Abstract
Petasites hybridus (Common butterbur) is extensively used in traditional medicine, and is currently gaining interest and popularity as a food supplement and for its medicinal properties. It contains a large number of active compounds of potential therapeutic activity, but also toxic pyrrolizidine alkaloids. Science-based information is needed to support the developing modern use of this plant, and to direct continued safe practice in traditional medicine. The present study focused on the essential oils from leaves and rhizomes of the understudied P. hybridus ssp. ochroleucus from the Balkans, and evaluated its phytochemistry and potential therapeutic activities (antimicrobial, antioxidant, anti-cholinesterase and anti-inflammatory), as well its toxicology potential (acute toxicity in insects and mice). We studied the essential oils, which are not commonly used in traditional practices, but have a potential for safe use since the toxic pyrrolizidine alkaloids, which are non-volatiles, are usually not present in the distilled essential oils. Pyrrolizidine alkaloids were indeed not detected in the essential oils; ingestion of the essential oils did not induce toxicity signs in mice, and topical application did not elicit skin irritation in humans. The essential oils had no antimicrobial properties against 20 pathogenic bacterial strains, but demonstrated good local anti-inflammatory activity in a Carrageenan-induced paw edema test. An insect toxicity test demonstrated that the leaf essential oil is an efficient insect repellent, and the demonstrated anti-cholinesterase activity suggests a potential for the treatment of neurological conditions. Isopetasin, a sesquiterpene found in plants of the genus Petasites, known to have anti-inflammatory effects, was present only in the rhizomes essential oil (3.9%), and sesquiterpene lactones concentrations were high, likely contributing to the antioxidant activity.
1. Introduction
Common butterbur [Petasites hybridus (L.) G.Gaertn., B.Mey. & Scherb] is a perennial herbaceous flowering plant of the daisy (Asteraceae) family. It has long rhizomes and large leaves, which develop after flowering and can reach up to 60 cm in diameter, and it grows up to 3 feet in height and has a history of use in traditional medicine [1]. It is native to Europe, and is present as an introduced species in North America and West and North Asia. It is common on riverbanks, in wet meadows and in other damp and shady locations [2]. Herbal preparations of P. hybridus have been practiced in traditional medicine in Europe for over 900 years, for the treatment of a broad spectrum of human aliments [3,4]. The rhizomes with roots (Rhizoma cum radicibus Petasiti) and the leaves (Folium Petasiti) are used to treat spastic cough, bronchitis, allergic rhinitis, asthma, migraine, dysmenorrhea, hypertension, ulcers, loss of appetite, as well as inflammatory gastrointestinal and genitourinary diseases [4,5,6,7,8]. In addition to its traditional use, P. hybridus is increasingly used today for its medicinal properties as an alternative medicine [9], and is rapidly gaining popularity as a dietary supplement [4]. Commercial preparations of P. hybridus capsules, extracts, powders, tinctures and softgels are available nowadays in many countries around the world.
Although the extracts of P. hybridus are approved by the USA Food and Drug Administration for use as dietary supplement, negative side effects (e.g., gastrointestinal problems, nausea, headache, drowsiness and halitosis) are known to occur [4]. Toxic effects are mainly attributed to alkaloid compounds—pyrrolizidine alkaloids, which are known to cause liver damage and may induce cancer [10]—and it is therefore not recommended for self-medication [11]. The concentrations of pyrrolizidine alkaloids in commercial preparations are usually below the limit of detection due to the production processes [4]. However, traditional processing, which is exercised in the Balkan Peninsula, results in potential exposure of the users to high concentrations of pyrrolizidine alkaloids. Following the Balkan’s ethnobotanical tradition, preparations of P. hybridus are widespread for the treatment of gastrointestinal and parasitic-induced hepatobiliary and respiratory disorders, as well as migraine and tension headaches [12,13,14,15].
The present study focused on Petasites hybridus subsp. ochroleucus, (i.e., a subspecies of Petasites hybridus) which is spread and used in traditional medicine mainly in the eastern, central and southern parts of the Balkans [1,16], and is endemic to the southern Balkan region. Very little information is available on this sub-species, which is distinguished from the more common and widely distributed, red-flowered subspecies P. hybridus subsp. hybridus, which is common in the northern and western parts of the Balkans, as well as in the rest of Europe. We focused on the essential oil of this plant, which is a herbal preparation not commonly used in traditional practices of Petasites hybridus and therefore has attracted little research-attention so far, but that has a potential for safe use since the toxic pyrrolizidine alkaloids are non-volatiles and are therefore usually not present in essential oils.
To the best of our knowledge, the chemical composition of the essential oil of P. hybridus, subsp. ochroleucus has not been studied before, and only limited information is available regarding its biological activities, despite the common use of the plant by the people of the Balkans. Variability in chemical composition within and between P. hybridus species is known to occur [11,17]. We therefore analyzed the chemical profile of the essential oils, as well as their effect on a range of biological and toxicological activities. Chemical composition may also vary between plant organs, and we have therefore comparatively evaluated the composition and activities of essential oils from leaves and rhizomes.
External application of P. hybridus is uncommon, and it could potentially be a safer mode of application. There are indications for external use of P. hybridus in the western Balkan Peninsula, for the treatment of rheumatism and musculoskeletal ailments and pains [14]. Additionally, according to reports in the 19th century literature from Serbia, it was well known and respected as an external remedy, with anti-inflammatory and wound healing properties, and as an effective treatment for swellings and skin ulcers [18]. We have therefore also evaluated the skin irritant potential of topical applications of the essential oil.
The overall aim of this project was to evaluate therapeutic and toxicology activities of the essential oil of P. hybridus subsp. ochroleucus. The hypotheses guiding the workplan were: (1) Essential oils from various plant parts vary in chemical composition and hence in therapeutic and toxicological effects. (2) The essential oils will not cause skin irritation when applied externally. To evaluate these hypotheses, we studied: (A) Pytochemistry (chemical profile) of essential oils from the leaves (above ground tissue) and rhizomes (below ground tissue) of P. hybridus subsp. ochroleucus. (B) Antimicrobial, antioxidant, anti-inflammatory and anti-cholinesterase activities, as well as toxicological effects (in mouse and Drosophila), comparatively, for essential oils from the leaves and rhizomes. (C) Skin irritation potential in humans, with external application of the leaf and rhizome essential oils. Antimicrobial activity was evaluated against 20 pathogenic bacterial strains (10 laboratory reference strains and 10 strains isolated from human wound swabs). Anti-inflammatory activity was studied in a Carrageenan-induced paw edema test with female Sprague Dawley rats.
2. Results
The chemical profiles of the essential oils of P. hybridus subsp. ochroleucus are presented in Table 1. Essential oil from the leaves [yield = 0.015% (v/w), d = 848.0 µg/µL] contained 42 components (94.62% of the total oil). Only five compounds had content higher than 3%. These are, in decreasing order: Fukinanolide (33.42%), 7-epi-α-Eudesmol (16.14%), Linalool (9.03%), Eremophilene (4.31%) and Geramacrene D (4.26%). Essential oil from the rhizome [yield = 0.067% (v/w), d = 914.0 µg/µL] contained 60 components (92.97% of total oil). Nine compounds had content higher than 3%. These are, in decreasing order: (2E)-Nonenal (11.23%), 1-Nonene (8.57%), Germacrene D (5.01%), α-Eudesmol (4.52%), Isopetasin (3.93%), α-Bisabolol oxide (3.38%), epi-β-Santalene (3.45%), α-Santalene (3.36%) and β-Cubebene (3.13%).

Table 1.
Chemical composition of the essential oils of Petasites hybridus subsp. ochroleucus extracted by hydrodistillation from rhizome and leaves.
Essential oils from both leaves and the rhizomes of P. hybridus subsp. ochroleucus demonstrated antioxidant activity (Table 2), with the activity of the leaves’ essential oils greater than that of the rhizomes. Neither of the two essential oils expressed antimicrobial activity against any of the tested bacterial strains (20 pathogenic bacterial strains, e.g., 10 laboratory reference strains and 10 strains isolated from human wound swabs), and certain bacterial strains even appeared to grow better in the presence of the essential oils (although not statistically significant; data not shown). External (topical) application of the essential oils did not cause skin irritation in any of the participating human volunteers, and all of the experimental Albino Swiss Webster mice survived the acute toxicity trial.

Table 2.
Antioxidant activity of the essential oils obtained from rhizomes and leaves of Petasites hybridus subsp. ochroleucus.
Results obtained from the screening of the interaction of the essential oils with cholinesterase from pooled human serum are presented in Table 3. Essential oils from the leaves and rhizomes of P. hybridus subsp. ochroleucus inhibited cholinesterase activity, but to a lesser extent then Neositgmin bromide, a synthetic cholinesterase inhibitor. The inhibition by the leaves’ essential oil (33.24%) was lower than the inhibition by the essential oil from the rhizomes (26.37%).

Table 3.
Anti-cholinesterase activity of essential oils obtained from rhizome and leaves of P. hybridus subsp. ochroleucus.
The anti-inflammatory activity results are presented in Table 4. Essential oil from the rhizome significantly decreased edema thickness at the concentrations of 10% (at 1, 4 and 6 h) and 20% (at 4 and 6 h), and the anti-inflammatory activity increased similarly to the referent indomethacin group. Essential oil from leaves decreased edema thickness only at the concentration of 40%, with high efficiency throughout the examined duration and a high percent of anti-inflammatory activity, similar to the indomethacin group. Lower concentrations of the essential oil from the leaves did not show significant results.

Table 4.
Anti-inflammatory activity (in %) of essential oils obtained from rhizome and leaves of P. hybridus subsp. ochroleucus.
The toxicity test on D. melanogaster revealed that the essential oils from both the rhizomes and the leaves is toxic for developing larvae. For both essential oils, significant larvae mortality was noted, starting at the concentration of 0.38% (Fischer’s exact test, p < 0.01). Estimated 48 h and 96 h LC50s for the rhizome oil were 3.40% and 3.13%, respectively. By comparison, the leaves’ essential oil was four times more toxic, with estimated 48 h and 96 h LC50s of 0.84% and 0.80%, respectively (Table 5). Prolonged monitoring of pupae (until all individuals reached imago stadium) did not show any further significant increase in mortality at the stage of pupa with rhizome essential oil. Only a few individuals that turned into pupae could not achieve imago stadium. However, for the leaves’ essential oil, prolonged mortality was evident during the pupa stage. In the two highest concentrations tested, for both oil types, it was also noted that the larvae grew slower, and both the larvae pupae, and adults were visibly smaller compared to the other groups.

Table 5.
Effects of essential oils of Petasites hybridus subsp. ochroleucus obtained from rhizomes and leaves on D. melanogaster survival.
The rhizome oil also affected the development of D. melanogaster larvae, and significantly delayed achievement of pupa stadium (Figure 1). The effect was dose-dependent and highly significant when compared to the control (ANOVA overall effect p < 0.001 with significant Dunnett’s procedure, for concentrations of 0.75% and higher; chi-square test overall effect p < 0.0001 with Fischer’s exact test for each concentration vs. control p << 0.05). Development time is presented in Table 5. Compared to the control group, development time for the rhizome essential oil treatment was delayed by 24–60 h depending on the concentration used. In the case of the leaf essential oil, it was not always possible to estimate the development time due to the high mortalities. At the lower concentrations, however, the trend of a delayed pupa stadium was the same as that for the rhizome essential oil.
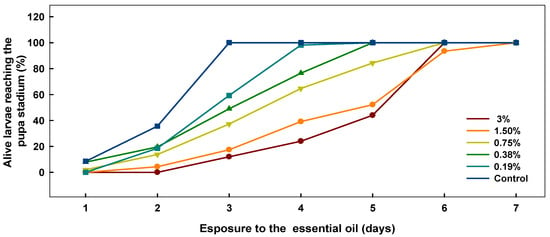
Figure 1.
The effect of essential oil from rhizomes of P. hybridus subsp. ochroleucuson on the development of D. melanogaster larvae into pupae. The effect is statically significant for each tested concentration when compared to the control group (Fischer’s exact test p < 0.05).
3. Discussion
Plants of the genus Petasites contain large amounts of active compounds such as sesquiterpene esters, sesquiterpene lactones and pyrrolizidine alkaloids [3]. Pyrrolizidine alkaloids are toxic [3,4], while some of the sesquiterpene esters and sesquiterpene lactones have medicinal properties. Primarily petasin and isopetasin sesquiterpene esters are considered as the most valuable pharmaceutical components in Petasites plants [3]. Therefore, P. hybridus plants used for pharmaceutical purposes, and especially the preparations made from these plants, should ideally contain high amounts of petasin/isopetasin, and, most importantly, low amounts of pyrrolizidine alkaloids [17,19]. The chemical analysis of the essential oils of P. hybridus ssp. ochroleucus from the Balkans revealed low concentrations of isopetasin, no petasin and no pyrrolizidine alkaloids. Concentrations of sesquiterpene lactones were, however, high. Isopetasin was present only in the rhizome essential oil (3.9%) and absent from the essential oil of the leaves. This concentration is below the average petasin/isopetasin concentration found in other preparations of Petasites plants [3]. The distribution and the concentration of petasin/isopetasin is often higher in rhizomes than in leaves [19], which is in accordance with the present results. Lower concentrations of sesquiterpenes (for example, farnesene, bisabolene, cyperene, santalene and germacrene, as well as many others) were detected in the rhizome essential oil, compared to the leaves. All the well-known pyrrolizidine alkaloids commonly present in Petasites (senecionine, integerrimine, seneciphylline or senkirkine) [3], as well as other pyrrolizidine alkaloids common in other genera [20], were below the detection limit in the essential oils. This is in accord with pyrrolizidine alkaloids being non-volatiles, which was the drive for the selection of essential oils (that are produced by distillation) as the medium of study. Therefore, essential oils of P. hybridus subsp. ochroleucus from the Balkans are of low toxicity, and satisfy the pharmaceutical requirement for low pyrrolizidine alkaloids content. However, as discussed above, they contain only moderate concentrations of the pharmaceutically active petasin/isopetasin, and their medicinal properties are therefore based on the activity of other compounds.
Absence of pyrrolizidine alkaloids in the essential oils is in accordance with the zero mortality rate observed, and the lack of behavioral changes, in the albino Swiss Webster mice in the acute toxicity trial, as well as with the lack of any adverse reaction following contact with human skin, as demonstrated with the skin irritation assay. The observed toxicity in Drosophila flies is therefore related to the presence of various terpene and terpene-like compounds. Plants produce many terpenes as a natural defense against insects [21]. The essential oil of the P. hybridus subsp. ochroleucus leaf, which was four times more toxic to Drosophila than the rhizome essential oil was, contained high concentrations of linalool (9.03%) (Table 1), which is used as a fruit fly and mosquito insecticide [22,23]. Given that the 48 h LC50 for Drosophila larvae was 0.84% (Table 5), and that additional prolonged toxicity hindered pupae ability to reach imago stadium, essential oil from the leaves of P. hybridus subsp. ochroleucus can be considered as an efficient insect repellent.
The anti-inflammatory activity of the essential oils of P. hybridus, and the role of petasin/isopetasin, have already been described [24]. In this paper, we examined the anti-inflammatory activity of essential oils obtained from rhizomes and leaves on the Carrageenan-induced paw edema model in rats. Anti-inflammatory activity of oil obtained from rhizome, starting from the concentration of 10%, is high and immediate, while concentration of 20% leads to significant inhibition of edema only after 4 h and repeated applications (Table 4), probably due to the slower dermal absorption of higher concentrations and the accompanying slower effects. Although low, the concentration of the sesquiterpene ester, isopetasine, in the essential oil obtained from the rhizome (3.93%) showed an anti-inflammatory effect after local treatment. The local application mode to the inflammation site probably contributed to the anti-inflammatory activity, because it has been shown that presence of petasin/isopetasin below 15% is inefficient for medical use. Rhizome essential oil at the concentration of 40% did not show significant reduction in paw thickness, which can be associated with problem of dermal absorption of high concentrations of this oil, or an inadequately effective dose. Unlike the essential oil from the rhizome, essential oil from the leaves was effective in suppressing paw edema only at the higher concentrations (statistical significance was obtained at the concentration of 40%). The differences in the effects of the essential oils obtained from rhizomes and leaves are due to different groups of active compounds present in those two plant organs. Sesquiterpenes lactones, which are present at high concentration in leaves’ essential oils (fukinanolide, 33.42%), have some medical properties as well [25], so the absence of petasin/isopetasin in leaves’ essential oils may be offset by high concentrations of sesquiterpenes lactones. This explains the anti-inflammatory effect of the essential oil from the leaves, as well as the higher concentration required to obtain the corresponding response. The efficiency of high concentrations may also indicate better dermal absorption of the essential oil obtained from the leaves.
Essential oil produced by the leaves had a higher antioxidative activity compared to essential oils from the underground parts of the plant, likely due to different chemical composition. Our results for the antioxidant activity of the leaves’ essential oil are supported by results for Petasites albus, a different species in the Petasites genus, from Iran [26], which also reported moderate antioxidant activity for the essential oil of the aerial part of the plant. The essential oil obtained from the leaves of P. hybridus subsp. ochroleucus contained high concentrations of sesquiterpene lactones (fukinanolide 33.42%) (Table 1). Sesquiterpene lactones of different Petasites species are known to have antioxidant activities [25,27,28,29], therefore it is likely that the higher antioxidant activity of the leaves’ vs the rhizome’s essential oil is due to the higher fukinanolide concentration.
Essential oils from the leaves and rhizomes of P. hybridus had a moderate anti-cholinesterase activity, with 33.24% and 26.37% inhibition for leaves and rhizomes essential oil, respectively (Table 3). Cholinesterase inhibitors are considered to delay the progression of dementia, and for now only several such inhibitors are officially registered. There is therefore a strong interest in identifying such compounds. Species from the Petasites genus are traditionally used for the treatment of migraine, which suggested their potential for the treatment of other neurological disorders including Alzheimer’s disease e.g., acting as acetylcholinesterase and butyrylcholinesterase inhibitors. The results indeed demonstrate an anticholinergic activity of the tested essential oils, and therefore their a potential value for the the treatment of neurological disorders, acting as AChE and BuChE inhibitors.
4. Materials and Methods
4.1. Plant Material
Petasites hybridus subsp. ochroleucus, (a subspecies of Petasites hybridus) was the plant material studied in the project. This subspecies is spread mainly in the eastern, central and southern parts of the Balkan Peninsula [1,16], and is endemic to the southern Balkan region. It is distinguished from the more common and widely distributed, red-flowered subspecies P. hybridus subsp. hybridus, which is related more to the northern and western parts of the Balkans, as well as to the rest of Europe.
Leaves and rhizomes of P. hybridus subsp. ochroleucus were collected in September 2014 from natural habitats, close to the village of Petačinci (42°52′50 N; 22°40′19.17 E), Dimitrovgrad, southeastern Serbia. Voucher specimens were deposited in the “Herbarium Moesiacum Niš” (HMN) of the Department of Biology and Ecology, Faculty of Science and Mathematics, University of Niš under the acquisition number 10,800. The official name of the species and status has been checked with http://www.theplantlist.org on 20 October 2017.
4.2. Essential-Oil Extraction and Chemical Analyses
The essential oil was extracted from fresh rhizomes and leaves by hydrodistillation for 4 h using a Clevenger-type apparatus. The extracted oil was dried over anhydrous sodium sulfate and stored at 4 °C until further use. Chemical profile of the essential oils was obtained by GC and GC-MS analyses. The GC analysis was performed on a GC HP-5890 II apparatus, equipped with a split–splitless injector, an HP-5MS capillary column (30 m × 0.25 mm, 0.25 μm film thickness) using helium as the carrier gas (1 mL/min), and a flame ionization detector. Operating conditions were as previously reported [30].
Comparison of Kovats retention indices [applying calibrated automated mass spectral deconvolution and identification system software (AMDIS ver. 2.64)], in combination with the SIA resolution method [31], was used for detection of the compounds. Spectral data were compared with the available literature [32], while obtained mass spectra were compared to those from Wiley 275 and NIST/NBS libraries using various search engines. The retention indices were obtained by co-injection with a standard aliphatic hydrocarbons C7–C40 mixture.
Essential oils from leaves and rhizome are complex systems containing numerous compounds, as is shown in Figure S1 (supplemental). Occurrence of the overlapped and embedded peaks is evident in the total ion chromatogram (TIC), as shown in the result of gas chromatography-mass spectrometry (GC-MS) analysis, especially with regard to teropenoids/sesquiterpenoids (compounds 16–62). First, the peaks from TICs obtained by GC-MS were identified by applying SIA. AMDIS was used for the deconvolution of these peaks and extraction of characteristic ion peaks originating from the specific compounds. After deconvolution, the purified mass spectrum of each peak was compared with the National Institute of Standard and Technology (NIST) 08 database or the mass spectra of standards.
Three examples of this procedure are presented for compounds No. 54, 61 and 62 (Figures S1–S3).
4.3. Antioxidant Activity of the Essential Oils
Antioxidant activity of the essential oils from leaves and rhizomes was tested by DPPH (2,2-diphenyl-1-picrylhydrazyl) and ABTS [2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)] assays, as previously described [30], using a Shimadzu, UV-Visible PC 1650 spectrophotometer (Tokyo, Japan). Data analysis was performed with OriginPro 8.0 software (Northampton, MA, USA).
4.4. Antimicrobial Activity of the Essential Oils
The antimicrobial activity of the essential oils extracted from P. hybridus subsp. ochroleucus was evaluated against 20 pathogenic bacterial strains: (1) A total of 10 laboratory reference strains from the American Type Culture Collection (ATCC)—Staphylococcus aureus ATCC 6538, S. epidermidis ATCC 12228, Streptococcus pyogenes ATCC 19615, Enterococcus faecalis ATCC 19433, Propionibacterium acnae ATCC 11827 [from group of Gram-positive bacteria], Escherichia coli ATCC 9863, Pseudomonas aeruginosa ATCC 9027, Acinetobacter boumanii ATCC 196060, Proteus mirabilis ATCC 12453 and Klebsiella pneumoniae ATCC 10031 (from group of Gram-negative bacteria); and (2) A total of 10 bacterial strains isolated from human wound swabs—Staphylococcus aureus, S. epidermidis, Streptococcus pyogenes, Enterococcus faecalis, Propionibacterium acnae, Escherichia coli, Pseudomonas aeruginosa, Acinetobacter sp., Proteus mirabils and Klebsiella sp. (Sorce: Institute of Public Health in Novi Sad, Serbia).
The antibacterial activity of the essential oils against the selected bacteria was evaluated in vitro, by Micro-Well Dilution Assay [33], as was previously described [30]. All experiments were performed in triplicates, and two growth controls, consisting of the corresponding medium with 10% DMSO (as a negative control) and antibiotic Doxycycline (as a positive control), were included (the concentration of Doxycycline ranged from 0.005 to 10.0 mg/mL). Bacterial growth was determined by adding 20 μL of 0.5% triphenyl tetrazolium chloride (TTC) aqueous solution to the plate [34]. Minimal inhibitory concentration (MIC) was defined as the lowest concentration of the oil that inhibited visible growth (red colored pellet on the bottom of the wells after the addition of TTC). For the determination of the minimal bactericidal concentration (MBC), broth was taken from each well that did not exhibit visible growth, and inoculated in Mueller Hinton agar for 24 h at 37 °C. MBC was defined as the lowest oil concentration that killed 99.9% of the bacterial cells.
4.5. Determination of the Skin Irritation Potential of the Essential Oils
The skin irritation potential of P. hybridus subsp. ochroleucus oil was determined in 30 male and female healthy volunteers (aged 18–30 years) who showed no signs of dermatological diseases. All volunteers signed an informed consent form after having received a full explanation of the test objectives, procedures and foreseeable risks to subjects. A piece of filter paper saturated (30 µL/cm2) either with undiluted P. hybridus oils or aqueous solution of sodium lauryl sulfate (20% w/v, positive control substance) was applied on the skin of the antecubital area of the arm and tightly covered by surgical tape. After 4 h of exposure, the patches were removed, and the sites of application were gently washed with water. The skin was examined and scored at 24, 48 and 72 h after patch removal. Skin reactions were scored, as previously suggested [35], by using a four-point scale.
4.6. Acute Toxicity
Albino Swiss Webster mice of either sex (males, 39–46 g; and females, 31–35 g) from the Oswaldo Cruz Foundation breeding stock were used for the study. Mice were housed, cared and handled in accordance with the Faculty of Medicine, University of Niš-approved guidelines and regulations from 03.07.2007, directed by the government of Serbia, Republic of Serbia Official Gazette No. 37/91, 50/92, 33/93, 52/93, 53/95, 52/96, 48/94, and 25/2000 (health protection of animals). The animals were separated by sex and housed as is described by Mihajilov-Krstev (2014) [30]. All mice had free access to tap water and were fed ad libitum. A single dose of oil of P. hybridus obtained from leaves and rhizome, diluted in corn oil, was given by gavages to male (0, 1250, 1500 and 2000 mg/kg body weight) and female (0, 1250, 1500, 2000, 2500 and 5000 mg/kg body weight) mice. An untreated control group was included as well. The mice were observed over 14 days for signs of toxicity or mortality. The procedure was repeated, for the oils extracted from both the underground and the aerial plant parts, separately.
4.7. Anti-Cholinesterase Activity
Inhibition of human serum cholinesterase was measured spectrophotometrically using a Konelab 20 analyzer (Thermofisher Scientific, Helsinki, Finland) with flow thermostated cells, length 7 mm (at 405 nm wavelength). Anti-cholinesterase activity was measured as described before [36]. Butyrylthiocholine iodide (purity > 99%), dithio-nitro benzoic acid (DTNB) and neostygmine bromide were purchased from Sigma Co., St. Louis, Missouri, USA. All other chemicals and reagents used [NaH2PO4-Na2HPO4, butylhydroxytoluene (BHT), dimethilsulphoxid (DMSO)] were purchased from Merck, Darmstadt, Germany.
4.8. Anti-Inflammatory Activity: Carrageenan-Induced Paw Edema
Anti-inflammation activity of the essential oils was determined on Carrageenan-induced paw edema in Sprague Dawley rats, following Amdekar et al. (2012) [37,38]. Female Sprague Dawley rats (180–250 g) were divided into nine groups of five animals per group. In all groups, 0.1 mL, 1% w/v λ-carrageenan analytical grade (Sigma-Aldrich Chemie Gmbh., Munich, Germany) in normal saline was injected into the subplantar tissue of the right hind paw. Plant essential oils, obtained from rhizomes and leaves, were dissolved separately in 2.5% DMSO (final volume 50 µL) and applied on the right hind paw of rats from the test groups, in concentrations of 10%, 20% or 40%. Oil was applied twice: first, immediately after the carrageenan injection, and again 3 hours later. The same volume (50 µL) of DMSO was applied to the right paw of animals from the control group. Paw thickness was measured before the carrageenan injection and 1, 2, 4 and 6 h after, using a digital caliper.
Edema thickness (mm) represents increases in the paw thickness at different time intervals after the carrageenan injection, relative to the values obtained before injection. Anti-inflammatory activity (edema inhibition) was calculated as anti-inflammatory activity (%) = (C–T)/(C) ×100, where C represents edema thickness in the control group, and T is the edema thickness in the test group. Indomethacin (10 mg/kg, p.o.) dissolved in saline was used as a standard anti-inflammatory drug, and its anti-inflammatory activity was measured as compared with the control group of animals that received the same amount of normal saline. Indomethacin and saline were given orally, 1 h before the carrageenan was injected. Data is expressed as mean ± S.D. The data was subjected to one–way analysis of variance (ANOVA) followed by Dunnett’s test. Differences between two means were detected by Student t-test. Data were considered significantly different for p < 0.05.
4.9. Drosophila Toxicity Tests
Larvae of Drosophila melanogaster Oregon-RC (wild-type flies, stock no. 5) ("Bloomington Drosophila Stock Center", Indiana University, USA) fruit flies were used for toxicity testing. Fruit flies were cared for, grown, fed, mated and transferred to the treatment media as previously described [30]. Larvae aged 72 ± 4 h were washed with distilled water and transferred to the standard feeding media containing 0, 0.19, 0.38, 0.75, 1.5 or 3% v/v of either rhizome or leaf oil extract from P. hybridus. Each experimental/control group consisted of 3 replicas. Each replica contained 20 larvae. Number of pupae, hatched adults and adult mortality were recorded daily. The exposure period lasted until all individuals that were alive emerged from pupae, i.e., 7 and 13 days for the rhizome and leaf essential oil extracts, respectively. As each of the adults emerged from its pupae it was immediately transferred to a new essential oil extract-free feeding medium. Hatched adults were monitored for an additional 7 (rhizome oil group) or 14 (leaf oil group) days after emergence for any prolonged effects on adulthood survival.
Risk Assessment Tool Analysis Software RA V1.0 was used to predict the short term LC50 (the lethal concentration required to kill 50% of the experimental population at a given time) by using surface response model analysis of the results for the P. hybridus rhizome and leaf extracts after subchronic exposure. Furthermore, the chronic toxicity threshold (LC1) was derived for the D. melanogaster (larva to adult). Development time (larva to pupa; larva to adult) was calculated for each replica of 20 eggs (N = 3) according to the following equation: DT = ∑nd*d/nt, where nd is the number of pupating larvae/emerging flies d days after the eggs were laid, and nt is the total number of individuals pupating/emerging at the end of experiment. Results were analyzed by one-way ANOVA followed by Tukey’s test and Dunnett’s procedure if significant. In addition, frequency analysis was performed by pooling together three replicas (total N = 60 per concentration) and results were expressed as categorical data (1—pass, or 0—fail). A chi-square test was conducted to assess significant differences in the relative mortality or pupation frequencies. If significant, after residual analysis, Fischer’s exact test was used to compare specific 2 × 2 tables and assess differences in the relative frequencies. Only a p value of ≤ 0.05 was considered statistically significant.
5. Conclusions
Pyrrolizidine alkaloids were not present in the essential oil of P. hybridus subsp. ochroleucus; concentrations of sesquiterpene esters were moderate, and sesquiterpene esters were present only in the rhizome’s essential oil, while essential oil from the leaves had high concentrations of sesquiterpene lactones. The essential oils showed no signs of toxicity to mice and no skin irritation potential in humans, and had no antimicrobial properties. However, they were found to have good local anti-inflammatory activity, a potential for use as an efficient insect repellent (leaves’ essential oils) and a potential value for the treatment of neurological disorders, due to their anticholinergic activity.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/6/700/s1, Figure S1. TIC Chromatogram of rhizome essential oil (Numbers above the peaks corresponding to the numbers in Table 1, Figure S2: Extracted spectrum for the compound No. 54, identified as 7-epi-α-Eudesmol, Figure S3: Extracted spectrum for the compound No. 60, identified as Isopetasine, Figure S4: Extracted spectrum for the compound No. 61, identified as Fukinanolide.
Author Contributions
Conceptualization, T.M.-K.; Formal analysis, T.M.-K., B.J., J.M., J.V., V.C., B.I., N.J., N.B., D.M., T.J., V.S.J., L.Đ. and N.S.; Funding acquisition, T.M.-K., B.Z., V.S.J. and N.B.; Resources, T.M.-K., B.Z., and J.M.; Writing—review and editing, N.B., T.M.-K., B.J., B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Education and Sciences of the Republic of Serbia, contracts number 451-03-68/2020-14. The APC was funded by Volcani Center, Israel.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dingwall, I. Petasites Miller. In Flora Europaea, 4th ed.; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; University Press: Cambridge, UK, 1976; pp. 186–188. [Google Scholar]
- Meusel, H.; Jäger, E.; Weinert, E. Vergleichende Chorologie der Zentraleuropäischen Flora «2»; Karten: Gustav Fischer, Jena, 1978. [Google Scholar]
- Aydın, A.A.; Zerbes, V.; Parlar, H.; Letzel, T. The medical plant butterbur (Petasites): Analytical and physiological (re)view. J. Pharm. Biomed. Anal. 2013, 75, 220–229. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Chemical Information Review Document for Butterbur (Petasites hybridus, ext.) [CAS No. 90082-63-6]; Supporting Nomination for Toxicological Evaluation by the National Toxicology Program; U.S. Department of Health and Human Services Research: Triangle Park, NC, USA, 2009.
- Asenov, I.; Gusev, C.; Kitanov, G.; Nikolov, S.; Petkov, T. Bilkosabiranie—Rakovodstvo za Brane i Parvična Prerabotka na Lečebni Rastenia; Biler: Sofia, Bulgaria, 1998. [Google Scholar]
- Danesch, U. Petasites hybridus (Butterbur root) extract in the treatment of asthma-an open trial. Altern. Med. Rev. 2004, 9, 54–62. [Google Scholar] [PubMed]
- Sutherland, A.; Sweet, B.V. Butterbur: An alternative therapy for migraine prevention. Am. J. Health Syst. Pharm 2010, 67, 705. [Google Scholar] [CrossRef]
- Thomet, O.A.R.; Schapowal, A.; Heinisch, I.V.W.M.; Wiesmann, U.N.; Simon, H.-U. Anti-inflammatory activity of an extract of Petasites hybridus in allergic rhinitis. Int. Immunopharmacol. 2002, 2, 997–1006. [Google Scholar] [CrossRef]
- Arkko, P.J.; Arkko, B.L.; Kari-Koshinen, O.; Taskinen, P.J. A survey of unproven cancer remedies and their users in an outpatient clinic for cancer therapy in Finland. Soc. Sci. Med. 1980, 14, 511–514. [Google Scholar]
- Hirono, I.; Mori, H.; Yamada, K.; Hirata, Y.; Haga, M.; Tatematsu, H.; Kanie, S. Carcinogenic activity of petasitenine, a new pyrrolizidine alkaloid isolated from Petasites japonicus Maxim. J. Natl. Cancer Inst. 1977, 58, 1155–1157. [Google Scholar] [CrossRef]
- Tasić, S.; Šavikin-Fodulović, K.; Menković, N. A Guide to the World of Medicinal Plants; Agency Valjevac: Valjevo, Serbia, 2004. [Google Scholar]
- Menković, N.; Šavikin, K.; Tasić, S.; Zdunić, G.; Stešević, D.; Milosavljević, S.; Vincek, D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro). J. Ethnopharmacol. 2011, 133, 97–107. [Google Scholar] [CrossRef]
- Redžić, S.S. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Coll. Antropol. 2007, 31, 869–890. [Google Scholar]
- Šarić-Kundalić, B.; Dobeš, C.; Klatte-Asselmeyer, V.; Saukel, J. Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J. Ethnopharmacol. 2010, 131, 33–55. [Google Scholar] [CrossRef]
- Tasić, S. Ethnobotany in SEE-WB countries; traditional uses of indigenous plants. Lek. Sirovine 2012, 32, 71–81. [Google Scholar]
- Zlatković, B. Carlina corymbosa L., Petasites hybridus subsp. ochroleucus (Boiss. & A. Huet) Šourek. In Euro+Med Notulae: 2: 709, 713—Willdenowia; Greuter, W., Raab-Straube, E., Eds.; BGBM: Berlin, Germany, 2006; Volume 36, pp. 707–717. [Google Scholar]
- Chizzola, R.; Ozelsberger, B.; Langer, T. Variability in chemical constituents in Petasites hybridus from Austria. Biochem. Syst. Ecol. 2000, 28, 421–432. [Google Scholar] [CrossRef]
- Petrović, S. Medicinal Herbs in Serbia, Serbian Archives for All Medicine, 1st ed.; Section 2, Book 16; Royal Serbian State Printing Office: Belgrade, Serbia, 1883. [Google Scholar]
- Wildi, E.; Langer, T.; Schaffner, W.; Büter, K.B. Quantitative analysis of petasin and pyrrolizidine alkaloids in leaves and rhizomes of in situ grown Petasites hybridus plants. Planta Med. 1998, 64, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Stelljes, M.E.; Kelley, R.B.; Molyneux, R.J.; Seiber, J.N. Gc-ms determination of pyrrolizidine alkaloids in four Senecio species. J. Nat. Prod. 1991, 54, 759–773. [Google Scholar] [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ling Chang, C.; Kyu Cho, I.; Li, Q.X. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 2009, 102, 203. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.C.; Junnila, A.; Butler, J.; Kravchenko, V.D.; Revay, E.E.; Weiss, R.W.; Schlein, Y. Efficacy of the botanical repellents geraniol, linalool, and citronella against mosquitoes. J. Vector Ecol. 2009, 34, 2–8. [Google Scholar] [CrossRef]
- Thomet, O.A.R.; Wiesmann, U.N.; Schapowal, A.; Bizer, C.; Simon, H.U. Role of petasin in the potential anti-inflammatory activity of a plant extract of Petasites hybridus. Biochem. Pharmacol. 2001, 61, 1041–1047. [Google Scholar] [CrossRef]
- Zhang, F.J.; Wang, Q.; Wang, Y.; Guo, M.L. Anti-allergic effects of total bakkenolides from Petasites tricholobus in ovalbumin-sensitized rats. Phytother. Res. 2011, 25, 116–121. [Google Scholar] [CrossRef]
- Mohammadi, M.; Yousefi, M.; Habibi, Z.; Dastan, D. Chemical composition and antioxidant activity of the essential oil of aerial parts of Petasites albus from Iran: A good natural source of euparin. Nat. Prod. Res. 2012, 26, 291–297. [Google Scholar] [CrossRef]
- Sun, Z.L.; Gao, G.L.; Luo, J.Y.; Zhang, X.L.; Zhang, M.; Feng, J. A new neuroprotective bakkenolide from the rhizome of Peatasites tatewakianus. Fitoterapia 2011, 82, 401–404. [Google Scholar] [CrossRef]
- Watanabe, S.; Hashimoto, K.; Tazaki, H.; Iwamoto, Y.; Shinohara, N.; Satoh, K.; Sakagami, H. Radical scavenging activity and inhibition of macrophage NO production by fukinolic acid, a main phenolic constituent in Japanese butterbur Petasites japonicus. Food Sci. Technol. Res. 2007, 13, 366–371. [Google Scholar] [CrossRef]
- Zhang, N.; Guo, M.L.; Zhang, G.; Li, R.P. A new neuroprotective bakkenolide from the rhizome of Petasites tricholobus. Chin. Chem. Lett. 2008, 19, 841–844. [Google Scholar] [CrossRef]
- Mihajilov-Krstev, T.; Jovanović, B.; Jović, J.; Ilić, B.; Miladinović, D.; Matejić, J.; Rajković, J.; Đorđević, L.; Cvetković, V.; Zlatković, B. Antimicrobial, antioxidative, and insect repellent effects of Artemisia absinthium essential oil. Planta Med. 2014, 80, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Liang, Y.; Yi, L.; Li, H.; Zhou, Z.; Ji, X.; Deng, J. Identification of free fatty acids profiling of type 2 diabetes mellitus and exploring possible biomarkers by GC–MS coupled with chemometrics. Metabolomics 2010, 6, 219–228. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, Approved Standard—9th ed.; CLSI Document M07-A9, 3(2); Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Sartoratto, A.; Machado, A.L.M.; Delarmelina, C.; Figueira, G.M.; Duarte, M.C.T.; Rehder, V.L.G. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz. J. Microbiol. 2004, 35, 275–280. [Google Scholar] [CrossRef]
- Basketter, D.A.; Chamberlain, M.; Griffiths, H.A.; Rowson, M.; Whittle, E.; York, M. The classification of skin irritants by human patch test. Food Chem. Toxicol. 1997, 35, 845–852. [Google Scholar] [CrossRef]
- Stankov-Jovanovic, V.P.; Nikolic-Mandic, S.D.; Mandic, L.M.; Mitic, V.D. A modification of the kinetic determination of pancuronium bromide based on its inhibitory effect on cholinesterase. J. Clin. Lab. Anal. 2007, 21, 124–131. [Google Scholar] [CrossRef]
- Amdekar, S.; Roy, P.; Singh, V.; Kumar, A.; Singh, R.; Sharma, P. Anti-inflammatory activity of Lactobacillus on carrageenan induced paw edema in male Wistar rats. Int. J. Inflamm. 2012, 75, 2015. [Google Scholar]
- Bernstein, N.; Akram, M.; Daniyal, M.; Koltai, H.; Fridlender, M.; Gorelick, J. Anti-inflammatory potential of medicinal plants: A source for therapeutic secondary metabolites. Adv. Agron. 2018, 150, 131–183. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).