Enhanced Production of Bryonolic Acid in Trichosanthes cucumerina L. (Thai Cultivar) Cell Cultures by Elicitors and Their Biological Activities
Abstract
1. Introduction
2. Results and Discussions
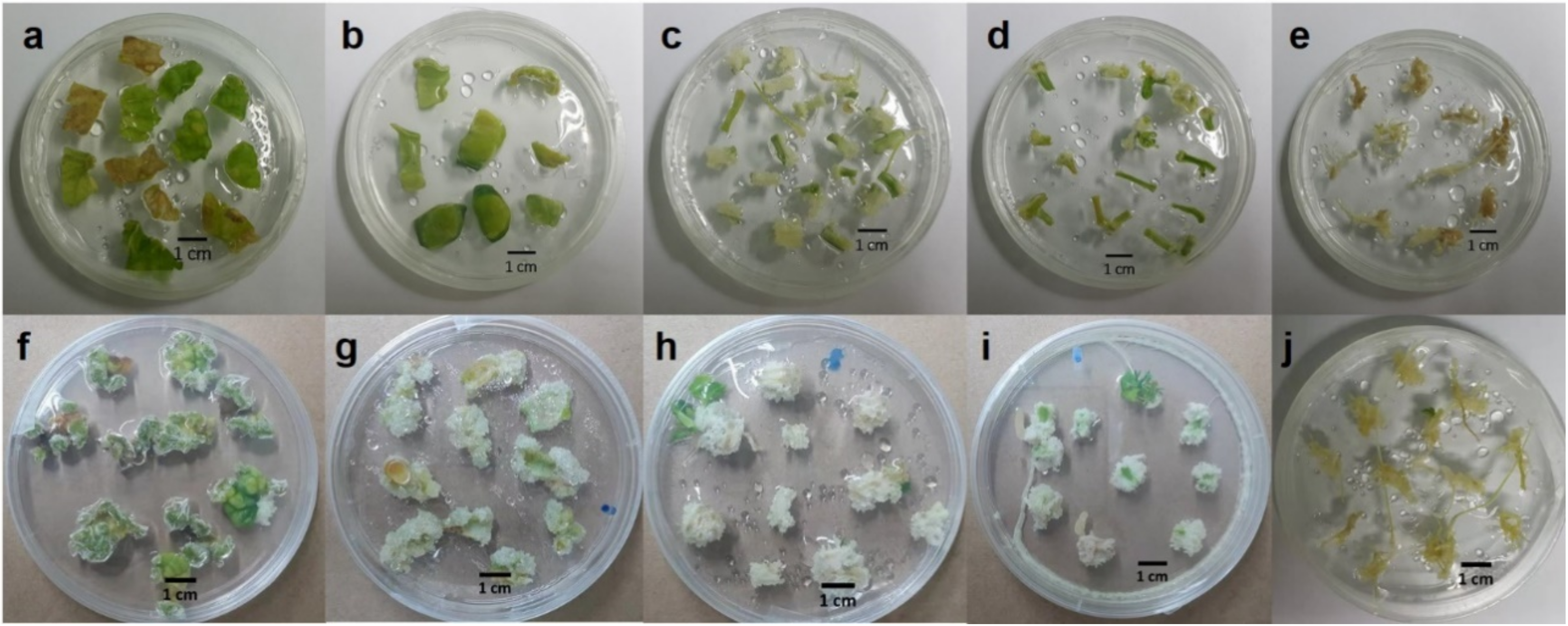
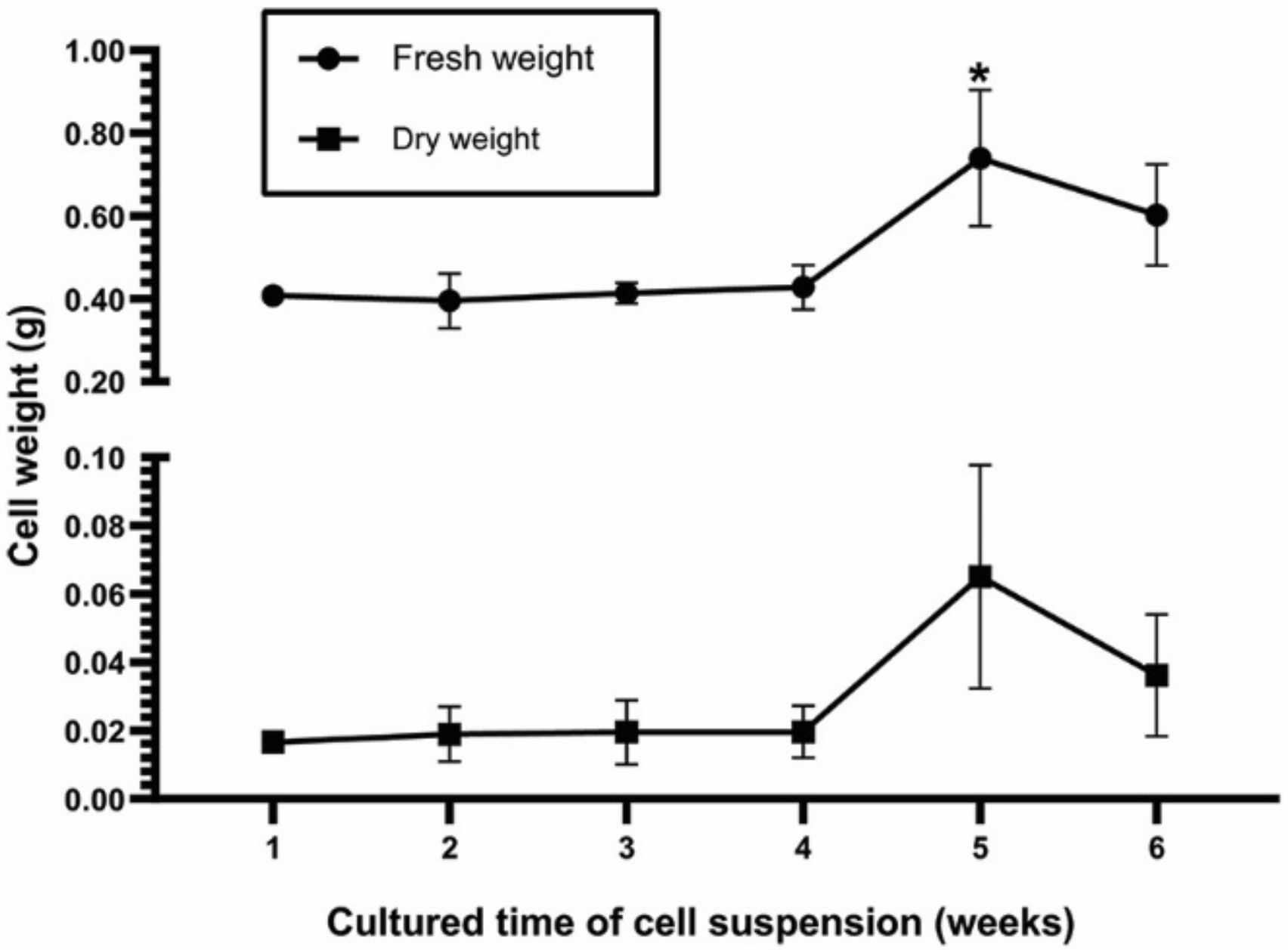
2.1. Effect of Explants on Callus and Cell Suspension Induction
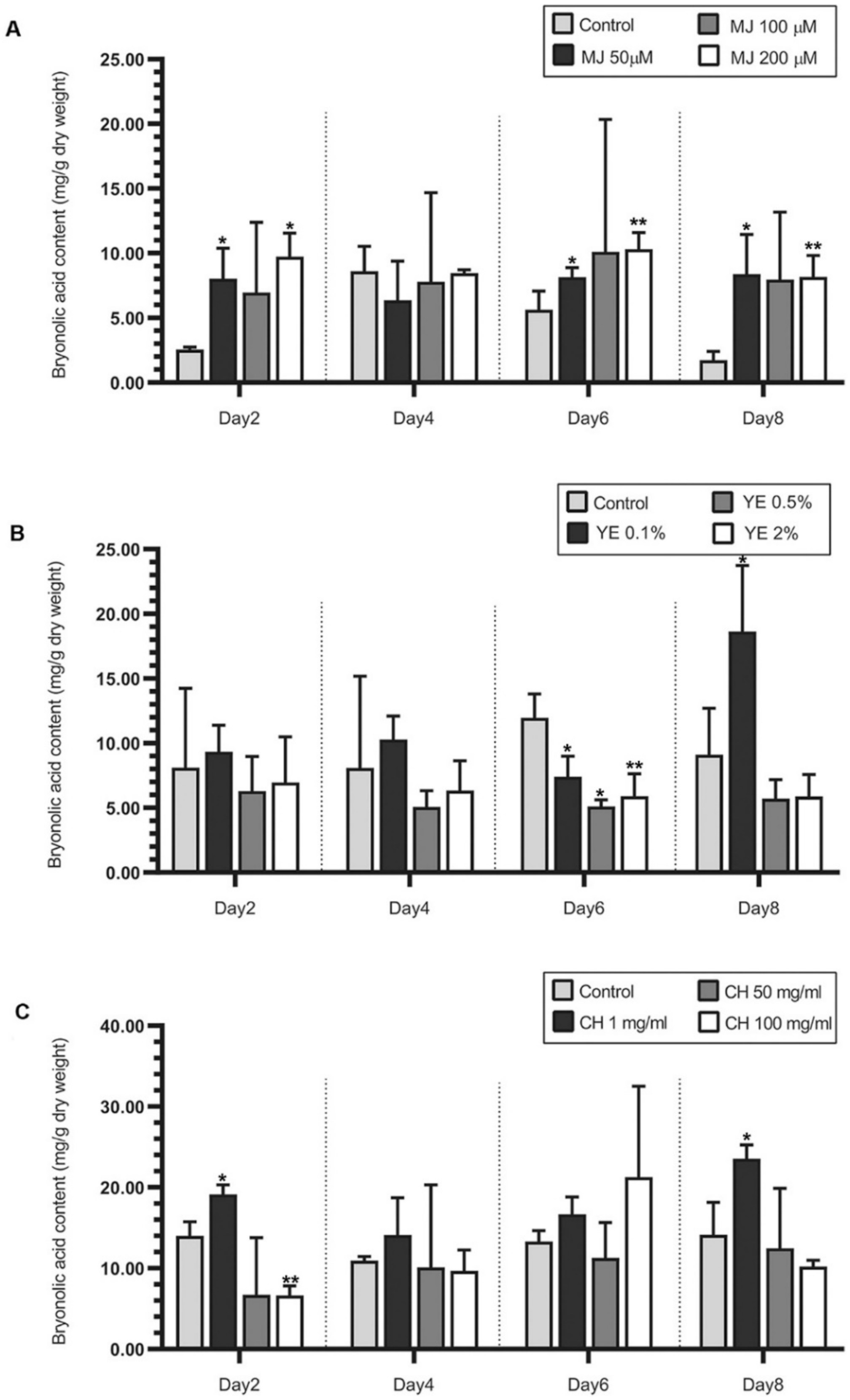
2.2. Effect of Elicitors on Bryonolic Acid Production
2.3. Metabolic Extraction
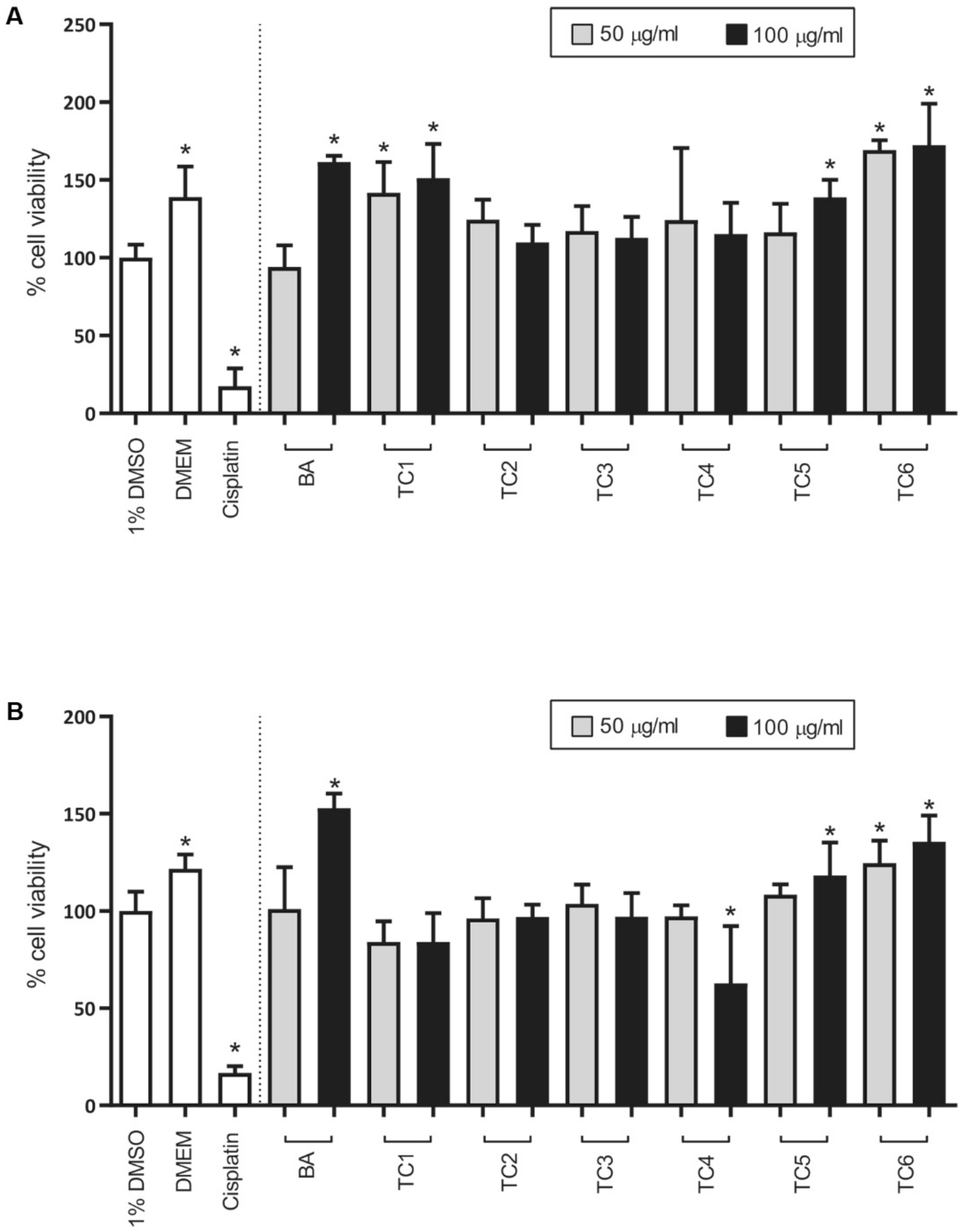
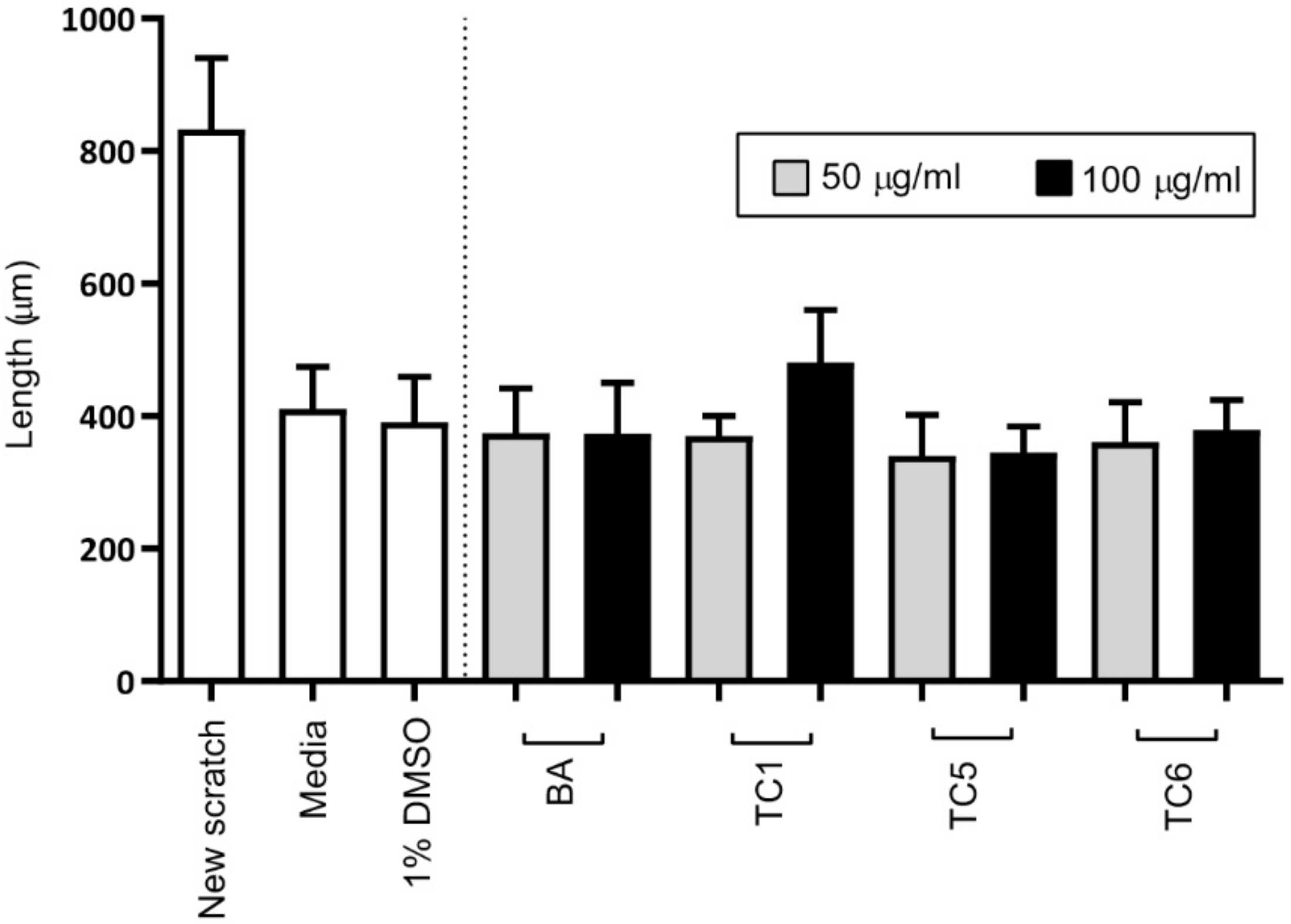
2.4. Biological Activities
3. Materials and Methods
3.1. Chemicals
3.2. Plant Materials
3.3. Callus Induction
3.4. Cell Suspension Initiation
3.5. Effect of Elicitors on Bryonolic Acid Accumulation
3.6. Active Compound Analysis Using High-Performance Liquid Chromatography (HPLC)
3.7. Cytotoxicity Test
3.8. Wound-Healing Test
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tanaka, S.; Uno, C.; Akimoto, M.; Tabata, M.; Honda, C.; Kamisako, W. Anti-Allergic Effect of Bryonolic Acid from Luffa cylindrica Cell Suspension Cultures. Planta Med. 1991, 57, 527–530. [Google Scholar] [CrossRef]
- Gatbonton-Schwager, T.N.; Letterio, J.J.; Tochtrop, G.P. Bryonolic Acid Transcriptional Control of Anti-inflammatory and Antioxidant Genes in Macrophages In Vitro and In Vivo. J. Nat. Prod. 2012, 75, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Que, J.; Ye, M.; Zhang, Y.; Xu, W.; Li, H.; Xu, W.; Chu, K. Bryonolic Acid, a Triterpenoid, Protect Against N-methyl-D-Aspartate-Induced Neurotoxicity in PC12 Cells. Molecules 2016, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Barker, E.C.; Gatbonton-Schwager, T.N.; Han, Y.; Clay, J.E.; Letterio, J.J.; Tochtrop, G.P. Bryonolic Acid: A Large-Scale Isolation and Evaluation of Heme Oxygenase 1 Expression in Activated Macrophages. J. Nat. Prod. 2010, 73, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, W.J.J.O.; Duyfjes, B.E.E. Keys to and checklist of species of the genus Trichosanthes L. (Cucurbitaceae) in Indochina. Adansonia 2012, 34, 265–278. [Google Scholar] [CrossRef]
- Saboo, S.; Thorat, P.; Tapadiya, G.G.; Khadabadi, S.S. Distribution and ancient-recent medicinal uses of Trichosanthes species. Int. J. Phytopharm. 2012, 2, 2. [Google Scholar] [CrossRef]
- Duyfjes, B.E.E.; Pruesapan, K. The genus Trichosanthes L. (Cucurbitaceae) in Thailand. Thai For. Bull. (Bot.) 1970, 32, 76–109. [Google Scholar]
- Ekeke, C.; Agogbua, J.U. Morphological and Anatomical Studies on Trichosanthes cucumerina L. (Cucurbitaceae). Int. J. Plant Soil Sci. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2019, 226, 1240–1255. [Google Scholar] [CrossRef]
- Kar, A.; Choudhary, B.K.; Bandyopadhyay, N.G. Comparative evaluation of hypoglycaemic activity of some Indian medicinal plants in alloxan diabetic rats. J. Ethnopharmacol. 2003, 84, 105–108. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kirana, H. Trichosanthes cucumerina Linn. improves glucose tolerance and tissue glycogen in non insulin dependent diabetes mellitus induced rats. Indian J. Pharmacol. 2008, 40, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.L.; Mali, V.R.; Zambare, G.N.; Bodhankar, S. Cardioprotective Activity of Methanol Extract of fruit of Trichosanthes cucumerina on Doxorubicin-induced Cardiotoxicity in Wistar Rats. Toxicol. Int. Former. Indian J. Toxicol. 2012, 19, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Arawwawala, L.; Thabrew, M.; Arambewela, L. Gastroprotective activity of Trichosanthes cucumerina in rats. J. Ethnopharmacol. 2010, 127, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.; Kumar, B.R.; Mohan, G.K. Hepatoprotective effect of Trichosanthes cucumerina Var cucumerina L. on carbon tetrachloride induced liver damage in rats. J. Ethnopharmacol. 2009, 123, 347–350. [Google Scholar] [CrossRef]
- Kongtun, S.; Jiratchariyakul, W.; Kummalue, T.; Tan-Ariya, P.; Kunnachak, S.; Frahm, A.W. Cytotoxic Properties of Root Extract and Fruit Juice of Trichosanthes cucumerina. Planta Med. 2009, 75, 839–842. [Google Scholar] [CrossRef]
- Smetanska, I. Production of Secondary Metabolites Using Plant Cell Cultures. Plant Cells 2008, 111, 187–228. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusido, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Hayashi, H.; Huang, P.; Inoue, K. Up-regulation of Soyasaponin Biosynthesis by Methyl Jasmonate in Cultured Cells of Glycyrrhiza glabra. Plant Cell Physiol. 2003, 44, 404–411. [Google Scholar] [CrossRef]
- Veerashree, V.; Anuradha, C.M.; Kumar, V. Elicitor-enhanced production of gymnemic acid in cell suspension cultures of Gymnema sylvestre R. Br. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 108, 27–35. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Fang, J.; Cai, W.; Tang, Z. Chitosan treatment raises the accumulation of saponin and the transcriptional Level of genes encoding the key enzymes of saponin synthesis in cultured panaxginseng cells. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2002, 28, 485–490. [Google Scholar]
- Al-Khayri, J.M. Abiotic and Biotic Elicitors—Role in Secondary Metabolites Production through In Vitro Culture of Medicinal Plants. In Abiotic and Biotic Stress in Plants; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Dev, G.K.; Jeyaraj, M.; Rajesh, M.; Arjunan, A.; Muthuselvam, M.; Manickavasagam, M.; Selvaraj, N.; Ganapathi, A. Increased production of withanolide A, withanone, and withaferin A in hairy root cultures of Withania somnifera (L.) Dunal elicited with methyl jasmonate and salicylic acid. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 114, 121–129. [Google Scholar] [CrossRef]
- Halitschke, R.; Baldwin, I.T. Jasmonates and related compounds in plant-insect interactions. J. Plant Growth Regul. 2004, 23, 238–245. [Google Scholar] [CrossRef]
- Sánchez-Sampedro, M.A.; Fernández-Tárrago, J.; Corchete, P. Yeast extract and methyl jasmonate-induced silymarin production in cell cultures of Silybum marianum (L.) Gaertn. J. Biotechnol. 2005, 119, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sivanandhan, G.; Selvaraj, N.; Ganapathi, A.; Manickavasagam, M. Enhanced Biosynthesis of Withanolides by Elicitation and Precursor Feeding in Cell Suspension Culture of Withania somnifera (L.) Dunal in Shake-Flask Culture and Bioreactor. PLoS ONE 2014, 9, e104005. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Ito, M.; Tanaka, S.; Kamisako, W.; Tabata, M. Biosynthesis of bryonolic acid in cultured cells of watermelon. Phytochemistry 1993, 33, 1407–1413. [Google Scholar] [CrossRef]
- Takeda, T.; Kondo, T.; Mizukami, H.; Ogihara, Y. Bryonolic Acid Production in Hairy Roots of Trichosanthes kirilowii MAX. var. japonica KITAM. Transformed with Agrobacterium rhizogenes and Its Cytotoxic Activity. Chem. Pharm. Bull. 1994, 42, 730–732. [Google Scholar] [CrossRef]
- Go, R.-E.; Kim, C.-W.; Choi, K. Effect of fenhexamid and cyprodinil on the expression of cell cycle- and metastasis-related genes via an estrogen receptor-dependent pathway in cellular and xenografted ovarian cancer models. Toxicol. Appl. Pharmacol. 2015, 289, 48–57. [Google Scholar] [CrossRef]
- Go, R.-E.; Kim, C.-W.; Jeon, S.-Y.; Byun, Y.-S.; Jeung, E.-B.; Nam, K.-H.; Choi, K. Fludioxonil induced the cancer growth and metastasis via altering epithelial-mesenchymal transition via an estrogen receptor-dependent pathway in cellular and xenografted breast cancer models. Environ. Toxicol. 2016, 32, 1439–1454. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Bolla, S.R.; Al-Subaie, A.M.; Al-Jindan, R.Y.; Balakrishna, J.P.; Ravi, P.K.; Veeraraghavan, V.P.; Pillai, A.A.; Gollapalli, S.S.R.; Joseph, J.P.; Surapaneni, K.M. In vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression. Heliyon 2019, 5, e01648. [Google Scholar] [CrossRef]
- Pandith, H.; Zhang, X.; Liggett, J.; Min, K.-W.; Gritsanapan, W.; Baek, S. Hemostatic and Wound Healing Properties of Chromolaena odorata Leaf Extract. ISRN Dermatol. 2013, 2013, 168269. [Google Scholar] [CrossRef] [PubMed]
- Nyegaard, S.; Christensen, B.; Rasmussen, J.T. An optimized method for accurate quantification of cell migration using human small intestine cells. Metab. Eng. Commun. 2016, 3, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Devendra, N.K.; Everaldo, G.A.; Raghunandan, D. In Vitro Production of Cucurbitacins From Trichosanthes cucumerina L. var. cucumerina. Adv. Life Sci. 2012, 2, 108–111. [Google Scholar] [CrossRef]
- Azis, H.; Taher, M.; Ahmed, A.; Sulaiman, W.; Susanti, D.; Chowdhury, S.; Zakaria, Z.A. In vitro and In vivo wound healing studies of methanolic fraction of Centella asiatica extract. S. Afr. J. Bot. 2017, 108, 163–174. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lertphadungkit, P.; Suksiriworapong, J.; Satitpatipan, V.; Sirikantaramas, S.; Wongrakpanich, A.; Bunsupa, S. Enhanced Production of Bryonolic Acid in Trichosanthes cucumerina L. (Thai Cultivar) Cell Cultures by Elicitors and Their Biological Activities. Plants 2020, 9, 709. https://doi.org/10.3390/plants9060709
Lertphadungkit P, Suksiriworapong J, Satitpatipan V, Sirikantaramas S, Wongrakpanich A, Bunsupa S. Enhanced Production of Bryonolic Acid in Trichosanthes cucumerina L. (Thai Cultivar) Cell Cultures by Elicitors and Their Biological Activities. Plants. 2020; 9(6):709. https://doi.org/10.3390/plants9060709
Chicago/Turabian StyleLertphadungkit, Pornpatsorn, Jiraphong Suksiriworapong, Veena Satitpatipan, Supaart Sirikantaramas, Amaraporn Wongrakpanich, and Somnuk Bunsupa. 2020. "Enhanced Production of Bryonolic Acid in Trichosanthes cucumerina L. (Thai Cultivar) Cell Cultures by Elicitors and Their Biological Activities" Plants 9, no. 6: 709. https://doi.org/10.3390/plants9060709
APA StyleLertphadungkit, P., Suksiriworapong, J., Satitpatipan, V., Sirikantaramas, S., Wongrakpanich, A., & Bunsupa, S. (2020). Enhanced Production of Bryonolic Acid in Trichosanthes cucumerina L. (Thai Cultivar) Cell Cultures by Elicitors and Their Biological Activities. Plants, 9(6), 709. https://doi.org/10.3390/plants9060709




