The Effect of Mechanical Stress on Plant Susceptibility to Pests: A Mini Opinion Review
Abstract
1. Introduction
2. Mechanical Stimuli Have an Impact on a Wide Variety of Plants, Pests and Pathogens
3. Quantifying Mechanical Stimulation in the Case of Non-wounding Stimuli: Spatial and Temporal Aspects
3.1. Quantifying the Mechanical State of Plants Due to Mechanical Stimulus
3.2. Timing between the Application of Mechanical Stimulation and Inoculation
3.3. Persistence of the Effect of Mechanical Stress on Plant Susceptibility to Pests
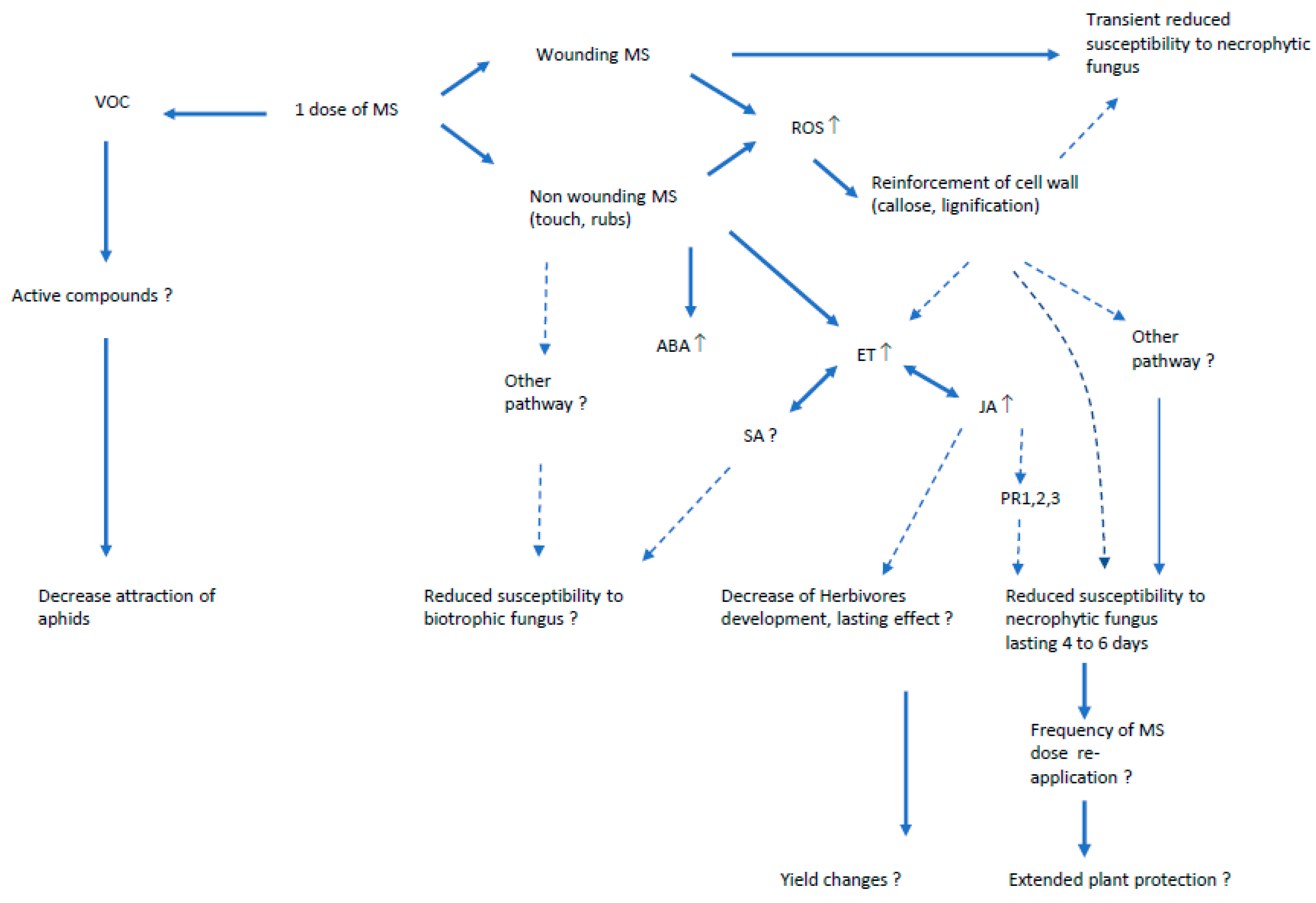
4. Pathways Involved in Mechanically-Induced Resistance to Pests
4.1. Oxidative Stress
4.2. Hormones and Defense-Related Genes
4.3. Volatile Organic Compounds (VOC)
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Achuo, E.A.; Prinsen, E.; Hofte, M. Influence of drought, salt stress and abscisic acid on the resistance of tomato to botrytis cinerea and oidium neolycopersici. Plant Pathol. 2006, 55, 178–186. [Google Scholar] [CrossRef]
- Lecompte, F.; Nicot, P.C.; Ripoll, J.; Abro, M.A.; Raimbault, A.K.; Lopez-Lauri, F.; Bertin, N. Reduced susceptibility of tomato stem to the necrotrophic fungus Botrytis cinerea is associated with a specific adjustment of fructose content in the host sugar pool. Ann. Bot. 2017, 119, 931–943. [Google Scholar]
- Lecompte, F.; Abro, M.A.; Nicot, P.C. Can sugar mediate the effect of nitrogen fertilization on lettuce susceptibility to two necrotrophic pathogens: Botrytis cinerea and Sclerotinium sclerotinium? Plant Soil 2013, 369, 387–401. [Google Scholar] [CrossRef]
- Ben-Issa, R.; Gomez, L.; Gautier, H. Companion Plants for Aphid Pest Management. Insects 2017, 8, 112. [Google Scholar] [CrossRef]
- Marolleau, B.; Gaucher, M.; Heintz, C.; Degrave, A.; Warneys, R.; Orain, G.; Lemarquand, A.; Brisset, M.-N. When a Plant Resistance Inducer leaves the lab for the field: Integrating ASM into routine apple protection practices. Front. Plant 2017, 8. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Chassot, C.; Buchala, A.; Schoonbeek, H.-J.; Metraux, J.-P.; Lamotte, O. Wounding of Arabidopsis leaves causes a powerful but transient protection against Botrytis infection. Plant J. 2008, 55, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Cipollini, D.F. Wind-induced mechanical stimulation increases pest resistance in common bean. Oecologia 1997, 111, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Chehab, E.W.; Yao, C.; Henderson, Z.; Kim, S.; Braam, J. Arabidopsis Touch-Induced Morphogenesis Is Jasmonate Mediated and Protects against Pests. Curr. Biol. 2012, 22, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Markovic, D.; Glinwood, R.; Olsson, U.; Ninkovic, V. Plant response to touch affects the behaviour of aphids and ladybirds. Arthropod-Plant Interact. 2014, 8, 171–181. [Google Scholar] [CrossRef]
- Benikhlef, L.; Floriane L’Haridon, F.; Abou-Mansour, E.; Serrano, M.; Binda, M.; Costa, A.; Silke Lehmann, S.; Métraux, J.-P. Perception of soft mechanical stress in Arabidopsis leaves activates disease resistance. BMC Plant Biol. 2013, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Grau, R.H.; Requena-Serra, F.J.; Hael-Conrad, V.; Martinez-Zamora, M.G.; Guerrero-Molina, M.F.; Diaz-Ricci, J.C. Soft mechanical stimulation induces a defense response against Botrytis cinerea in strawberry. Plant Cell Rep. 2017, 37, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Coutand, C.; Chevolot, M.; Lacointe, A.; Rowe, N.; Scotti, I. Mechanosensing of stem bending and its interspecific variability in five neotropical rainforest species. Ann. Bot. 2010, 105, 341–347. [Google Scholar] [CrossRef]
- Coutand, C.; Moulia, B. Biomechanical study of the effect of a controlled bending on tomato stem elongation: Local strain sensing and spatial integration of the signal. J. Exp. Bot. 2000, 51, 1825–1842. [Google Scholar] [CrossRef]
- Moulia, B.; Coutand, C.; Julien, J.-L. Mechanosensitive control of plant growth: Bearing the load, sensing, transducing and responding. Front. Plant Sci. 2015, 6, 52. [Google Scholar] [CrossRef]
- Coutand, C.; Martin, L.; Leblanc-Fournier, N.; Decourteix, M.; Julien, J.-L.; Moulia, B. Strain Mechanosensing Quantitatively Controls Diameter Growth and PtaZFP2 Gene Expression in Poplar. Plant Physiol. 2009, 151, 223–232. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martin, L.; Leblanc-Fournier, N.; Julien, J.-L.; Moulia, B.; Coutand, C. Acclimation kinetics of physiological and molecular responses of plants to multiple mechanical loadings. J. Exp. Bot. 2010, 61, 2403–2412. [Google Scholar] [CrossRef]
- Windram, O.; Madhou, P.; McHattie, S.; Hill, C.; Hickman, R.; Cooke, E.; Jenkins, D.J.; Penfold, C.A.; Baxter, L.; Breeze, E.; et al. Arabidopsis Defense against Botrytis cinerea: Chronology and Regulation Deciphered by High-Resolution Temporal Transcriptomic Analysis. Plant Cell 2012, 24, 3530–3557. [Google Scholar] [CrossRef]
- Asselbergh, B.; Curvers, K.; Franca, S.C.; Audenaert, K.; Vuylsteke, M.; Van Breusegem, F.; Hoefte, M. Resistance to botrytis cinerea in sitiens; an abscisic acid-deficient tomato mutant; involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 2007, 144, 1863–1877. [Google Scholar] [CrossRef]
- Mengiste, T. Plant immunity to necrotrophs. Annu. Rev. Phytopathol. 2012, 50, 267–294. [Google Scholar] [CrossRef]
- Perchepied, L.; Balague, C.; Riou, C.; Claudel-Renard, C.; Riviere, N.; Grezes-Besset, B.; Roby, D. Nitric oxide participates in the complex interplay of defense-related signaling pathways controlling disease resistance to sclerotinia sclerotiorum in arabidopsis thaliana. Mol. Plant-Microbe Interact. 2010, 23, 846–860. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Kabbage, M.; Kim, H.-J.; Britt, R.; Dickman, M.B. Tipping the balance: Sclerotinia sclerotiorum secreted oxalic acid suppresses host defenses by manipulating the host redox environment. PLoS Pathog. 2011, 7, e1002107. [Google Scholar] [CrossRef] [PubMed]
- Audenaert, K.; De Meyer, G.B.; Hofte, M.M. Abscisic acid determines basal susceptibility of tomato to botrytis cinerea and suppresses salicylic acid-dependent signalling mechanisms. Plant Physiol. 2002, 128, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Asselbergh, B.; Hoefte, M. Basal tomato defences to botrytis cinerea include abscisic acid-dependent callose formation. Physiol. Mol. Plant Pathol. 2007, 71, 33–40. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- L’Haridon, F.; Besson-Bard, A.; Binda, M.; Serrano, M.; Abou-Mansour, E.; Balet, F.; Schoonbeek, H.-J.; Hess, S.; Mir, R.; Leon, J.; et al. A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity. PLoS Pathog. 2011, 7, e1002148. [Google Scholar] [CrossRef]
- Orozco-Cardenas, M.; Ryan, C.A. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 6553–6557. [Google Scholar] [CrossRef]
- Govrin, E.M.; Levine, A. The hypersensitive response facilitates plant infection by necrophytic pathogen Botrytis cinereal. Curr. Biol. 2000, 10, 751–757. [Google Scholar] [CrossRef]
- Torres, M.A. ROS in biotic interactions. Physiol. Plant. 2010, 138, 414–429. [Google Scholar] [CrossRef]
- Guerrero-Molina, M.F.; Lovaisa, N.C.; Salazar, S.M.; Martinez-Zamora, M.G.; Diaz-Ricci, J.C.; Predaza, R.O. Physiological, structural and molecular traits activated in strawberry plants after inoculation with the plant growth-promoting bacterium Azospirillum brasilense REC3. Plant Biol. 2014, 17, 766–773. [Google Scholar] [CrossRef]
- Chehab, E.W.; Eich, E.; Braam, J. Thigmomorphogenesis: A complex plant response to mechano-stimulation. J. Exp. Bot. 2009, 60, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Mauch, F.; Kmecl, A.; Schaffrath, U.; Volrath, S.; Gorlach, J.; Ward, E.; Ryals, J.; Dudler, R. Mechanosensitive expression of a lipoygenase gene in wheat. Plant Physiol. 1997, 114, 1561–1566. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Diana, H.; Polisensky, D.H.; Braam, J. Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: A focus on calmodulin-like and XTH genes. New Phytol. 2005, 165, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Tretner, C.; Huth, U.; Hause, B. Mechanostimulation of Medicago truncatula leads to enhanced levels of jasmonic acid. J. Exp. Bot. 2008, 59, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A.; Myers, P.N. Mechanical stress regulation of plant growth and development. Hortic. Rev. 1995, 17, 1–42. [Google Scholar]
- Coutand, C. Mechanosensing and thigmomorphogenesis, a physiological and biomechanical point of view. Plant Sci. 2010, 179, 168–182. [Google Scholar] [CrossRef]
- Jehong, Y.; Ota, Y. A relationship between growth inhibition and abscisic acid content by mechanical stimulation in rice plant. Jpn. J. Crop Sci. 1990, 49, 615–616. [Google Scholar] [CrossRef]
- Erner, Y.; Jaffe, M.J. Thigmomorphogenesis: The involvement of auxin and abscisic acid in growth retardation due to mechanical perturbation. Plant Cell Physiol. 1982, 23, 935–941. [Google Scholar]
- Cipollini, D.F.; Redman, A.M. Age-dependent effects of jasmonic acid treatment and wind exposure on foliar oxidase activity and insect resistance in tomato. J. Chem. Ecol. 1999, 25, 271–281. [Google Scholar] [CrossRef]
- Cipollini, D.F. The induction of soluble peroxidase activity in bean leaves by wind-induced mechanical perturbation. Am. J. Bot. 1998, 85, 1586–1591. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J.; Eggermont, K.; Penninckx, I.A.M.A.; Mauch-Mani, B.; Voselsang, R.; Cammue, B.P.A.; Broekaert, W.F. Separate jasmonate-depedent and salicylate-dependent defense-response pathways in Arabidopsis are essential for resistance to distinct microbial pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed]
- Botella, J.R.; Arteca, R.N.; Frangos, J.A. A mechanical strain-induced 1-aminocyclopropane-1-carboxylic acid synthase gene. Proc. Natl. Acad. Sci. USA 1995, 92, 1595–1598. [Google Scholar] [CrossRef] [PubMed]
- Kissoudis, C.; Sunarti, S.; van de Wiel, C.; Visser, R.G.F.; van der Linden, G.; Bai, Y. Responses to combined abiotic and biotic stress in tomato are governed by stress intensity and resistance mechanism. J. Exp. Bot. 2016, 67, 119–5132. [Google Scholar] [CrossRef] [PubMed]
- Kissoudis, C.; Seifi, A.; Yan, Z.; Islam, A.T.M.T.; van der Schoot, H.; van de Wiel, C.C.M.; Visser, R.G.F.; van der Linden, C.G.; Bai, Y. Ethylene and Abscisic Acid Signaling Pathways Differentially Influence Tomato Resistance to Combined Powdery Mildew and Salt Stress. Front. Plant Sci. 2017, 7, 2009. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sunarti, S.; Kissoudis, C.; Visser, R.G.F.; van der Linden, C.G. The Role of Tomato WRKY Genes in Plant Responses to Combined Abiotic and Biotic Stresses. Front. Plant Sci. 2018. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef]
- Graham, M.Y.; Weifer, J.; Wheeler, K.; Pelow, M.J.; Graham, T.L. Induced expression of pathogenesis-related protein genes in soybean by wounding and the Phythophthora sojae cell well glucan elicitor. Physiol. Mol. Plant Pathol. 2003, 63, 141–149. [Google Scholar] [CrossRef]
- Conrath, U. Systemic acquired resistance. Plant Signal. Behav. 2006, 1, 179–184. [Google Scholar] [CrossRef]
- Ishihara, K.L.; Eric, K.W.; Lee, E.K.W.; Borthakur, D. Thigmomorphogenesis: Changes in morphology; biochemistry; and levels of transcription in response to mechanical stress in Acacia koa. Can. J. For. Res. 2017, 47, 583–593. [Google Scholar] [CrossRef]
- Gus-Mayer, S.; Naton, B.; Hahlbrock, K.; Schmelzer, E. Local mechanical stimulation induces components of the pathogen defense response in parsley. Proc. Natl. Acad. Sci. USA 1998, 95, 8398–8403. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Liu, C.P.; Liang, T.W. Fermented and enzymatic production of chitin/chitosan oligosaccharides by extracellular chitinases from Bacillus cereus TKU027. Carbohydr. Polym. 2012, 90, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; ten Have, A.; van Kan, J.A.L. The role of ethylene and wound signaling in resistance of tomato to botrytis cinerea. Plant Physiol. 2002, 129, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Dardouri, T.; Gautier, H.; Ben Issa, R.; Costagliola, G.; Gomez, L. Repellence of Myzus persicae (Sulzer): Evidence of two modes of action of volatiles from selected living aromatic plants. Pest Manag. Sci. 2018, 75, 1571–1584. [Google Scholar] [CrossRef]
- Dardouri, T.; Gomez, L.; Dcheny, A.; Costagliola, G.; Gautier, H. Behavioural response of green peach aphid Myzus persicae (Sulzer). to volatiles from different rosemary (Rosmarinus officinalis L.) clones. Agric. For. Entomol. 2019, 21, 336–345. [Google Scholar] [CrossRef]
- Bengtsson, G.B. Effect of Postharvest Conditions and Treatments on Health-Related Quality of Vegetables and Fruits. III INTERNATIONAL CONFERENCE POSTHARVEST UNLIMITED 2008. Acta Hortic. 2010, 858, 113–120. [Google Scholar] [CrossRef]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coutand, C. The Effect of Mechanical Stress on Plant Susceptibility to Pests: A Mini Opinion Review. Plants 2020, 9, 632. https://doi.org/10.3390/plants9050632
Coutand C. The Effect of Mechanical Stress on Plant Susceptibility to Pests: A Mini Opinion Review. Plants. 2020; 9(5):632. https://doi.org/10.3390/plants9050632
Chicago/Turabian StyleCoutand, Catherine. 2020. "The Effect of Mechanical Stress on Plant Susceptibility to Pests: A Mini Opinion Review" Plants 9, no. 5: 632. https://doi.org/10.3390/plants9050632
APA StyleCoutand, C. (2020). The Effect of Mechanical Stress on Plant Susceptibility to Pests: A Mini Opinion Review. Plants, 9(5), 632. https://doi.org/10.3390/plants9050632



