Isolation and Screening of Extracellular PGPR from the Rhizosphere of Tomato Plants after Long-Term Reduced Tillage and Cover Crops
Abstract
1. Introduction
2. Material and Methods
2.1. Field Site and Samples Collection
2.2. Isolation of Putative Diazotrophic Vacteria
2.3. Rep-PCR
2.4. Taxonomic Identification of Unique Isolates
2.5. Phosphate Solubilization Ability Assessment
2.6. Determination of the Indole Acetic Acid Production
2.7. Siderophores Production Assay
2.8. In Vitro Assessment of Antifungal Activity
2.9. Data Analysis
3. Results
3.1. Isolation, Putative Diazotrophic Bacteria Identification, Rep-PCR, and Molecular Characterization of Rhizobacteria
3.2. Qualitative Estimation of Phosphate Solubilization Ability
3.3. Determination of the Indole Acetic Acid Production
3.4. Siderophores Production Assay
3.5. In Vitro Assessment of Antifungal Activity
3.6. Ranking of Different Plant Growth Promoting Traits
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Virk, H.K.; Singh, G.; Sharma, P. Effect of Tillage, Crop Residues of Preceding Wheat Crop and Nitrogen Levels on Biological and Chemical Properties of Soil in the Soybean–Wheat Cropping System. Commun. Soil Sci. Plant. Anal. 2017, 48, 1764–1771. [Google Scholar] [CrossRef]
- Mishra, P.; Singh, P.P.; Singh, S.K.; Verma, H. Sustainable agriculture and benefits of organic farming to special emphasis on PGPR. In Role of Plant Growth Promoting Microorganisms in Sustainable Agriculture and Nanotechnology; Elsevier Inc.: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Fiorini, A.; Boselli, R.; Maris, S.C.; Santelli, S.; Ardenti, F.; Capra, F.; Tabaglio, V. May conservation tillage enhance soil C and N accumulation without decreasing yield in intensive irrigated croplands? Results from an eight-year maize monoculture. Agric. Ecosyst. Environ. 2020, 296, 106926. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Kloepper, J.W. Plant Growth-Promoting Rhizobacteria and Plant Growth Under Gnotobiotic Conditions. Phytopathology 1981, 81, 97–168. [Google Scholar] [CrossRef]
- Boyetchko, S. Plant Growth Promoting Rhizobacteria. Encycl. Pest. Manag. 2002, 2011, 1–30. [Google Scholar]
- Glick, B.R. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995, 41, 109–117. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World, J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Saia, S.; Rappa, V.; Ruisi, P.; Abenavoli, M.R.; Sunseri, F.; Giambalvo, D.; Frenda, A.S.; Martinelli, F. Soil inoculation with symbiotic microorganisms promotes plant growth and nutrient transporter genes expression in durum wheat. Front. Plant. Sci. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Ali, S.; Hameed, S.; Shahid, M.; Iqbal, M.; Lazarovits, G.; Imran, A. Functional characterization of potential PGPR exhibiting broad-spectrum antifungal activity. Microbiol. Res. 2020, 232, 126389. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone Mediation of Interactions Between Plants and Non-Symbiotic Growth Promoting Bacteria Under Edaphic Stresses. Front. Plant. Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Mun, B.G.; Kang, S.M.; Hussain, A.; Shahzad, R.; Seo, C.W.; Kim, A.Y.; Lee, S.U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef] [PubMed]
- Ilangumaran, G.; Smith, D.L. Plant growth promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant. Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Kerry, R.G.; Das, G.; Paramithiotis, S.; Shin, H.S.; Patra, J.K. Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol. Res. 2018, 206, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef]
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next generation of microbial inoculants for agriculture and bioremediation. Microb. Biotechnol. 2017, 10, 19–21. [Google Scholar] [CrossRef]
- Zhang, L.-N.; Wang, D.-C.; Hu, Q.; Dai, X.-Q.; Xie, Y.-S.; Li, Q.; Liu, H.-M.; Guo, J.-H. Consortium of Plant Growth-Promoting Rhizobacteria Strains Suppresses Sweet Pepper Disease by Altering the Rhizosphere Microbiota. Front. Microbiol. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Kalita, M.; Bharadwaz, M.; Dey, T.; Gogoi, K.; Dowarah, P.; Unni, B.G.; Ozah, D.; Saikia, I. Developing novel bacterial based bioformulation having PGPR properties for enhanced production of agricultural crops. Indian, J. Exp. Biol. 2015, 53, 56–60. [Google Scholar]
- Pastor, N.; Carlier, E.; Andrés, J.; Rosas, S.B.; Rovera, M. Characterization of rhizosphere bacteria for control of phytopathogenic fungi of tomato. J. Environ. Manag. 2012, 95, S332–S337. [Google Scholar] [CrossRef]
- Title of Site: Il Comparto del Pomodoro da Industria, Dati ISMEA. Available online: https://terraevita.edagricole.it/wp-content/uploads/sites/11/2019/08/Il-comparto-del-pomodoro-da-industria.pdf (accessed on 8 April 2020).
- Title of Site: I.Stat. Available online: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_COLTIVAZIONI (accessed on 8 April 2020).
- Vaikuntapu, P.R.; Dutta, S.; Samudrala, R.B.; Rao, V.R.V.N.; Kalam, S.; Podile, A.R. Preferential Promotion of Lycopersicon esculentum (Tomato) Growth by Plant Growth Promoting Bacteria Associated with Tomato. Indian J. Microbiol. 2014, 54, 403–412. [Google Scholar] [CrossRef]
- Seleim, M.A.A.; Saead, F.A.; Abd-El-Moneem, K.M.H.; Abo-Elyousr, K.A.M. Biological control of bacterial wilt of tomato by plant growth promoting rhizobacteria. Plant. Pathol. J. 2011, 10, 146–153. [Google Scholar] [CrossRef]
- Narendra Babu, A.; Jogaiah, S.; Ito, S.; Kestur Nagaraj, A.; Tran, L.S.P. Improvement of growth, fruit weight and early blight disease protection of tomato plants by rhizosphere bacteria is correlated with their beneficial traits and induced biosynthesis of antioxidant peroxidase and polyphenol oxidase. Plant. Sci. 2015, 231, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.P.K.; Agrawal, S. Original Research Article Characterization of Bacillus sp. strains isolated from rhizosphere of tomato plants, (Lycopersicon esculentum) for their use as potential plant growth promoting rhizobacteria. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 406–417. [Google Scholar]
- Manfredi, G. Technologies for the Seismic Isolation and Control. Engineering 2009, 1, 271–320. [Google Scholar]
- Damodaran, T.; Sah, V.; Rai, R.B.; Sharma, D.K.; Mishra, V.K.; Jha, S.K.; Kannan, R. Isolation of salt tolerant endophytic and rhizospheric bacteria by natural selection and screening for promising plant growth-promoting rhizobacteria (PGPR) and growth vigour in tomato under sodic environment. Afr. J. Microbiol. Res. 2013, 7, 5082–5089. [Google Scholar]
- Vacheron, J.; Moënne-Loccoz, Y.; Dubost, A.; Gonçalves-Martins, M.; Muller, D.; Prigent-Combaret, C. Fluorescent pseudomonas strains with only few plant-beneficial properties are favored in the maize Rhizosphere. Front. Plant. Sci. 2016, 7, 1212. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.Y.A.; Milani, K.M.L.; Gonçalves, L.S.A.; De Oliveira, A.L.M. Diversity and plant growth-promoting functions of diazotrophic/N-scavenging bacteria isolated from the soils and rhizospheres of two species of Solanum. PLoS ONE 2020, 15, e0227422. [Google Scholar] [CrossRef]
- Abdeljalil, N.O.-B.; Vallance, J. Characterization of Tomato-associated Rhizobacteria Recovered from Various Tomato-growing Sites in Tunisia. J. Plant. Pathol. Microbiol. 2016, 7, 12. [Google Scholar] [CrossRef]
- Fernando, W.G.D.; Nakkeeran, S.; Zhang, Y.; Savchuk, S. Biological control of Sclerotinia sclerotiorum (Lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop. Prot. 2007, 26, 100–107. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Bai, Y.; Wang, J.; Nie, M.; Li, B.; Xiao, M. The use of Pseudomonas fluorescens P13 to control sclerotinia stem rot (Sclerotinia sclerotiorum) of oilseed rape. J. Microbiol. 2011, 49, 884–889. [Google Scholar] [CrossRef]
- Massawe, V.C.; Hanif, A.; Farzand, A.; Mburu, D.K.; Ochola, S.O.; Wu, L.; Tahir, H.A.S.; Gu, Q.; Wu, H.; Gao, X. Volatile compounds of endophytic Bacillus spp. have biocontrol activity against Sclerotinia sclerotiorum. Phytopathology 2018, 108, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Gray, E.J.; Smith, D.L. Intracellular and extracellular PGPR: Commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBryun, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Finney, D.M.; Buyer, J.S.; Kaye, J.P. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 2017, 72, 361–373. [Google Scholar] [CrossRef]
- Degrune, F.; Theodorakopoulos, N.; Dufrêne, M.; Colinet, G.; Bodson, B.; Hiel, M.P.; Taminiau, B.; Nezer, C.; Daube, G.; Vandenbol, M. No favorable effect of reduced tillage on microbial community diversity in a silty loam soil (Belgium). Agric. Ecosyst. Environ. 2016, 224, 12–21. [Google Scholar] [CrossRef]
- Barillot, C.D.C.; Sarde, C.O.; Bert, V.; Tarnaud, E.; Cochet, N. A standardized method for the sampling of rhizosphere and rhizoplan soil bacteria associated to a herbaceous root system. Ann. Microbiol. 2013, 63, 471–476. [Google Scholar] [CrossRef]
- Baldani, J.I.; Reis, V.M.; Videira, S.S.; Boddey, L.H.; Baldani, V.L.D. The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: A practical guide for microbiologists. Plant. Soil 2014, 384, 413–431. [Google Scholar] [CrossRef]
- Kloos, K.; Mergel, A.; Rösch, C.; Bothe, H. Denitrification within the genus azospirillum and other associative bacteria. Funct. Plant Biol. 2001, 28, 991–998. [Google Scholar] [CrossRef]
- Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Bacteria (PGPB): Isolation and Screening of PGP Activities. Curr. Protoc. Plant Biol. 2017, 2, 190–209. [Google Scholar] [CrossRef]
- Ishii, S.; Sadowsky, M.J. Applications of the rep-PCR DNA fingerprinting technique to study microbial diversity, ecology and evolution: Minireview. Environ. Microbiol. 2009, 11, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Healy, M.; Huong, J.; Bittner, T.; Lising, M.; Frye, S.; Raza, S.; Schrock, R.; Manry, J.; Renwick, A.; Nieto, R.; et al. Microbial DNA typing by automated repetitive-sequence-based PCR. J. Clin. Microbiol. 2005, 43, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Heras, J.; Domínguez, C.; Mata, E.; Pascual, V.; Lozano, C.; Torres, C.; Zarazaga, M. GelJ—a tool for analyzing DNA fingerprint gel images. BMC Bioinform. 2015, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Di Cello, F.; Fani, R. A molecular strategy for the study of natural bacterial communities by PCR-based techniques. Minerva Biotecnol. 1996, 8, 126–134. [Google Scholar]
- Glickmann, E.; Dessaux, Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl. Environ. Microbiol. 1995, 61, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Schwyn, B.; Neilands, J.B. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 1987, 160, 47–56. [Google Scholar] [CrossRef]
- Arora, N.K.; Verma, M. Modified microplate method for rapid and efficient estimation of siderophore produced by bacteria. 3 Biotech. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Dikin, A.; Sijam, K.; Kadir, J.; Semanz, I.A. Antagonistic bacteria against Schizophyllum Commune FR. In Peninsular Malaysia. Biotropia 2006, 13, 111–121. [Google Scholar]
- El-Sayed, W.S.; Akhkha, A.; El-Naggar, M.Y.; Elbadry, M. In vitro antagonistic activity, plant growth promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front. Microbiol. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Deka, H.; Deka, S.; Baruah, C.K. Plant Growth Promoting Rhizobacteria for Value Addition: Mechanism of Action. In Plant-Growth-Promoting Rhizobacteria (PGPR) and Medicinal Plants; Springer International Publishing: Gewerbestrasse, Switzerland, 2015; pp. 305–321. [Google Scholar]
- Kennedy, A.C.; de Luna, L.Z. Rhizosphere. In Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780080547954. [Google Scholar]
- Santi, C.; Bogusz, D.; Franche, C. Biological nitrogen fixation in non-legume plants. Ann. Bot. 2013, 111, 743–767. [Google Scholar] [CrossRef]
- Islam, M.R.; Sultana, T.; Joe, M.M.; Yim, W.; Cho, J.C.; Sa, T. Nitrogen-fixing bacteria with multiple plant growth-promoting activities enhance growth of tomato and red pepper. J. Basic Microbiol. 2013, 53, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Hurek, T.; Reinhold, B.; Grimm, B.; Fendrik, I.; Niemann, E.G. Occurrence of effective nitrogen-scavenging bacteria in the rhizosphere of kallar grass. Plant. Soil 1988, 110, 339–348. [Google Scholar] [CrossRef]
- Brooke, J.S. Stenotrophomonas maltophilia: An emerging global opportunistic pathogen. Clin. Microbiol. Rev. 2012, 25, 2–41. [Google Scholar] [CrossRef] [PubMed]
- Diancourt, L.; Passet, V.; Verhoef, J.; Grimont, P.A.D.; Brisse, S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 2005, 43, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Maul, J.E.; Buyer, J.S.; Lehman, R.M.; Culman, S.; Blackwood, C.B.; Roberts, D.P.; Zasada, I.A.; Teasdale, J.R. Microbial community structure and abundance in the rhizosphere and bulk soil of a tomato cropping system that includes cover crops. Appl. Soil Ecol. 2014, 77, 42–50. [Google Scholar] [CrossRef]
- Legrand, F.; Picot, A.; Cobo-Díaz, J.F.; Carof, M.; Chen, W.; Le Floch, G. Effect of tillage and static abiotic soil properties on microbial diversity. Appl. Soil Ecol. 2018, 132, 135–145. [Google Scholar] [CrossRef]
- Piazza, G.; Ercoli, L.; Nuti, M.; Pellegrino, E. Interaction Between Conservation Tillage and Nitrogen Fertilization Shapes Prokaryotic and Fungal Diversity at Different Soil Depths: Evidence From a 23-Year Field Experiment in the Mediterranean Area. Front. Microbiol. 2019, 10, 1–20. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 59, 215–223. [Google Scholar] [CrossRef]
- Buyer, J.S.; Teasdale, J.R.; Roberts, D.P.; Zasada, I.A.; Maul, J.E. Factors affecting soil microbial community structure in tomato cropping systems. Soil Biol. Biochem. 2010, 42, 831–841. [Google Scholar] [CrossRef]
- Hariprasad, P.; Niranjana, S.R. Isolation and characterization of phosphate solubilizing rhizobacteria to improve plant health of tomato. Plant. Soil 2009, 316, 13–24. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.S.; Ahemad, M.; Oves, M. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung. 2009, 56, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Wang, L.T.; Tai, C.J.; Wu, Y.C.; Chen, Y.B.; Lee, F.L.; Wang, S.L. Pseudomonas taiwanensis sp. nov., isolated from soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 2094–2098. [Google Scholar] [CrossRef] [PubMed]
- Volmer, J.; Neumann, C.; Bühler, B.; Schmid, A. Engineering of Pseudomonas taiwanensis VLB120 for constitutive solvent tolerance and increased specific styrene epoxidation activity. Appl. Environ. Microbiol. 2014, 80, 6539–6548. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.; Ramírez-Bahena, M.H.; José Cuesta, M.; Velázquez, E.; Peix, A. Pseudomonas guariconensis sp. nov., isolated from rhizospheric soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 4413–4420. [Google Scholar] [CrossRef]
- Dixit, S.; Kuttan, K.K.A.; Shrivastava, R. Isolation and characterization of phosphorus solubilizing bacteria from manganese mining area of Balaghat and Chhindwara. Curr. Sci. 2017, 113, 500–504. [Google Scholar] [CrossRef]
- Shahab, S.; Ahmed, N.; Khan, N.S. PSB_tested in plant growth. Afr. J. Agric. Res. 2009, 4, 1312–1316. [Google Scholar]
- Kumari, P.; Meena, M.; Upadhyay, R.S. Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal. Agric. Biotechnol. 2018, 16, 155–162. [Google Scholar] [CrossRef]
- Majeed, A.; Kaleem Abbasi, M.; Hameed, S.; Imran, A.; Rahim, N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Obafemi, Y.D.; Ajayi, A.A.; Taiwo, O.S.; Olorunsola, S.J.; Isibor, P.O. Isolation of polygalacturonase-producing bacterial strain from tomatoes (Lycopersicon esculentum Mill.). Int. J. Microbiol. 2019, 2019, 7505606. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole Acetic Acid Production by the Indigenous Isolates of Azotobacter and Fluorescent Pseudomonas in the Presence and Absence of Tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Anwar, S.; Ali, B.; Sajid, I. Screening of rhizospheric actinomycetes for various in-vitro and in-vivo plant growth promoting (PGP) traits and for agroactive compounds. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Dutta, J.; Thakur, D. Evaluation of multifarious plant growth promoting traits, antagonistic potential and phylogenetic affiliation of rhizobacteria associated with commercial tea plants grown in Darjeeling, India. PLoS ONE 2017, 12, e0182302. [Google Scholar] [CrossRef]
- Tian, F.; Ding, Y.; Zhu, H.; Yao, L.; Du, B. Genetic diversity of siderophore-producing bacteria of tobacco rhizosphere. Braz. J. Microbiol. 2009, 40, 276–284. [Google Scholar] [CrossRef]
- Mehnaz, S.; Weselowski, B.; Lazarovits, G. Sphingobacterium canadense sp. nov., an isolate from corn roots. Syst. Appl. Microbiol. 2007, 30, 519–524. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, J.C.; Peraza-Echeverría, L.; Soto-Hernández, R.M.; San Miguel-Chávez, R.; Pérez-Brito, D.; Tapia-Tussell, R.; Ortiz-Vázquez, E.; Rodríguez-García, C.M. Diospyros cuneata Inhibition of Fusarium oxysporum: Aqueous Extract and its Encapsulation by Ionic Gelation. Plant Pathol. Microbiol. 2016, 7, 1–11. [Google Scholar]
- Kim, Y.M.; Ahn, K.J.; Beppu, T.; Uozumi, T. Nucleotide sequence of the nifLA operon of Klebsiella oxytoca NG13 and characterization of the gene products. MGG Mol. Gen. Genet. 1986, 205, 253–259. [Google Scholar] [CrossRef]
- Sajjad Mirza, M.; Ahmad, W.; Latif, F.; Haurat, J.; Bally, R.; Normand, P.; Malik, K.A. Isolation, partial characterization, and the effect of plant growth-promoting bacteria (PGPB) on micro-propagated sugarcane in vitro. Plant. Soil 2001, 237, 47–54. [Google Scholar] [CrossRef]
- Pavlova, A.S.; Leontieva, M.R.; Smirnova, T.A.; Kolomeitseva, G.L.; Netrusov, A.I.; Tsavkelova, E.A. Colonization strategy of the endophytic plant growth-promoting strains of Pseudomonas fluorescens and Klebsiella oxytoca on the seeds, seedlings and roots of the epiphytic orchid, Dendrobium nobile Lindl. J. Appl. Microbiol. 2017, 123, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Park, M.R.; Kim, Y.C.; Lee, S.; Kim, I.S. Identification of an ISR-related metabolite produced by rhizobacterium Klebsiella oxytoca C1036 active against soft-rot disease pathogen in tobacco. Pest. Manag. Sci. 2009, 65, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant. Sci. 2018, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
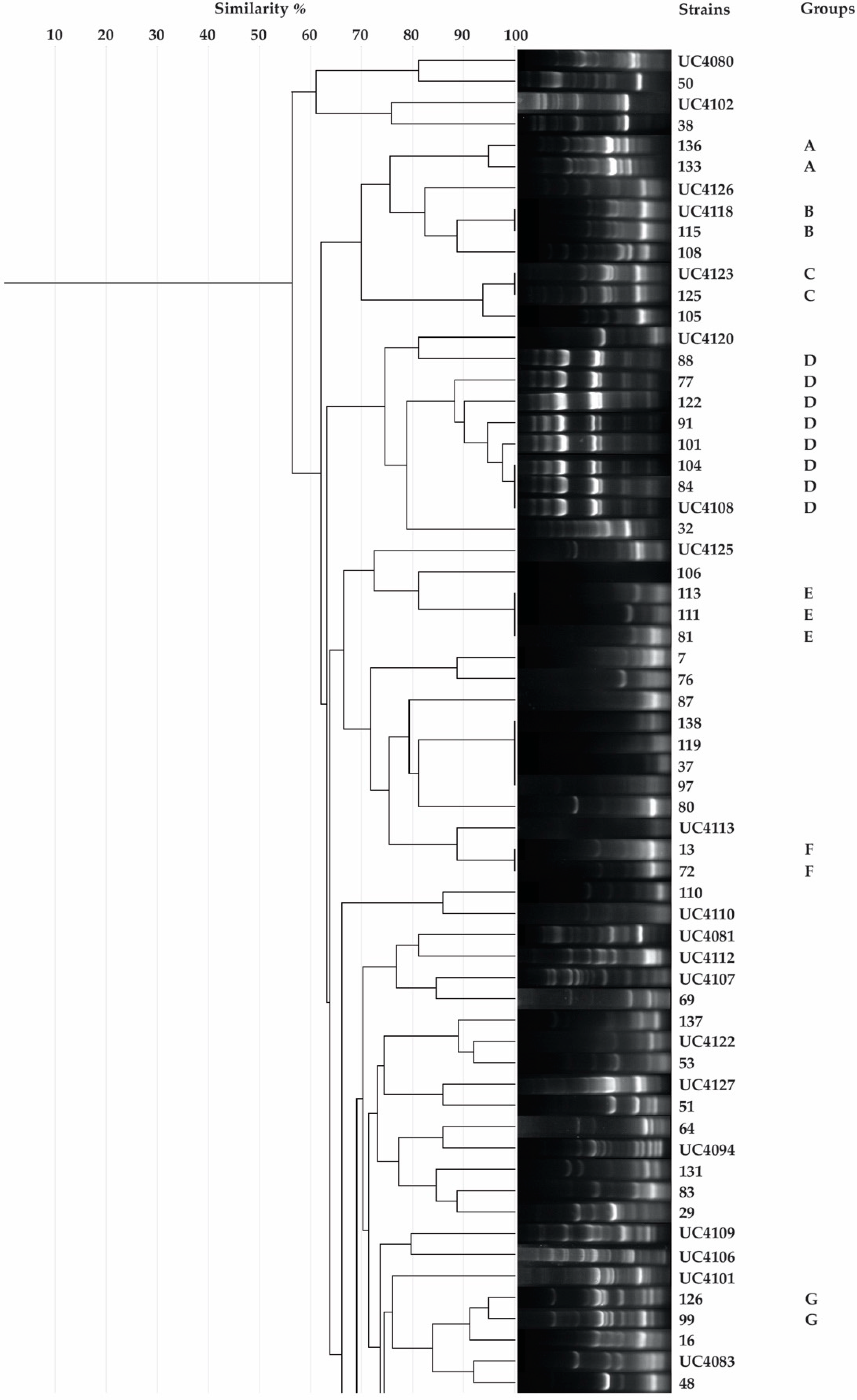
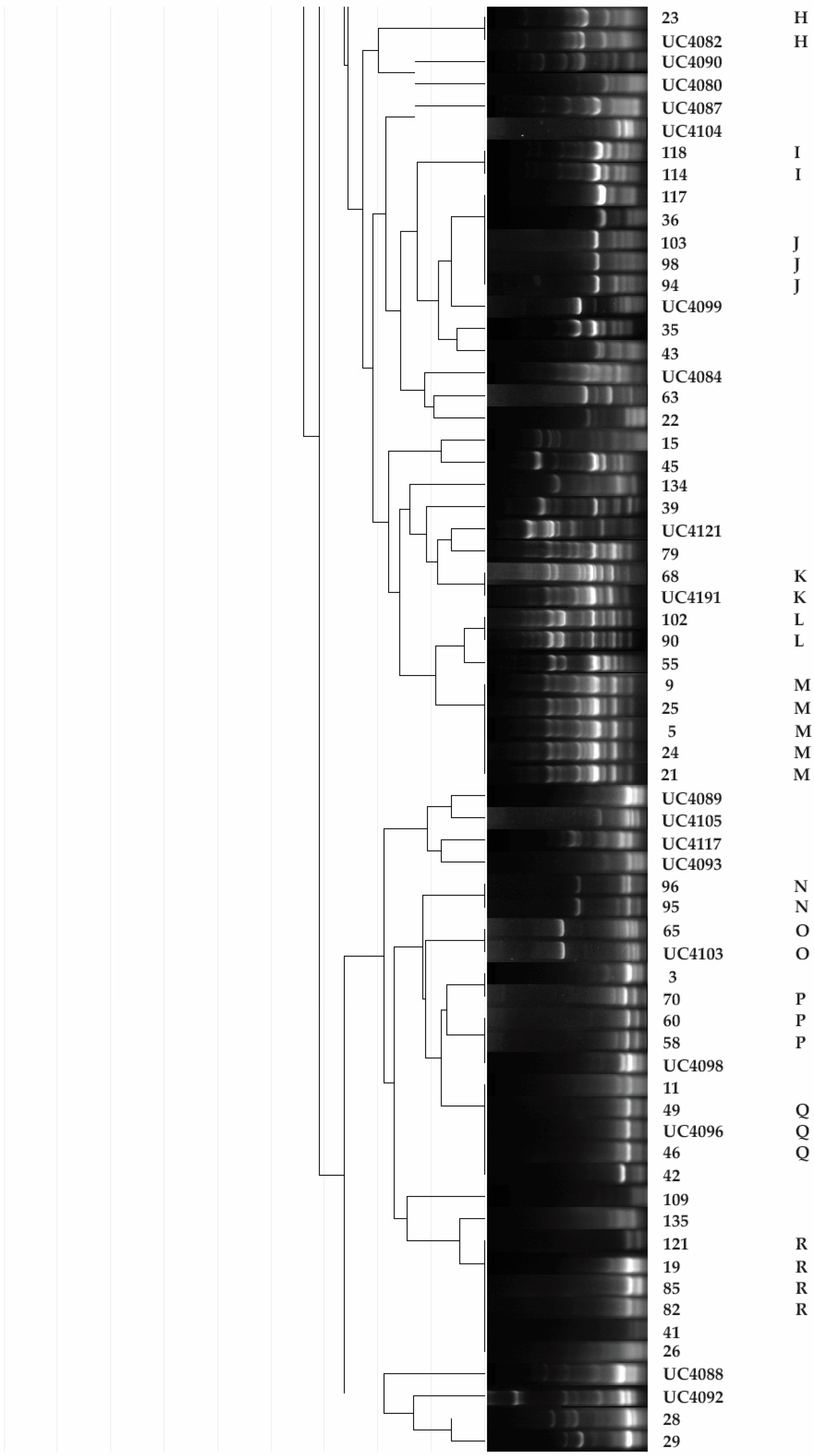
| Code a | Blast Match | ||||||
|---|---|---|---|---|---|---|---|
| Identity | Query Lenght | Bit-Score | Query Cover (%) | E-Value | Ident (%) | Accession No. b | |
| UC4080 | Sphingobacterium detergens | 816 | 1485 | 100 | 0.0 | 99.51 | MT435019 |
| UC4081 | Chryseobacterium oranimense | 1073 | 1940 | 99 | 0.0 | 99.35 | MT435020 |
| UC4082 | Pseudomonas pseudoalcaligenes | 1041 | 1855 | 99 | 0.0 | 99.13 | MT435021 |
| UC4083 | Stenotrophomonas acidaminiphila | 1182 | 2132 | 100 | 0.0 | 99.24 | MT435022 |
| UC4084 | Kosakonia radicincintans | 1123 | 2061 | 99 | 0.0 | 99.82 | MT435023 |
| UC4086 | Klebsiella oxytoca | 1155 | 2093 | 99 | 0.0 | 99.56 | MT435024 |
| UC4087 | Pseudomonas indoloxydans | 1204 | 2176 | 99 | 0.0 | 99.33 | MT435025 |
| UC4088 | Pseudomonas indoloxydans | 1243 | 2268 | 100 | 0.0 | 99.60 | MT435026 |
| UC4089 | Stenotrophomonas pictorum | 1136 | 2076 | 100 | 0.0 | 99.65 | MT435027 |
| UC4090 | Aeromonas caviae | 800 | 1458 | 99 | 0.0 | 99.87 | MT435028 |
| UC4091 | Pseudomonas pseudoalcaligenes | 1178 | 2109 | 100 | 0.0 | 98.98 | MT435029 |
| UC4092 | Kosakonia radicincitans | 1068 | 1954 | 100 | 0.0 | 99.72 | MT435030 |
| UC4093 | Stenotrophomonas pictorum | 1184 | 2145 | 99 | 0.0 | 99.58 | MT435031 |
| UC4094 | Enterobacter tabaci | 1102 | 2021 | 99 | 0.0 | 99.82 | MT435032 |
| UC4096 | Stenotrophomonas pavanii | 996 | 1808 | 100 | 0.0 | 99.40 | MT435033 |
| UC4098 | Stenotrophomonas rhizophila | 990 | 1810 | 99 | 0.0 | 99.70 | MT435034 |
| UC4099 | Enterobacter tabaci | 1075 | 1965 | 99 | 0.0 | 99.81 | MT435035 |
| UC4101 | Klebsiella grimontii | 1056 | 1914 | 99 | 0.0 | 99.52 | MT435036 |
| UC4102 | Chryseobacterium ureilyticum | 1143 | 2025 | 99 | 0.0 | 98.77 | MT435037 |
| UC4103 | [Pseudomonas] hibiscicola | 1159 | 2080 | 99 | 0.0 | 99.30 | MT435038 |
| UC4104 | Stenotrophomonas rhizophila | 1023 | 1873 | 100 | 0.0 | 99.71 | MT435039 |
| UC4105 | Stenotrophomonas pictorum | 1188 | 2128 | 99 | 0.0 | 99.32 | MT435040 |
| UC4106 | Enterobacter ludwigii | 1155 | 2115 | 99 | 0.0 | 99.91 | MT435041 |
| UC4107 | Sphingobacterium canadense | 1152 | 2080 | 99 | 0.0 | 99.13 | MT435042 |
| UC4108 | Chryseobacterium rhizosphaerae | 1162 | 2117 | 99 | 0.0 | 99.83 | MT435043 |
| UC4109 | Enterobacter tabaci | 1164 | 2102 | 99 | 0.0 | 99.48 | MT435044 |
| UC4110 | Kosakonia oryzendophytica | 1150 | 2045 | 98 | 0.0 | 99.12 | MT435045 |
| UC4112 | Pseudomonas taiwanensis | 1036 | 1890 | 99 | 0.0 | 99.81 | MT435046 |
| UC4113 | [Pseudomonas] hibiscicola | 1251 | 2244 | 99 | 0.0 | 99.04 | MT435047 |
| UC4117 | Pseudomonas taiwanensis | 1066 | 1949 | 99 | 0.0 | 99.81 | MT435048 |
| UC4118 | Klebsiella oxytoca | 1185 | 2158 | 99 | 0.0 | 99.66 | MT435049 |
| UC4120 | Chryseobacterium rhizosphaerae | 1212 | 2215 | 99 | 0.0 | 99.75 | MT435050 |
| UC4121 | Sphingobacterium siyangense | 985 | 1725 | 99 | 0.0 | 98.28 | MT435051 |
| UC4122 | Pseudomonas taiwanensis | 1162 | 2102 | 99 | 0.0 | 99.31 | MT435052 |
| UC4123 | Klebsiella oxytoca | 1150 | 2084 | 99 | 0.0 | 99.56 | MT435053 |
| UC4125 | Delftia tsuruhatensis | 1022 | 1869 | 99 | 0.0 | 99.90 | MT435054 |
| UC4126 | Pseudomonas japonica | 1151 | 2087 | 99 | 0.0 | 99.31 | MT435055 |
| UC4127 | Klebsiella oxytoca | 1187 | 2165 | 99 | 0.0 | 99.83 | MT435056 |
| Code | Identity | N Fixation | P Solubilization | IAA Production (μg/mL) | |||
|---|---|---|---|---|---|---|---|
| Growth on Nfb | w/Try | w/o Try | |||||
| s.e.m | Tukey | s.e.m | Tukey | ||||
| UC4080 | Sphingobacterium detergens | − | − | 1.42 ± 1.11 | ab | 0.39 ± 0.14 | b |
| UC4081 | Chryseobacterium oranimense | − | Level 1 | 1.34 ± 0.01 | ab | 0.86 ± 0.02 | b |
| UC4082 | Pseudomonas pseudoalcaligenes | + | Level 1 | 0.50 ± 0.05 | b | 0.84 ± 0.09 | b |
| UC4083 | Stenotrophomonas acidaminiphila | − | − | 1.61 ± 1.35 | ab | 1.45 ± 1.11 | b |
| UC4084 | Kosakonia radicincintans | + | Level 2 | 1.20 ± 0.91 | ab | 0.50 ± 0.25 | b |
| UC4086 | Klebsiella oxytoca | − | Level 2 | 17.84 ± 0.94 | ab | 10.30 ± 0.55 | ab |
| UC4087 | Pseudomonas indoloxydans | + | − | 1.62 ± 0.97 | ab | 2.05 ± 0.26 | b |
| UC4088 | Pseudomonas indoloxydans | + | − | 1.88 ± 0.71 | ab | 1.99 ± 0.20 | b |
| UC4089 | Stenotrophomonas pictorum | + | − | 5.82 ± 5.01 | ab | 0.66 ± 0.43 | b |
| UC4090 | Aeromonas caviae | + | Level 2 | 5.82 ± 2.37 | ab | 3.99 ± 0.26 | ab |
| UC4091 | Pseudomonas pseudoalcaligenes | + | − | 7.32 ± 5.71 | ab | 1.61 ± 0.46 | b |
| UC4092 | Kosakonia radicincitans | + | Level 2 | 4.20 ± 0.51 | ab | 4.17 ± 0.91 | ab |
| UC4093 | Stenotrophomonas pictorum | + | Level 1 | 0.50 ± 0.12 | b | 0.38 ± 0.25 | b |
| UC4094 | Enterobacter tabaci | + | Level 2 | 15.33 ± 11.40 | ab | 13.88 ± 11.54 | ab |
| UC4096 | Stenotrophomonas pavanii | + | Level 1 | 1.42 ± 1.04 | ab | 0.90 ± 0.34 | b |
| UC4098 | Stenotrophomonas rhizophila | + | Level 2 | 5.20 ± 1.72 | ab | 33.07 ± 29.25 | a |
| UC4099 | Enterobacter tabaci | + | Level 2 | 11.84 ± 7.00 | ab | 3.05 ± 0.03 | ab |
| UC4101 | Klebsiella grimontii | + | Level 2 | 8.14 ± 0.18 | ab | 8.20 ± 0.35 | ab |
| UC4102 | Chryseobacterium ureilyticum | − | Level 1 | 1.23 ± 1.23 | ab | 1.13 ± 0.17 | b |
| UC4103 | [Pseudomonas] hibiscicola | + | Level 1 | 1.24 ± 1.23 | ab | 1.16 ± 0.51 | b |
| UC4104 | Stenotrophomonas rhizophila | + | Level 1 | 1.25 ± 1.15 | ab | 0.81 ± 0.37 | b |
| UC4105 | Stenotrophomonas pictorum | + | Level 1 | 5.65 ± 5.51 | ab | 0.93 ± 0.15 | b |
| UC4106 | Enterobacter ludwigii | + | Level 2 | 11.95 ± 3.33 | ab | 8.51 ± 0.91 | ab |
| UC4107 | Sphingobacterium canadense | − | − | 1.90 ± 1.77 | ab | 0.67 ± 0.26 | b |
| UC4108 | Chryseobacterium rhizosphaerae | − | − | 1.22 ± 0.90 | ab | 0.90 ± 0.18 | b |
| UC4109 | Enterobacter tabaci | + | Level 2 | 33.00 ± 27.14 | a | 3.71 ± 1.12 | ab |
| UC4110 | Kosakonia oryzendophytica | + | Level 2 | 2.05 ± 1.12 | ab | 1.27 ± 0.83 | b |
| UC4112 | Pseudomonas taiwanensis | + | Level 2 | 0.44 ± 0.06 | b | 1.02 ± 0.12 | b |
| UC4113 | [Pseudomonas] hibiscicola | + | Level 1 | 1.41 ± 1.37 | ab | 1.05 ± 0.61 | b |
| UC4117 | Pseudomonas taiwanensis | + | Level 2 (+) | 2.21 ± 1.18 | ab | 2.90 ± 2.37 | ab |
| UC4118 | Klebsiella oxytoca | + | Level 2 | 8.55 ± 1.11 | ab | 7.37 ± 0.75 | ab |
| UC4120 | Chryseobacterium rhizosphaerae | − | − | 5.80 ± 5.70 | ab | 0.72 ± 0.10 | b |
| UC4121 | Sphingobacterium siyangense | − | − | 0.03 ± 0.03 | b | 0.05 ± 0.05 | b |
| UC4122 | Pseudomonas taiwanensis | + | Level 2 (+) | 0.00 ± 0.00 | b | 1.00 ± 0.00 | b |
| UC4123 | Klebsiella oxytoca | + | Level 2 | 8.35 ± 1.71 | ab | 7.11 ± 1.04 | ab |
| UC4125 | Delftia tsuruhatensis | + | − | 0.17 ± 0.10 | b | 0.49 ± 0.20 | b |
| UC4126 | Pseudomonas japonica | + | Level 2 | 5.94 ± 5.38 | ab | 4.30 ± 3.78 | ab |
| UC4127 | Klebsiella oxytoca | + | Level 2 | 8.38 ± 1.21 | ab | 7.12 ± 0.72 | ab |
| Code | Identity | Antifungal Activity vs. Sclerotinia sclerotiorum | Siderophore | ||
|---|---|---|---|---|---|
| s.e.m | Tukey | s.e.m | Tukey | ||
| UC4080 | Sphingobacterium detergens | 21.40 ± 10.02 | ab | 41.50 ± 4.04 | abcdef |
| UC4081 | Chryseobacterium oranimense | 31.77 ± 10.45 | ab | 47.66 ± 4.94 | abcd |
| UC4082 | Pseudomonas pseudoalcaligenes | 43.18 ± 2.45 | ab | 31.40 ± 3.11 | bcdefghi |
| UC4083 | Stenotrophomonas acidaminiphila | 37.00 ± 7.74 | ab | 42.91 ± 1.48 | abcde |
| UC4084 | Kosakonia radicincintans | 31.03 ± 2.83 | ab | 30.38 ± 4.42 | cdefghij |
| UC4086 | Klebsiella oxytoca | 20.00 ± 11.55 | ab | 10.08 ± 3.55 | jklm |
| UC4087 | Pseudomonas indoloxydans | 39.20 ± 4.39 | ab | 28.80 ± 1.25 | defghijkl |
| UC4088 | Pseudomonas indoloxydans | 41.08 ± 2.81 | ab | 27.58 ± 0.78 | defghijkl |
| UC4089 | Stenotrophomonas pictorum | 39.08 ± 5.44 | ab | 51.36 ± 3.76 | ab |
| UC4090 | Aeromonas caviae | 35.31 ± 13.80 | ab | 15.91 ± 7.60 | hijklm |
| UC4091 | Pseudomonas pseudoalcaligenes | 44.19 ± 2.48 | ab | 29.50 ± 2.57 | defghijk |
| UC4092 | Kosakonia radicincitans | 31.79 ± 6.41 | ab | 3.21 ± 2.00 | mn |
| UC4093 | Stenotrophomonas pictorum | 28.92 ± 13.80 | ab | 47.73 ± 2.16 | abcd |
| UC4094 | Enterobacter tabaci | 24.53 ± 4.55 | ab | 26.86 ± 1.27 | efghijkl |
| UC4096 | Stenotrophomonas pavanii | 36.49 ± 8.46 | ab | 40.17 ± 1.08 | abcdefg |
| UC4098 | Stenotrophomonas rhizophila | 27.24 ± 9.31 | ab | 0.00 ± 0.00 | n |
| UC4099 | Enterobacter tabaci | 21.38 ± 6.48 | ab | 26.51 ± 2.84 | efghijkl |
| UC4101 | Klebsiella grimontii | 29.19 ± 9.22 | ab | 0.56 ± 0.56 | n |
| UC4102 | Chryseobacterium ureilyticum | 35.46 ± 3.22 | ab | 51.20 ± 3.11 | ab |
| UC4103 | [Pseudomonas] hibiscicola | 45.10 ± 9.59 | ab | 35.41 ± 0.83 | abcdefgh |
| UC4104 | Stenotrophomonas rhizophila | 26.96 ± 9.46 | ab | 30.51 ± 2.80 | cdefghij |
| UC4105 | Stenotrophomonas pictorum | 35.98 ± 8.49 | ab | 40.90 ± 2.22 | abcdef |
| UC4106 | Enterobacter ludwigii | 29.03 ± 5.49 | ab | 3.95 ± 2.36 | mn |
| UC4107 | Sphingobacterium canadense | 22.12 ± 8.13 | ab | 52.28 ± 2.65 | a |
| UC4108 | Chryseobacterium rhizosphaerae | 31.40 ± 0.36 | ab | 42.59 ± 8.01 | abcde |
| UC4109 | Enterobacter tabaci | 18.07 ± 6.69 | ab | 8.79 ± 3.65 | lmn |
| UC4110 | Kosakonia oryzendophytica | 29.02 ± 9.36 | ab | 9.64 ± 4.47 | klm |
| UC4112 | Pseudomonas taiwanensis | 8.09 ± 0.26 | ab | 12.25 ± 1.14 | ijklm |
| UC4113 | [Pseudomonas] hibiscicola | 40.85 ± 7.09 | ab | 35.53 ± 1.69 | abcdefgh |
| UC4117 | Pseudomonas taiwanensis | 28.17 ± 5.93 | ab | 20.29 ± 8.28 | ghijklm |
| UC4118 | Klebsiella oxytoca | 20.59 ± 6.29 | ab | 5.86 ± 4.65 | mn |
| UC4120 | Chryseobacterium rhizosphaerae | 39.36 ± 8.38 | ab | 50.69 ± 1.16 | abc |
| UC4121 | Sphingobacterium siyangense | 19.02 ± 6.57 | ab | 45.71 ± 5.14 | abcde |
| UC4122 | Pseudomonas taiwanensis | 9.73 ± 3.34 | ab | 11.30 ± 2.24 | ijklm |
| UC4123 | Klebsiella oxytoca | 44.56 ± 5.46 | ab | 1.24 ± 1.24 | n |
| UC4125 | Delftia tsuruhatensis | 42.31 ± 3.95 | ab | 14.39 ± 1.44 | ijklm |
| UC4126 | Pseudomonas japonica | 5.23 ± 2.62 | b | 21.77 ± 5.78 | fghijklm |
| UC4127 | Klebsiella oxytoca | 48.01 ± 1.97 | a | 4.93 ± 3.18 | mn |
| Code | Identity | N Fixation | P Solubilization | IAA Production | Antifungal Activity vs. S. sclerotiorum | Siderophore | Rank | |
|---|---|---|---|---|---|---|---|---|
| w/Try | w/o Try | |||||||
| UC4094 | Enterobacter tabaci | 1 | 0.5 | 0.46 | 0.42 | 0.51 | 0.51 | 3.41 |
| UC4098 | Stenotrophomonas rhizophila | 1 | 0.5 | 0.16 | 1,00 | 0.57 | 0.00 | 3.22 |
| UC4109 | Enterobacter tabaci | 1 | 0.5 | 1.00 | 0.11 | 0.38 | 0.17 | 3.16 |
| UC4127 | Klebsiella oxytoca | 1 | 0.5 | 0.25 | 0.22 | 1.00 | 0.09 | 3.06 |
| UC4089 | Stenotrophomonas pictorum | 1 | 0.5 | 0.25 | 0.22 | 1.00 | 0.09 | 2.99 |
| UC4105 | Stenotrophomonas pictorum | 1 | 0.25 | 0.17 | 0.03 | 0.75 | 0.78 | 2.98 |
| UC4103 | [Pseudomonas] hibiscicola | 1 | 0.25 | 0.04 | 0.04 | 0.94 | 0.68 | 2.94 |
| UC4123 | Klebsiella oxytoca | 1 | 0.5 | 0.25 | 0.21 | 0.93 | 0.02 | 2.92 |
| UC4099 | Enterobacter tabaci | 1 | 0.5 | 0.36 | 0.09 | 0.45 | 0.51 | 2.90 |
| UC4117 | Pseudomonas taiwanensis | 1 | 0.75 | 0.07 | 0.09 | 0.59 | 0.39 | 2.88 |
| UC4113 | [Pseudomonas] hibiscicola | 1 | 0.25 | 0.04 | 0.03 | 0.85 | 0.68 | 2.86 |
| UC4096 | Stenotrophomonas pavanii | 1 | 0.25 | 0.04 | 0.03 | 0.76 | 0.77 | 2.85 |
| UC4090 | Aeromonas caviae | 1 | 0.5 | 0.18 | 0.12 | 0.74 | 0.30 | 2.84 |
| UC4106 | Enterobacter ludwigii | 1 | 0.5 | 0.36 | 0.26 | 0.60 | 0.08 | 2.80 |
| UC4093 | Stenotrophomonas pictorum | 1 | 0.25 | 0.02 | 0.01 | 0.60 | 0.91 | 2.79 |
| UC4082 | Pseudomonas pseudoalcaligenes | 1 | 0.25 | 0.01 | 0.03 | 0.90 | 0.60 | 2.79 |
| UC4084 | Kosakonia radicincitans | 1 | 0.5 | 0.04 | 0.02 | 0.65 | 0.58 | 2.78 |
| UC4091 | Pseudomonas pseudoalcaligenes | 1 | 0 | 0.22 | 0.05 | 0.92 | 0.57 | 2.76 |
| UC4101 | Klebsiella grimontii | 1 | 0.5 | 0.25 | 0.25 | 0.61 | 0.01 | 2.61 |
| UC4118 | Klebsiella oxytoca | 1 | 0.5 | 0.26 | 0.22 | 0.43 | 0.11 | 2.52 |
| UC4088 | Pseudomonas indoloxydans | 1 | 0 | 0.06 | 0.06 | 0.86 | 0.53 | 2.50 |
| UC4087 | Pseudomonas indoloxydans | 1 | 0 | 0.05 | 0.06 | 0.82 | 0.55 | 2.48 |
| UC4092 | Kosakonia radicincitans | 1 | 0.5 | 0.13 | 0.13 | 0.66 | 0.06 | 2.48 |
| UC4104 | Stenotrophomonas rhizophila | 1 | 0.25 | 0.04 | 0.02 | 0.56 | 0.58 | 2.46 |
| UC4110 | Kosakonia oryzendophytica | 1 | 0.5 | 0.06 | 0.04 | 0.60 | 0.18 | 2.39 |
| UC4126 | Pseudomonas japonica | 1 | 0.5 | 0.18 | 0.13 | 0.11 | 0.42 | 2.33 |
| UC4122 | Pseudomonas taiwanensis | 1 | 0.75 | 0.00 | 0.03 | 0.20 | 0.22 | 2.20 |
| UC4125 | Delftia tsuruhatensis | 1 | 0 | 0.01 | 0.01 | 0.75 | 0.28 | 2.05 |
| UC4102 | Chryseobacterium ureilyticum | 0 | 0.25 | 0.04 | 0.03 | 0.74 | 0.98 | 2.04 |
| UC4120 | Chryseobacterium rhizosphaerae | 0 | 0 | 0.18 | 0.02 | 0.82 | 0.97 | 1.99 |
| UC4086 | Klebsiella oxytoca | 0 | 0.5 | 0.54 | 0.31 | 0.42 | 0.19 | 1.96 |
| UC4112 | Pseudomonas taiwanensis | 1 | 0.5 | 0.01 | 0.03 | 0.17 | 0.23 | 1.95 |
| UC4081 | Chryseobacterium oranimense | 0 | 0.25 | 0.04 | 0.03 | 0.66 | 0.91 | 1.89 |
| UC4083 | Stenotrophomonas acidamiphila | 0 | 0 | 0.05 | 0.04 | 0.77 | 0.75 | 1.61 |
| UC4107 | Sphingobacterium canadense | 0 | 0 | 0.06 | 0.02 | 0.46 | 1.00 | 1.54 |
| UC4108 | Chryseobacterium rhizosphaerae | 0 | 0 | 0.04 | 0.03 | 0.65 | 0.81 | 1.53 |
| UC4080 | Sphingobacterium detergens | 0 | 0 | 0.04 | 0.01 | 0.45 | 0.79 | 1.29 |
| UC4121 | Sphingobacterium siyangense | 0 | 0 | 0.00 | 0.00 | 0.40 | 0.87 | 1.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrieri, M.C.; Fanfoni, E.; Fiorini, A.; Trevisan, M.; Puglisi, E. Isolation and Screening of Extracellular PGPR from the Rhizosphere of Tomato Plants after Long-Term Reduced Tillage and Cover Crops. Plants 2020, 9, 668. https://doi.org/10.3390/plants9050668
Guerrieri MC, Fanfoni E, Fiorini A, Trevisan M, Puglisi E. Isolation and Screening of Extracellular PGPR from the Rhizosphere of Tomato Plants after Long-Term Reduced Tillage and Cover Crops. Plants. 2020; 9(5):668. https://doi.org/10.3390/plants9050668
Chicago/Turabian StyleGuerrieri, Maria Chiara, Elisabetta Fanfoni, Andrea Fiorini, Marco Trevisan, and Edoardo Puglisi. 2020. "Isolation and Screening of Extracellular PGPR from the Rhizosphere of Tomato Plants after Long-Term Reduced Tillage and Cover Crops" Plants 9, no. 5: 668. https://doi.org/10.3390/plants9050668
APA StyleGuerrieri, M. C., Fanfoni, E., Fiorini, A., Trevisan, M., & Puglisi, E. (2020). Isolation and Screening of Extracellular PGPR from the Rhizosphere of Tomato Plants after Long-Term Reduced Tillage and Cover Crops. Plants, 9(5), 668. https://doi.org/10.3390/plants9050668







