2018, 67, 113–115. [Google Scholar]
- Koshovyi, O.N.; Vovk, G.V.; Akhmedov, E.Y.; Komissarenko, A.N. The study of the chemical composition and pharmacological activity of Salvia officinalis leaves extracts getting by complex processing. Azerb. Pharm. Pharmacother. J. 2015, 15, 30–34. [Google Scholar]
- Ilina, T.; Kashpur, N.; Granica, S.; Goryacha, O.; Koshovyi, O. Phytochemical profiles and in vitro immunomodulatory activity of hydroethanolicic extracts from Galium aparine L. Plants 2019, 8, 541. [Google Scholar] [CrossRef] [PubMed]
- Stefanov, O.V. Preclinical Studies of Drugs; Avitsenna: Kiev, Ukraine, 2001. [Google Scholar]
- European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes. Strasbourg, 18 March 1986. Available online: https://zakon.rada.gov.ua/laws/show/994_137 (accessed on 21 May 2020).
- Procedure for Carrying out Experiments, Experiments on Animals by Scientific Institutions: Order of the Ministry of Education and Science, Youth and Sports of Ukraine No. 249 dated 1 March 2012. Available online: http://zakon.rada.gov.ua/laws/show/z0416-12 (accessed on 21 May 2020).
- Svitlychnyi, O.; Berehelia, I. Administrative protection of animals used in scientific experiments, educational process and production of biological products from abuse. Entrep. Domest. Econ. Law 2017, 2, 150–154. [Google Scholar]
- Ostapets, M.O.; Volkovoi, V.A.; Fomina, H.P. The study of acute toxicity and effective dose of dry extract from Geranium palustre herb. Clin. Exp. Pathol. 2015, 1, 113–115. [Google Scholar]
- Veremchuk, O.A.; Moiseev, D.V. The study of the safety profile of Calluna vulgaris tincture. Bull. VSMU 2015, 14, 98–106. [Google Scholar]
- Hrytsyk, A.R.; Posatska, N.M.; Klymenko, A.O. Obtaining and investigation of the properties of Verbena officinalis extracts. Pharm. J. 2016, 3, 39–44. [Google Scholar]
- Khabriev, R.U. Guidelines for the Experimental (Preclinical) Study of New Pharmacological Substances, 2nd ed.; OJSC “Publishing house “Medicine”: Moscow, Russia, 2005. [Google Scholar]
- Kotov, O.O.; Hromov, L.O.; Yarosh, O.K. Formation of resistance (tolerance) to anxiolytic action of phenazepam after its prolonged administration to mice. Pharmacol. Drug Toxicol. 2014, 6, 27–31. [Google Scholar]
- Bezverkha, I.S.; Panteleimonova, T.M.; Sharabura, L.B. The antidepressant effect of isoflavone 5/09 at anxiety in male mice. Probl. Aging Longev. 2014, 23, 101–112. [Google Scholar]
- Avidzba, Y.N.; Zaliubovska, O.I.; Zlenko, V.V. Experimental study of the pharmacological action of Phytocardin. Sedative and diuretic effects. News Pharm. 2012, 2, 71–73. [Google Scholar]
- Lapach, S.M.; Chubenko, A.V.; Babich, P.M. Statistical Methods in Biomedical Research Using Exel; Morion: Kiev, Ukraine, 2000. [Google Scholar]
- Bondarenko, V.H.; Kanivska, I.Y.; Paramonova, S.M. Probability Theory and Mathematical Statistics, Part 1; NTUU “KPI”: Kiiv, Ukraine, 2006. [Google Scholar]
| Substances | Content, %, n = 5 | |
|---|---|---|
| Water Extract | Hydroethanolic Extract | |
| Phenols and their glycosides | ||
| Arbutin | 1.25 ± 0.05 | 0.83 ± 0.04 |
| Methylarbutin | 0.18 ± 0.03 | 0.23 ± 0.02 |
| Hydroxycinnamic acids | ||
| Chlorogenic | 1.25 ± 0.03 | 1.74 ± 0.03 |
| Caffeic | 0.02 ± 0.01 | 0.03 ± 0.01 |
| Ferulic | 0.11 ± 0.02 | 0.12 ± 0.01 |
| p-Coumaric | 0.03 ± 0.01 | 0.04 ± 0.01 |
| Flavonoids | ||
| Rutin | 0.65 ± 0.05 | 1.25 ± 0.05 |
| Hyperoside | 0.15 ± 0.05 | 0.2 ± 0.01 |
| Quercetin-3-D-glucoside | 0.17 ± 0.03 | 0.29 ± 0.01 |
| Luteolin | 0 | 0,05 ± 0.01 |
| Apigenin | 0 | 0,04 ± 0.01 |
| Kaempferol | 0.02 ± 0.01 | 0,05 ± 0.01 |
| Tannins metabolites | ||
| Gallic acid | 0.07 ± 0.01 | 0.13 ± 0.01 |
| (+)-Gallocatechin | 0.21 ± 0.01 | 0.94 ± 0.02 |
| (-)-Epigallocatechin | 0.95 ± 0.05 | 1.36 ± 0.09 |
| (+)-Catechin | 0.13 ± 0.03 | 0.21 ± 0.03 |
| (-)-Epicatechin | 0.09 ± 0.01 | 0.26 ± 0.02 |
| (-)-Catechin gallate | 0.11 ± 0.02 | 0.24 ± 0.01 |
| (-)Epicatechin gallate | 0.05 ± 0.01 | 0.07 ± 0.01 |
| The Group of BAS (Method of Analysis) | Content, , n = 9 | |
|---|---|---|
| Water Extract | Hydroethanolic Extract | |
| Total hydroquinone derivatives (spectrophotometry per arbutin) | 10.51 ± 0.04 | 7.86 ± 0.03 |
| Total hydroxycinnamic acids (spectrophotometry per chlorogenic acid) | 5.87 ± 0.02 | 7.92 ± 0.04 |
| Total flavonoids (spectrophotometry per rutin) | 0.89 ± 0.02 | 2.33 ± 0.07 |
| Total polyphenols (spectrophotometry per completely dry substance and pyrogallol) | 5.95 ± 0.11 | 11.08 ± 0.20 |
| Group of Animals | Indicators of Blood | ||
|---|---|---|---|
| Hemoglobin Content, g/L | Number of Red Blood Cells, ×1012/L | Number of Leukocytes, ×109/L | |
| Control | 128.6 ± 2.5 | 7.25 ± 0.14 | 17.56 ± 0.38 |
| Water extract | 126.08 ± 3.6 | 6.98 ± 0.24 | 14.62 ± 0.46 |
| Hydroethanolic extract | 127.71 ± 1.5 | 6.79 ± 0.09 | 12.14 ± 0.27 |
| Hypericum tincture | 138.6 ± 3.7 | 7.12 ± 0.11 | 12.26 ± 0.16 |
| Intact animals | 131.86 ± 4.8 | 7.78 ± 0.15 | 11.92 ± 0.25 |
| Indicator | Number of Animals | Groups | ||||
|---|---|---|---|---|---|---|
| Group I | Group II | Control | Group III | Intact Animals | ||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Total number of appearances in the dark chamber | 1 | 2 ± 0.24 *,** | 1.14 ± 0.36 ** | 1.14 | 1.43 ± 0.42 * | 1.71 |
| 2 | 1.71 ± 0.20 ** | 1.43 ± 0.10 ** | 2.14 | 0.57 ± 0.04 * | 1.71 | |
| 3 | 1.43 ± 0.29 * | 2.29 ± 0.05 * | 0.71 | 0.86 ± 0.71 * | 1.43 | |
| 4 | 1.86 ± 0.14 * | 1.29 ± 0.28 ** | 1.29 | 0.57 ± 0.41 * | 1.86 | |
| 5 | 1.57 ± 0.36 * | 1.29 ± 0.79 ** | 1.29 | 1.43 ± 1 * | 1.14 | |
| Σ 1–5 | 8.57 ± 0.31 ** | 7.43 ± 0.20 ** | 6.57 | 4.86 ± 0.29 * | 7.86 | |
| Total time of staying in the dark branches of the maze, s | 1 | 19 ± 0.01 ** | 18.14 ± 0.17 ** | 18.86 | 40.71 ± 0.10 * | 45.57 |
| 2 | 23.57 ± 0.09 ** | 18.43 ± 0.02 ** | 24.43 | 40.86 ± 0.17 * | 45.57 | |
| 3 | 23.71 ± 0.02 ** | 33.43 ± 0.12 ** | 22.71 | 44.43 ± 0.24 * | 51.86 | |
| 4 | 23.14 ± 0.02 ** | 32.71 ± 0.24 ** | 21.86 | 47.14 ± 0.11 * | 46.14 | |
| 5 | 13.86 ± 0.02 ** | 33.57 ± 0.17 ** | 22.71 | 42.57 ± 0.04 * | 54.57 | |
| Σ 1–5 | 103.29 ± 0.02 ** | 136.29 ± 0.04 ** | 110.57 | 215.71 ± 0.09 * | 243.71 | |
| Total number of appearances in the central location | 1 | 2.86 ± 0.24 ** | 2.29 ± 0.58 * | 2 | 2 ± 1 * | 1.43 |
| 2 | 3.14 ± 0.40 ** | 2.57 ± 0.34 ** | 2.86 | 1.86 ± 0.20 * | 2.43 | |
| 3 | 2.14 ± 0.35 ** | 2.29 ± 0.27 ** | 2 | 1.14 ± 0.10 * | 1.14 | |
| 4 | 3.43 ± 0.07 ** | 2 ± 0.60 * | 2.14 | 1.14 ± 0.22 * | 2.43 | |
| 5 | 2.57 ± 0.23 ** | 1.14 ± 0.20 * | 2.29 | 1.72 ± 0.68 * | 1 | |
| Σ 1–5 | 14.14 ± 0.12 ** | 10.29 ± 0.49 ** | 11.29 | 7.86 ± 0.31 * | 8.43 | |
| Total number of appearances in the illuminated branches of the maze | 1 | 1.57 ± 0.22 ** | 1 ± 0.36 ** | 1.14 | 0.71 ± 0.10 * | 0.86 |
| 2 | 0.86 ± 0.46 ** | 0.86 ± 0.42 ** | 0.71 | 0.57 ± 0.59 * | 0.14 | |
| 3 | 0.71 ± 0.41 ** | 0.71 ± 0.41 ** | 0.57 | 0.29 ± 0.36 * | 0.43 | |
| 4 | 0.86 ± 0.09 ** | 0.43 ± 0.36 *.** | 0.71 | 0.14 ± 0.17 * | 0.71 | |
| 5 | 1.14 ± 0.06 ** | 0.29 ± 0.27 * | 0.71 | 0.43 ± 0.42 * | 0.14 | |
| Σ 1–5 | 5.14 ± 0.04 ** | 3.29 ± 0.27 ** | 3.86 | 2.14 ± 0.07 * | 2.29 | |
| Total time of staying in the illuminated branches of the maze, s | 1 | 35.14 ± 0.02 ** | 34.71 ± 0.11 ** | 32.71 | 14.71 ± 0.11 * | 7.14 |
| 2 | 26.57 ± 0.20 ** | 36.29 ± 0.04 ** | 22.71 | 15 ± 0.60 * | 6 | |
| 3 | 27.43 ± 0.01 ** | 20.14 ± 0.11 ** | 25.86 | 7.14 ± 0.08 * | 3.86 | |
| 4 | 25.14 ± 0.07 ** | 19.43 ± 0.13 ** | 27.43 | 9.43 ± 0.24 | 8.86 | |
| 5 | 24.14 ± 0.11 ** | 18.29 ± 0.17 ** | 24.86 | 10.71 ± 0.11 * | 0.86 | |
| Σ 1–5 | 138.43 ± 0.04 ** | 128.86 ± 0.02 ** | 133.57 | 57 ± 0.11 * | 26.71 | |
| Total time of staying in the central location, s | 1 | 5.86 ± 0.86 ** | 7.14 ± 0.31 ** | 8.43 | 4.57 ± 1 * | 7.29 |
| 2 | 9.86 ± 0.15 ** | 5.29 ± 0.07 * | 12.86 | 3.14 ± 0.07 * | 8.43 | |
| 3 | 8.86 ± 0.73 ** | 6.43 ± 0.34 * | 11.43 | 8.43 ± 0.46 * | 4.29 | |
| 4 | 10.29 ± 0.12 ** | 7.86 ± 0.61 * | 10.71 | 3.43 ± 0.17 * | 5 | |
| 5 | 22 ± 0.06 ** | 8.14 ± 0.34 * | 12.43 | 6.71 ± 0.17 * | 4.57 | |
| Σ 1–5 | 56.86 ± 0.12 ** | 34.86 ± 0.23 * | 55.86 | 26.29 ± 0.23 * | 29.57 | |
| Number of cases of looking down (risk assessment) | 1 | 4.86 ± 0.09 ** | 2.57 ± 0.91 ** | 2.43 | 2.57 ± 1 * | 1.29 |
| 2 | 3.14 ± 0.40 * | 3.29 ± 0.10 * | 1.71 | 2.14 ± 0.78 * | 0.57 | |
| 3 | 3.71 ± 0.23 */** | 1.57 ± 0.10 * | 2.14 | 2.14 ± 0.89 * | 0.43 | |
| 4 | 3.57 ± 0.02 ** | 1.29 ± 0.20 * | 2.86 | 1.43 ± 0.34 * | 0.71 | |
| 5 | 4.57 ± 0.04 ** | 1.71 ± 0.05 * | 3.57 | 1.43 ± 0.07 * | 0.29 | |
| Σ 1–5 | 19.86 ± 0.02 ** | 10.43 ± 0.17 * | 12.71 | 9.71 ± 0.75 * | 3.29 | |
| The latent period of the first appearance in the branch of the maze | 0.07 ± 0.34 ** | 0.36 ± 0.28 * | 0.78 | 0.8 ± 0.79 * | 1.52 | |
| Groups of Animals | Latent Period of The First “Fading” | Active Swimming, s | Passive Swimming, s | Total Time of Immobility, s | Number of Dives | |
|---|---|---|---|---|---|---|
| Group I | Day 1 of experiment | 3.47 ± 0.46 * | 190.29 ± 0.17 * | 156 ± 0.86 * | 13.71 ± 0.10 ** | 3.43 ± 0.06 ** |
| Group II | 3.23 ± 0.46 * | 93.43 ± 0.12 ** | 217.71 ± 0.01 ** | 48.86 ± 0.11 * | 2.43 ± 0.28 ** | |
| Group III | 3.25 ± 0.39 * | 136.14 ± 0.73 * | 156.14 ± 0.73 * | 67.71 ± 0.23 * | 0.14 ± 0.10 * | |
| Control | 4.09 | 157.14 | 177 | 25.86 | 1.86 | |
| Intact animals | 3.07 | 98 | 201 | 61 | 1.29 | |
| Group I | Day 3 of experiment | 3.73 ± 0.75 * | 99.57 ± 0.11 ** | 245.29 ± 0.55 ** | 15.14 ± 0.46 ** | 0.86 |
| Group II | 2.98 ± 0.49 ** | 83.29 ± 0.61 ** | 226.71 ± 0.17 * | 50 ± 0.31 * | 0 | |
| Group III | 3.85 ± 0.61 * | 58.71 ± 0.61 * | 263.29 ± 0.86 * | 38 ± 0.49 * | 0 | |
| Control | 3.4 | 71.14 | 263.43 | 25.43 | 0.14 | |
| Intact animals | 2.4 | 78.86 | 141.57 | 107 | 2.43 | |
| Group I | Day 5 of experiment | 2.89 ± 0.34 * | 72.14 ± 0.17 * | 215 ± 0.39 * | 62.14 ± 0.61 ** | 0.57 |
| Group II | 3.26 ± 0.60 **/* | 45.29 ± 0.02 * | 260.14 ± 0.12 * | 45 ± 0.34 ** | 0.14 | |
| Group III | 2.22 ± 0.31 * | 58.57 ± 0.02 * | 215.14 ± 0.39 * | 86.29 ± 0.17 * | 0.57 | |
| Intact animals | 3.15 | 34.14 | 297 | 43.14 | 0 | |
| The Investigated Indicator | Group I | Group II | Control | Group III | Intact Animals |
|---|---|---|---|---|---|
| Latent period of the first approach to the cube, s | 0.46 ± 0.01 ** | 1.64 ± 0.46 * | 2.73 | 1.73 ± 0.34 * | 0.72 |
| Total number of approaches to the cube | 5.00 ± 0.07 * | 3.71 ± 0.46 * | 2.71 | 3.86 ± 0.27 * | 6.71 |
| Total time of the cube examination, s | 9.43 ± 0.34 * | 14.57 ± 0.02 | 4.43 | 7.43 ± 0.24 * | 8.86 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starchenko, G.; Hrytsyk, A.; Raal, A.; Koshovyi, O. Phytochemical Profile and Pharmacological Activities of Water and Hydroethanolic Dry Extracts of Calluna vulgaris (L.) Hull. Herb. Plants 2020, 9, 751. https://doi.org/10.3390/plants9060751
Starchenko G, Hrytsyk A, Raal A, Koshovyi O. Phytochemical Profile and Pharmacological Activities of Water and Hydroethanolic Dry Extracts of Calluna vulgaris (L.) Hull. Herb. Plants. 2020; 9(6):751. https://doi.org/10.3390/plants9060751
Chicago/Turabian StyleStarchenko, Galyna, Andriy Hrytsyk, Ain Raal, and Oleh Koshovyi. 2020. "Phytochemical Profile and Pharmacological Activities of Water and Hydroethanolic Dry Extracts of Calluna vulgaris (L.) Hull. Herb" Plants 9, no. 6: 751. https://doi.org/10.3390/plants9060751
APA StyleStarchenko, G., Hrytsyk, A., Raal, A., & Koshovyi, O. (2020). Phytochemical Profile and Pharmacological Activities of Water and Hydroethanolic Dry Extracts of Calluna vulgaris (L.) Hull. Herb. Plants, 9(6), 751. https://doi.org/10.3390/plants9060751








