Abstract
Breadfruit (Artocarpus altilis) is a traditional staple tree crop throughout the tropics. The species is an evergreen tree 15–20 m; there are currently no size-controlling rootstocks within the species. Through interspecific grafting, a dwarf phenotype was identified in breadfruit plants growing on Marang (Artocarpus odoratissimus) rootstocks, which displayed ~60% reduction in plant height with ~80% shorter internodes. To gain insight into the molecular mechanism underlying rootstock-induced dwarfing, we investigated the involvement of gibberellin (GA) in reduction of stem elongation. Expression of GA metabolism genes was analysed in the period from 18 to 24 months after grafting. In comparison to self-graft and non-graft, scion stems on marang rootstocks displayed decrease in expression of a GA biosynthetic gene, AaGA20ox3, and increase in expression of a GA catabolic genes, AaGA2ox1, in the tested 6-month period. Increased accumulation of DELLA proteins (GA-signalling repressors) was found in scion stems growing on marang rootstocks, together with an increased expression of a DELLA gene, AaDELLA1. Exogenous GA treatment was able to restore the stem elongation rate and the internode length of scions growing on marang rootstocks. The possibility that GA deficiency forms a component of the mechanism underlying rootstock-induced breadfruit dwarfing is discussed.
1. Introduction
Trees with reduced stature allow high-density planting and facilitate tree management and harvesting. In many species, tree dwarfing has been achieved through the widespread use of dwarfing rootstocks. The mechanism underlying rootstock-induced dwarfing has been extensively studied but remains poorly understood [1,2,3,4]. Breadfruit (Artocarpus altilis) is a traditional staple tree crop throughout the tropics. The species is an evergreen tree from 15 to 20 m. Breadfruits comprise fertile and sterile diploids and sterile triploids and has hundreds of cultivars [5], but there is currently no size-controlling rootstock within the species. Through interspecific grafting, a dwarf phenotype was recently identified in breadfruit plants growing on marang (Artocarpus odoratissimus) rootstocks [6]. Under the same genus of Artocarpus, marang is also a large tropical fruit tree to 25 m; no dwarf phenotype has been identified [7]. Little is known about the intriguing interaction by which marang greatly reduces the tree size of grafted scions when used as rootstocks.
The molecular mechanism by which dwarfing is conferred may differ between species as well as between annuals and perennials [4]. Several mechanisms have been proposed to explain how rootstocks cause dwarfing in scions. These include reduction of water and solute transport across graft union [3,8], anatomical change [2], and altered hormone signalling between scions and rootstocks [1,9]. There is considerable evidence to suggest that disruption in gibberellin (GA) metabolism plays a role in rootstock-induced dwarfing [4]. Previously dwarfing apple interstocks were found to limit the supply of [3H]GA4 to scion shoot tips as compared to non-dwarfing interstocks [10]. Dwarfing apple rootstocks also reduce the supply of the root-produced GA precursor, GA19, to scions [9,11]. Application of GA to scions on apple dwarfing rootstock restores the node number of both the primary axis and secondary shoots [12]. In ungrafted apple rootstocks, the level of GAs is the lowest in the dwarfing rootstock M.9 and the highest in the non-dwarfing rootstock MM111 [13]. Transcriptomic analysis revealed downregulation of GA biosynthetic genes in scions of apple trees gowning on dwarfing rootstocks [14] and upregulation of a GA catabolic gene together with decreased GA level in persimmon scion stems grafted on dwarfing interstocks [15]. Furthermore, a dwarf plum hybrid with elevated transcript levels of a major GA catabolic gene, GA2ox, exhibits shorter internodes and reduced stem elongation, and when used as rootstocks, it reduces the level of bioactive GAs in scions and reduces scion vigor [16].
GAs are a family of diterpenoid plant hormone involved in a wide range of plant growth and development [17]. In higher plants, the flux of active GAs is regulated by the balance between the rates of biosynthesis and deactivation [18]. Biosynthesis of GAs starts from geranylgeranyl diphosphate, a C20 precursor [18]. GA20-oxidase (GA20ox) is a multifunctional enzyme that converts GA12 or GA53 to GA9 or GA20 through three sequential oxidations, therefore representing one of the key enzymes controlling GA biosynthetic flux [18]. Suppression of GA20ox genes reduces endogenous active GA content and produces dwarfism in many species [17,19,20]. On the other hand, the main route for GA deactivation is through 2β-hydroxylation catalysed by 2-oxyglutarate dependent GA2-oxidases (GA2ox), leading to formation of biologically inactive GAs [17]. Overexpression of GA2ox genes therefore enhances GA deactivation and produces dwarf phenotype [16,21]. In parallel with the direct regulation of endogenous GA concentration mediated by GA biosynthetic and catabolic genes, GA signalling is regulated by the negative regulators, DELLA proteins [22]. DELLA proteins are characterized by a highly conserved N-terminal DELLA domain essential for GA-induced proteolysis [23]. Binding of GA molecule to its receptor, GA-INSENSITIVE DWARF1 (GID1) results in rapid degradation of DELLA proteins via the ubiquitin-proteasome pathway, as a result, it releases the DELLA repression of GA responses [23,24]. Mutants with over-accumulated DELLA display dwarf phenotype [23,25]. In plants, GA20-oxidase and GA2-oxidase are encoded by gene families [17], and DELLAs in dicot species are encoded by small gene families of various sizes [23]. While the overlap expression pattern suggests functional redundancy, the tissue-specific expression patterns among family members of GA2-orxidase genes in both poplar [26] and Arabidopsis [27] reflect specialised and functional divergence in their relative contribution to GA metabolism and signalling in a subset of organs.
Three predicted functional GA20-oxidases genes were isolated in breadfruit (Artocarpus altilis cv. Cannonball), with two genes, AaGA20ox1 and AaGA20ox3, predominantly expressed in green vegetative organs [28]. A cohort of four GA2-oxidase genes, AaGA2ox1–AaGA2ox4, was also cloned in the same cultivar, with three highly expressed in vegetative organs [29]. There are two DELLA genes, AaDELLA1 and AaDELLA2, isolated in “Mason” breadfruit species [30]. However, the lack of dwarf varieties and the scarcity of dwarfing rootstocks for breadfruits has limited our understanding of how vegetative growth is controlled by rootstocks in the species and any potential role of these genes in conferring breadfruit dwarfism.
Our current work focused on the involvement of GA in the scion stem elongation of breadfruits growing on marang rootstocks in relation to dwarf phenotype. We investigated GA response and the expression of GA biosynthetic and catabolic genes together with DELLA protein abundance and transcript levels in breadfruit scion stems in the period from 18 to 24 months after grafting. Our evidence suggests that GA deficiency may form a component in breadfruit dwarfing mechanism induced by marang rootstocks. The current work provided insight into the molecular mechanism modulating dwarfing in breadfruit through interspecific rootstocks.
2. Results
2.1. Effect of Rootstocks on Stem Elongation of Breadfruit Scions
Plants 20 months old after grafting were used for stem elongation analysis. The measurement was started from the emergence of an internode until the cessation of extension in the same segment. Significantly shorter internodes were observed in breadfruit plants growing on marang rootstocks, with 79.8% reduction in final internode length compared to those on self-grafts (Figure 1a). As a result, breadfruit plants on marang rootstocks displayed short stature with height reduction by 52.0% at 12 months and by 59.6% at 24 months after grafting (Figure 1b,c). There was no significant difference in both internode length and final plant height between the self-grafts and the non-graft (Figure 1). The results were consistent with previous growth observation in the 18-month period after grafting [6].
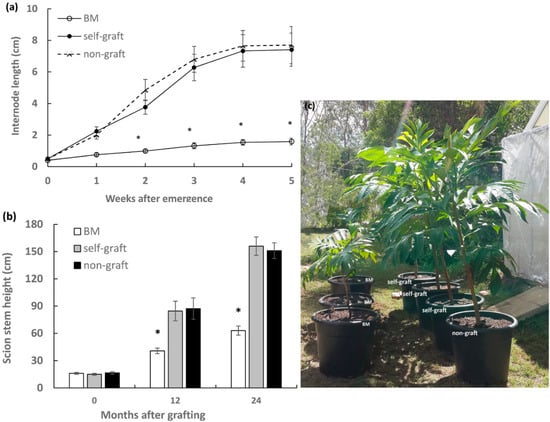
Figure 1.
Effect of rootstocks on stem elongation of breadfruit scions: (a) Internode elongation in breadfruit scions. Internodes were examined on grafted plants of 20 month olds (after grafting) with measurement initiated from the emergence of the internode under the terminal buds (week 0). (b) Height of scion stem growing on different rootstocks. (c) Representatives of breadfruit plants growing on different rootstocks at 24 months after grafting. BM, breadfruit plants on marang rootstocks. All values represent mean ± SE from five biological replicates (* p < 0.05).
2.2. Effect of Rootstocks on the Expression of GA Metabolic Genes
Six GA20-oxidase genes, AaGA20ox1–AaGA20ox6, were previously isolated in breadfruits, with three, AaGA20ox1–AaGA20ox3, predicted to encode functional GA20-oxidase and the other three, AaGA20ox4–AaGA20ox6, predicted to be unprocessed pseudogenes [28]. The expression of the three AaGA20oxs, AaGA20ox1–AaGA20ox3, were measured in scion stems, but only two genes, AaGA20ox1 and AaGA20ox3, showed expression in the current experiment condition. Similarly, of the four GA2ox genes previously identified in breadfruit [29], only two genes, AaGA2ox1 and AaGA2ox2, were detected in scion stem tissues under the current growth condition. All the detectable genes were analysed monthly in the period from 18 to 24 months after grafting. It was shown that the overall expression levels of AaGA20ox3 were over 1000 times higher than those of AaGA20ox1 and that the expression levels of AaGA2ox1 were over 10 times higher than those of AaGA2ox2 for all samples (Figure 2a–c). Over the six month period, scion stems on marang rootstocks showed no significant change in transcript levels of AaGA20ox1 but showed decreased transcript levels of AaGA20ox1 at serval time points, including 20, 21, 23, and 24 months after grafting when compared to those on self-grafts and non-grafts (Figure 2a,b). There was no significance difference between the self-graft and non-graft in the expression of these two genes (Figure 2a,b). For GA2-oxidase genes, higher levels of expression were detected every month from 18 to 24 months except for the 19-month time point for AaGA2ox1, but no significant change for AaGA2ox2 compared to those on self-grafts and non-graft was observed (Figure 2c,d). For both AaGA2ox1 and AaGA2ox2, the expression levels of the self-graft and non-graft were not significantly different (Figure 2c,d).
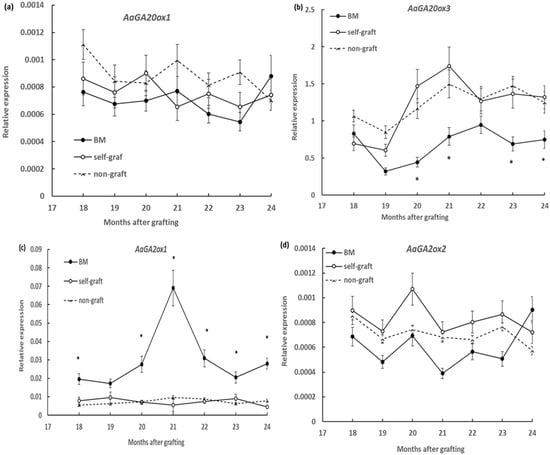
Figure 2.
Effect of rootstocks on the expression of gibberellin (GA) biosynthetic genes, AaGA20ox1 and AaGA20ox3, and GA catabolic genes, AaGA2ox1 and AaGA2ox2, in breadfruit scion stems: Expression level of each transcript was normalized to the expression of the actin gene. BM, breadfruit plants growing on marang rootstocks. All values represent mean ± SE from five separate RNA extractions (* p < 0.05). (a) AaGA20ox1; (b) AaGA20ox3; (c) AaGA2ox1 and (d) AaGA2ox2.
2.3. Effect of Rootstocks on DELLA Protein Abundance and Transcript Levels
Two DELLA genes, AaDELLA1 and AaDELLA2, were previously cloned from “Mason” breadfruit [30]. To confirm that the two genes or potentially more DELLA genes were present in stems of “Gold Noli” breadfruit, DELLA genes were first cloned from “Gold Noli” breadfruit by degenerate PCR using primers corresponding to two conserved regions of all known DELLAs, MDELLA(V/A) and AHFTANQA [23]. An expected fragment of 700 bp was amplified, and 40 degenerate PCR clones were sequenced. Only two distinct groups of cDNA clones were identified with one aligned to AaDELLA1 and the other aligned to AaDELLA2. Both alignments had 100% similarity to the previous AaDELLAs. Full lengths of the two DELLA genes were then isolated using primers corresponding to the 5’ and 3’ end of the AaDELLA1 and AaDELLA2 genes. Sequencing of the resulting full-length cDNA clones confirmed that “Mason” breadfruit had two DELLA genes, one identical to AaDELLA1 and the other identical to AaDELLA2 at the nucleic acid level.
To measure the DELLA protein abundance, a polyclonal anti-AaDELLA was raised which targeted the highly conserved region of AaDELLA1 and AaDELLA2 (see Materials and Methods). DELLA protein abundance was examined in scion stems growing on different rootstocks every 3 months in the period of 18 to 24 months after grafting. Compared to the self-graft and non-graft at the corresponding times, levels of immunologically detectable DELLA were obviously higher in scion stems grafted on marang rootstocks, indicating an increase in the accumulation of DELLA proteins at all the tested time points (Figure 3). The transcript levels of AaDELLA1 and AaDELLA2 were further analysed in scion stems growing on different rootstocks. When compared to those on the self-graft and non-graft over the 6-month period, scion stems on marang rootstocks were found to have increases in the expression of AaDELLA1 at three time points, including 18, 21, and 24 months, but to have relatively stable expression of AaDELLA2 (Figure 4). The expression levels between the self-graft and the non-graft were not significantly different for both genes (Figure 4).
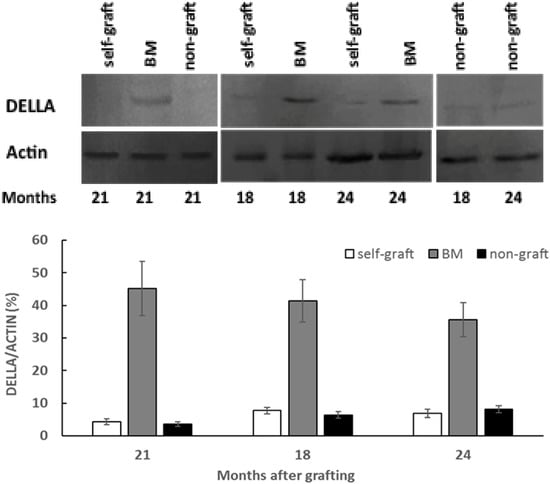
Figure 3.
Analyses of DELLA protein abundance in breadfruit scion stems on different rootstocks: Total proteins were extracted from scion stems at 18, 21, and 24 months after grafting. DELLA protein abundance was determined by Western blotting with affinity-purified polyclonal anti-AaDELLA. A representative immunoblot of DELLA protein with actin as a loading control is shown on top of the histogram showing the ratio of the band intensity of the DELLA to that of the corresponding actin. BM, breadfruit plants grafted on marang rootstocks. Vertical bars represent mean ± SE derived from three biological replicates.
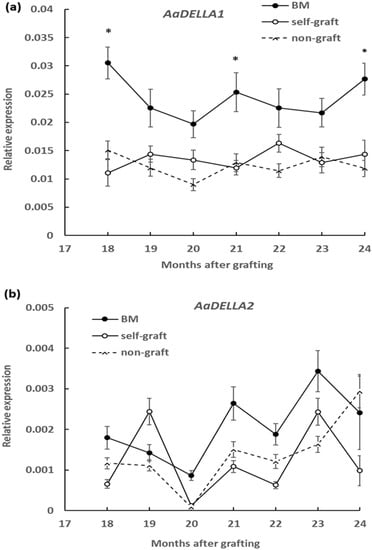
Figure 4.
Effect of rootstocks on the expression of DELLA genes, AaDELLA1 and AaDELLA2, in breadfruit scion stems: Expression level of each transcript was normalized to the expression level of the actin gene. BM, breadfruit plants growing on marang rootstocks. All values represent mean ± SE from five separate RNA extractions (* p < 0.05). (a) AaDELLA1; (b) AaDELLA2.
2.4. Restoration of Stem Elongation by GA Treatment
To confirm that the rootstock-induced short stature is due to GA deficiency, breadfruit plants growing on marang rootstocks were sprayed with GA3. For GA treatment, the plants were 18 months old (after grafting) with a single stem, characterised by the main active stem elongation from the terminal buds and minimal growth at the lower part of the stems. Following GA application, it was shown that their stem elongation rate was significantly increased, with 7.5-fold higher in the second month and 6.4-fold higher in the third month compared to those of the untreated plants on marang rootstocks at the same time (Figure 5a). By the 3rd month after exogenous GA application, the stem elongation rate was fully restored to normal with no significant difference to those on self-graft (Figure 5a). Furthermore, the internode length was increased by 2.2-fold after 3 months following GA treatment (Figure 5) and was also restored to nearly normal compared to those on self-graft (Figure 5b). There was no significant change in the number of internodes and the stem thickness within the four months after GA3 treatment.
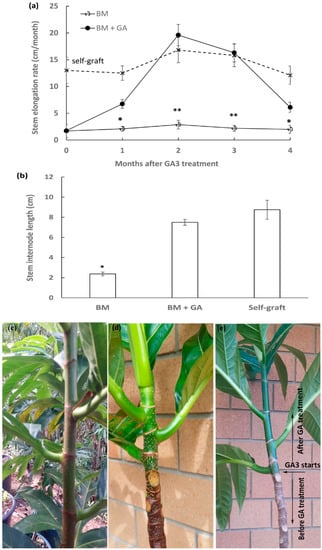
Figure 5.
Rescue of scion stem elongation by exogenous GA3 application in breadfruit plants on marang rootstocks: Comparison of stem elongation rate (a) and internode length (b) in GA3-treated and non-treated plants. Representatives of grafted plants displaying different scion stem internodes: (c) Untreated breadfruit scions on breadfruit rootstock (self-graft), (d) untreated breadfruit scions on marang rootstock, and (e) GA3-treated breadfruit scions on marang rootstock. The stem internode length was the averaged measurement of the second internodes at the third month after GA3 treatment. BM, breadfruit plants growing on marang rootstocks. All values represent mean ± SE from three biological replicates, with ** significant difference (p < 0.05) from the rest of the samples (non-graft and self-graft) and * significant difference from the non-graft only.
3. Discussion
Breadfruit plants growing on marang rootstocks were characterised by dwarf stature with ~60% reduction in total plant height and ~80% reduction in the length of internodes at the end of 24 months after grafting (Figure 1), consistent with the previous observation in the first 18-month period [6]. The phenotypes resemble the typical signs of GA deficiency [21,31]. The expression of GA biosynthetic genes and catabolic genes in scion stems growing on different rootstocks was compared over the period from 18 to 24 months after grafting. Of the two GA20ox genes expressed in stems, the predominant gene, AaGA20ox3, showed significantly reduced levels of expression several times during the six-month period (Figure 2). These suggest that the capacity of GA biosynthesis in scions of marang rootstocks may be affected by the reduced expression of the AaGA20ox3 gene during the period. Our results are in agreement with another study, where downregulation of the GA biosynthetic genes was found in scions gowning on dwarfing apple rootstocks of both M.9 and M.27 [14]. Suppression of GA20ox expression has been shown to produce plants with dwarf stature as a result of reduced endogenous GA contents in many species, inducing Arabidopsis, potato, tobacco, [17], apple trees [19], and citrus trees [20]. These suggest that downregulation of AaGA20ox3 may contribute to the dwarf phenotype of breadfruit plants grafted on marang rootstocks. Of the two GA catabolic genes, AaGA2ox1 and AaGA2ox2, a more consistent increase in the transcript levels of the major gene, AaGA2ox1, in the period suggests an upregulation of GA deactivation in scion stems on marang rootstocks in this period (Figure 2). Upregulation of a GA2ox gene accompanying a decreased GA level was previously reported in scions of persimmon trees grafted on dwarfing interstocks [15]. Overexpression of GA2ox enhances GA deactivation and produces dwarf phenotype in many species [16,17,21]. These results suggest that upregulation of the main GA catabolic gene, AaGA2ox1, may contribute to the rootstock-induced dwarf phenotype in breadfruit plants over the period from 18 to 24 months after grafting.
The combination of upregulation of a GA biosynthetic gene and downregulation of a GA catabolic gene suggests a decrease in GA signals in scion stems growing on marang rootstocks. In support of this hypothesis, an increase in the accumulation of DELLA proteins (GA-signalling repressors) was found in scion stems on marang rootstocks (Figure 3). Various evidences have previously indicated that dwarfing rootstocks reduce GA concentration in scions [9,10,11]; the change of DELLA protein abundance has rarely been reported. This work provides insight into the functional significance of the GA deficiency in relation to the repression of GA response. In plants, DELLA proteins are rapidly degraded in response to GA via the ubiquitin proteasome pathway [23,24]; an increased DELLA protein abundance therefore reflects an increased repression of GA response in scions grafted on marang rootstocks. DELLA proteins act as negative regulators of plant growth [23]. Plants carrying over-accumulated DELLA display dwarf phenotype [25,32,33]. DELLAs also interact with multiple transcription factors and key regulators of other pathways through direct protein–protein interactions [22]. These include integrating GA with brassinosteroid, ethylene, jasmonate, abscisic acid, and auxin signalling pathways [22,34,35,36]. Some of these signal pathways have long been proposed to be implicated in rootstock-induced dwarfism of other species [4]. In this context, the role of GA and its interaction with other growth regulators in regulating the dwarf traits of breadfruit on marang rootstocks deserves further investigation. Collectively, our results suggest that an increased repression of GA response contribute to the dwarf phenotype in breadfruit plants growing on marang rootstocks. These may be due to reduced GA signals as a result of the downregulation of GA biosynthesis and/or upregulation of GA deactivation. On the other hand, the increase in DELLA protein levels was reflected by the increased transcript levels of a DELLA gene, AaDELLA1, at all three occasions (Figure 4), suggesting that AaDELLA1 may be regulated at the transcription level. While most previous work has demonstrated the posttranscriptional regulation of DELLA protein [22,23], the transcriptional regulation of DELLA in response to various environmental signals has also been reported [35,37]. Upregulation of a DELLA gene was found in scions growing on persimmon dwarfing interstocks. In this context, it may be possible that transcriptional regulation of the AaDELLA1 plays a coordinative role in regulating GA response, thus contributing to the mechanism of rootstock-induced dwarfism in breadfruit plants.
Stem elongation of scions on marang rootstocks was restored to near normal by exogenous GA application as determined by both the stem elongation rate and stem internode length (Figure 5). The results provide evidence that GA deficiency may play a role in rootstock-induced dwarfing of breadfruit plants and support the association of scion GA metabolism genes and signalling genes, including AaGA20ox3, AaGA2ox1, and AaDELLA1, with the development of dwarf phenotype in breadfruit plants. The combined expression profiles of these genes therefore may represent potential markers for breadfruit dwarfing. Breadfruit tree height can be controlled though the use of the GA inhibitor paclobutrazol [29]. For woody species, this generally involves a long-term, repeated application of the chemicals in order to achieve effective tree-size reduction. Our current work may provide an opportunity to develop various size-controlling rootstocks through fast screening of GA-related gene markers in scions, leading to environmentally sustainable solutions for breadfruit dwarfing.
In the current study, we focused on stem elongation as a key phenotype in breadfruit plants on marang rootstocks. However, the rootstock-induced dwarf traits in breadfruits involve other morphological and biochemical components [6] which are not examined in the current experiment. Future characterisation of the roles of GA and other hormones/factors in these dwarf traits is required for the unravelling of the molecular mechanism underlying rootstock-induced breadfruit dwarfing. GA is also produced in roots [18], although the majority of the studies have suggested that GA precursors rather than active GA are involved in long-distance transport [38]. Previously, dwarfing apple rootstocks were shown to limit the root-produced GA precursor, GA19, to scions [9,10,11]. While our study suggests a reduction of GA response in scion stems as determined by the increase of DELLA protein abundance, the nature of root-derived GAs and their contribution to the GA response in scion stems need further investigation. On the other hand, phloem transport of DELLA mRNA from rootstocks to scions has been reported [39]. The possibility of marang DELLA gene transfer to breadfruit scions cannot be ruled out. The sequence of marang DELLA gene is not available, but it is possible that the polyclonal anti-DELLA in the current study also binds marang-derived DELLAs, given that the target region shares high homology to DELLA sequences of other species (NCBI BLAST search).
In conclusion, breadfruit scion stems growing on marang rootstocks displayed decreased expression of a major GA biosynthetic gene, AaGA20ox3, at several times over the 6-month period from 18 to 24 months after grafting, and had persistently higher expression of a major GA catabolic gene, AaGA2ox1. Increased DELLA protein abundance was shown in scion stems on marang rootstocks together with an increase in the expression of a DELLA gene, AaDELLA1. Exogenous GA treatment was able to restore the stem elongation rate and the internode length of scions growing on the dwarfing rootstocks.
4. Materials and Methods
4.1. Plant Materials and Treatments
Breadfruit (Artocarpus altilis cv. Cannonball, also called “Noli Gold”) and marang (Artocarpus odoratissimus) plants were obtained from a commercial nursery at Cairns, northern Queensland. Breadfruit plants as rooted cuttings and marang plants as seedlings were grown under glasshouse condition at 25 to 28 °C with natural daylight and daily water supply. Plants were grown in pots containing vermiculite and soil mixture as described previously [29]. Breadfruit scions selected from breadfruit plants 30 to 50 cm tall were grafted onto marang seedlings of similar sizes through approach grafting [40]. As a control, the breadfruit scions were also grafted onto breadfruit rootstocks of the same cultivar (self-graft). There were at least eight replicates for each grafting combination. Each established grafted plant was transferred to an 85-Litre pot six months after grafting and continued to grow under the same condition. The self-rooted breadfruit plants (non-graft) were grown alongside under the same condition. Plants were monitored for total height and stem elongation within the 24 months after grafting. Plants on marang rootstocks 18 months after grafting were used for GA treatment. For GA experiment, both foliage and soil surface were sprayed with 500 mg L−1 GA3 (Sigma, Sydney, NSW, Australia, dissolved in 0.1% ethanol, 0.1% Triton X-100). The bioactive form GA3 was chosen according to the previous experiment, showing a fast positive response to the chemical in stem growth of breadfruit trees [29]. Plants sprayed with the same concentration of ethanol and Triton X-100 were used for control. The treatment was applied once a week for 3 weeks. Three replicates were used for each treatment and control. Plants were monitored for stem elongation, and internode length in the second internode was counted from the top, with the first internode being defined as the one below the uppermost leaf.
4.2. Quantitative Real-Time PCR
Upon removal from plants, stem tissues (including epidermis, vascular tissues, and pith) in the second internodes were immediately immersed in RNAlater (Life Technologies, VIC, Australia) before stored at −80 °C. Total RNA was extracted by using RNeasy kit (Qiagen, VIC, Australia) and reverse transcribed with SuperScript reverse transcriptase and oligo(dT) (Life Technologies, VIC, Australia). Real-time PCR was performed on a Corbett Research Rotor-Gene 6000 cycler with the QuantiFast SYBR Green PCR Kit (Qiagen, VIC, Australia) as previously described [29]. Thermocycling was initiated with a 5-min incubation at 95 °C, followed by 40 cycles (95 °C for 10 s; 60 °C for 30 s). The specificity of amplification was confirmed by high-resolution melt curve analysis at the end of each run. The efficiency of each primer set was evaluated by standard curves using serial dilutions of plasmid DNA containing its amplified regions. Each reaction was carried out in duplicate (technical repeat) with non-reverse-transcribed cDNA (RT−) as negative controls (non-template control). Transcripts of AaGA20oxs and AaGA2oxs were amplified using their gene-specific primers as previously described [28,29]. Gene-specific primers were 5′-GAA AAA GATCAGAAGAAGAAGAATCATCATG-3′ and 5′-CCC AAA ACG GCC AGG AGCTCG-3′ for AaDELLA2 for AaDELLA1 and 5′-CAT CAG AAG AAGAAT CAT GAA AAG GGA AC-3′ and 5′-CAT GTC GGATGA CCT GAC CTT GTA G-3′ for AaDELLA2. Two housekeeping genes, actin and elongation factor 1-α (AaEFα-1) were tested for stability across the time points according to a previous protocol [41]. The actin gene was amplified using primers 5’-AATGGAACTGGAATGGTGAAG GC-3’ and 5’-TGCCAGATCTTCTCCATGTCATCC-3’, and the AaEFα-1 was amplified using primer 5’-GAAGCTCTTCGTCAAGAGAA-3’ and 5’-GAAATCTCTTGAAGTAACCATC-3’. The specificity of the primers was confirmed by amplicon sequencing. The actin gene, a more stable housekeeping gene compared to the elongation factor 1-α gene, was chosen to normalize to the expression of transcript abundance. The expression of each gene was an average of five biological replicates.
4.3. Cloning of DELLA cDNAs from “Gold Noli” Breadfruit
Total RNA was extracted from stem tissues of breadfruit plants by using RNeasy kit RNeasy kit (Qiagen, VIC, Australia) and reverse transcribed with reverse transcriptase and oligo(dT) (Life Technologies, VIC, Australia). The resulting cDNA was subjected to degenerate PCR using primers 5′-ATGGAYGARYTIYTIGCNG-3′ and 5′-GCNCAYTTYACNGCNAAYCARGCN-3′ as previously described [30]. The PCR reactions were performed at 35 cycles with annealing temperature at 52 °C. The PCR products were cloned into pGEMT vector (Promega, NSW, Australia) and sequenced. Full-length cDNA clones of AaDELLA genes were amplified with Advantage DNA polymerase (Takara Clontech, CA, USA). The thermocycling was initiated with a 2-min incubation at 92 °C, followed by 32 cycles (92 °C for 30 s and 68 °C for 2 min) and a final extension of 7 min at 68 °C. The gene-specific primers were 5′-GAAAAAGATCAGAAGAAGAAGAATCATCATG-3′ and 5′-GACCCGACTTAGCGAGCCACAG-3′ for AaDELLA1 and were 5′-GTGTTTGAGGAAAAAGAGGGCCTGTG-3′ and 5′-GGGTCCGGCCCGACTCAGAG-3′ for AaDELLA2. These primers were designed from the sequence information of AaDELLA genes isolated from the “Mason” breadfruit [30]. PCR products were cloned into pGEMT vector and sequenced as above. The resulting sequences were analysed by Sequencher (version 5.4.1, Gene Codes Corporation, Ann Arbor, MI, USA).
4.4. Western Blotting
The highly conserved sequence of AaDELLA1 and AaDELLA2 [30] between residues 37 and 59 (KMWEEDDGGMDELLAVLGYKVR) was synthesized, and the resulting peptide was used to raise antibodies in a rabbit (Mimotopes, Melbourne, Australia). Polyclonal anti-AaDELLA was purified by affinity chromatography. The titres of the affinity-purified antibodies were determined by enzyme-linked immunosorbent assay (ELISA). Proteins were extracted from the full stem tissues in the second internodes of scions according to the method previously described [42], and protein concentration was determined by a bicinchoninic acid protein assay (Sydney, NSW, Australia). The protein extracts were resolved on 10% SDS-PAGE gels (20 µg protein per lane) and electroblotted onto nitrocellulose membranes followed by immuno-detection [43]. Blots were probed with pre-immune serum as negative controls. Colour development was performed using an alkaline phosphatase-conjugated secondary antibody with Western blue (Promega, NSW, Australia). Each blot was first probed with polyclonal anti-AaDELLA; then stripped with 0.2 N glycine, pH 2.5; and re-probed with anti-actin (Sydney, NSW, Australia). The ratio of the band intensity of the DELLA to that of the corresponding actin was analysed by Quantity One 1-D analysis software in the Gel Doc system (BioRad, NSW, Australia).
4.5. Statistical Analyses
Significant differences were tested using analysis of variance (ANOVA) followed by Tukey’s multiple comparison test at p < 0.05 (IBM SPSS Statistics version 24).
Author Contributions
Conceptualization, Y.Z. and S.J.R.U.; methodology, Y.Z.; investigation, Y.Z.; writing—original draft preparation, Y.Z.; writing—review and editing, Y.Z. and S.J.R.U. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Australia Centre for International Agriculture Research (ACIAR) through project HORT 2014/077.
Acknowledgments
We thank the Plant Pathology and Horticulture group, Queensland Department of Agriculture and Fisheries at Ecosciences Precinct, Dutton Park, Queensland for laboratory support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lochard, R.G.; Schneider, G.W. Stock and scion growth relationships and the dwarfing mechanism in apple. Hortic. Rev. 1981, 3, 315–375. [Google Scholar] [CrossRef]
- Soumelidou, K.; Battey, N.H.; John, P.; Barnett, J.R. The Anatomy of the developing bud union and its relationship to dwarfing in apple. Ann. Bot. 1994, 74, 605–611. [Google Scholar] [CrossRef]
- Atkinson, C.J.; Else, M.A.; Taylor, L.; Dover, C.J. Root and stem hydraulic conductivity as determinants of growth potential in grafted trees of apple (Malus pumila Mill.). J. Exp. Biol. 2003, 54, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.; Atkinson, C.; Bengough, A.; Else, M.; Fernández-Fernández, F.; Harrison, R.; Schmidt, S. Contributions of roots and rootstocks to sustainable, intensified crop production. J. Exp. Bot. 2013, 64, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Ragone, D.; Leibniz Institute of Plant Genetics and Crop Plant Research (IPK). Breadfruit, Artocarpus altilis (Parkinson) Fosberg. Promot. Conserv. Use Underutilized Negl. Crop. 1997, 10, 77. [Google Scholar]
- Zhou, Y.; Underhill, S.J.R. A dwarf phenotype identified in breadfruit (Artocarpus altilis) plants growing on marang (A. odoratissimus) rootstocks. Horticulturae 2019, 5, 40. [Google Scholar] [CrossRef]
- Forster, J.R.; Forster, G. Characteres Generum Plantarum, Quas in Itinere Ad Insulas Maris Australis, Collegerunt, Descripserunt, Delinearunt, Annis 1772–1775; Missouri Botanical Garden: Saint Louis, MO, USA, 1776. [Google Scholar]
- Basile, B.; Marsal, J.; Solar, L.I.; Tyree, M.T.; Bryla, D.R.; Dejong, T.M. Hydraulic conductance of peach trees grafted on rootstocks with differing size-controlling potentials. J. Hortic. Sci. Biotech. 2003, 78, 768–774. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Hormone and growth interactions of scions and size-controlling rootstocks of young apple trees. Plant Growth Regul. 2016, 78, 105–119. [Google Scholar] [CrossRef]
- Richards, D.; Thompson, W.K.; Pharis, R.P. The influence of dwarfing interstocks on the distribution and metabolism of xylem-applied [3H]gibberellin A4 in apple. Plant Physiol. 1986, 82, 1090–1095. [Google Scholar] [CrossRef]
- van Hooijdonk, B.; Woolley, D.; Warrington, I.; Tustin, S. Rootstocks Modify Scion Architecture, Endogenous Hormones, and Root Growth of Newly Grafted ‘Royal Gala’ Apple Trees. J. Am. Soc. Hortic. Sci. 2011, 136, 93–102. [Google Scholar] [CrossRef]
- Van Hooijdonk, B.M.; Woolley, D.J.; Warrington, I.J.; Tustin, D.S. Initial alteration of scion architecture by dwarfing apple rootstocks may involve shoot-root-shoot signalling by auxin, gibberellin, and cytokinin. J. Hortic. Sci. Biotech. 2010, 85, 59–65. [Google Scholar] [CrossRef]
- Yadava, U.L.; Lockard, R.G. Abscisic Acid and Gibberellin-in Three Ungrafted Apple (Malus sylvestris) Rootstock Clones. Physiol. Plant 1977, 40, 225–229. [Google Scholar] [CrossRef]
- Foster, T.; Kirk, C.; Jones, W.T.; Allan, A.C.; Espley, R.; Karunairetnam, S.; Rakonjac, J. Characterisation of the DELLA subfamily in apple (Malus x domestica Borkh.). Tree Genet. Genomes 2007, 3, 187–197. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Zhuang, W.B.; Tu, X.T.; Gao, Z.H.; Xiong, A.S.; Yu, X.Y.; Li, X.H.; Li, F.H.; Qu, S.C. Transcriptomic analysis of interstock-induced dwarfism in Sweet Persimmon (Diospyros kaki Thunb.). Hortic. Res. 2019, 6, 51. [Google Scholar] [CrossRef]
- El-Sharkawy, I.; El Kayal, W.; Prasath, D.; Fernandez, H.; Bouzayen, M.; Svircev, A.M.; Jayasankar, S. Identification and genetic characterization of a gibberellin 2-oxidase gene that controls tree stature and reproductive growth in plum. J. Exp. Bot. 2012, 63, 1225–1239. [Google Scholar] [CrossRef]
- Hedden, P.; Phillips, A. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 2000, 5, 523–530. [Google Scholar] [CrossRef]
- Hedden, P.; Kamiya, Y. Gibberellin biosynthesis: Enzymes, genes and their regulation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 431–460. [Google Scholar] [CrossRef]
- Bulley, S.M.; Wilson, F.M.; Hedden, P.; Phillips, A.L.; Croker, S.J.; James, D.J. Modification of gibberellin biosynthesis in the grafted apple scion allows control of tree height independent of the rootstock. Plant Biotechnol. J. 2005, 3, 215–223. [Google Scholar] [CrossRef]
- Fagoaga, C.; Tadeo, F.R.; Iglesias, D.J.; Huerta, L.; Lliso, I.; Vidal, A.M.; Talon, M.; Navarro, L.; Garcia-Martinez, J.L.; Pena, L. Engineering of gibberellin levels in citrus by sense and antisense overexpression of a GA 20-oxidase gene modifies plant architecture. J. Exp. Bot. 2007, 58, 1407–1420. [Google Scholar] [CrossRef]
- Busov, V.B.; Meilan, R.; Pearce, D.W.; Ma, C.; Rood, S.B.; Strauss, S.H. Activation tagging of a dominant gibberellin catabolism gene (GA 2-oxidase) from poplar that regulates tree stature. Plant Physiol. 2003, 132, 1283–1291. [Google Scholar] [CrossRef]
- Hauvermale, A.L.; Ariizumi, T.; Steber, C.M. Gibberellin signaling: A theme and variations on DELLA repression. Plant Physiol. 2012, 160, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Gubler, F. Molecular mechanism of gibberellin signaling in plants. Annu. Rev. Plant Biol. 2004, 55, 197–223. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Ueguchi-Tanaka, M.; Sata, Y.; Ashikari, M.; Matsuoka, M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 2002, 14, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Boss, P.K.; Thomas, M.R. Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature 2002, 416, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.Q.; Ma, C.; Kadmiel, M.; Gai, Y.; Strauss, S.; Jiang, X.N.; Busov, V. Tissue-specific expression of Populus C(19) GA 2-oxidases differentially regulate above- and below-ground biomass growth through control of bioactive GA concentrations. New Phytol. 2011, 192, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Eriksson, S.; Powers, S.J.; Gong, F.; Griffiths, J.; Woolley, L.; Benlloch, R.; Nilsson, O.; Thomas, S.G.; Hedden, P.; et al. Genetic analysis reveals that C(19)-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell 2008, 20, 2420–2436. [Google Scholar] [CrossRef]
- Zhou, Y.; Underhill, S. Breadfruit (Artocarpus altilis) gibberellin 20-oxidase genes: Sequence variants, stem elongation and abiotic stress response. Tree Genet. Genomes 2015, 11, 1–13. [Google Scholar] [CrossRef]
- Zhou, Y.; Underhill, S. Breadfruit (Artocarpus altilis) gibberellin 2-oxidase genes in stem elongation and abiotic stress response. Plant Physiol. Biochem. 2016, 98, 81–88. [Google Scholar] [CrossRef]
- Zhou, Y.; Underhill, S. Breadfruit (Artocarpus altilis) DELLA genes: Gibberellin-regulated stem elongation and response to high salinity and drought. Plant Growth Regul. 2017, 83, 375–383. [Google Scholar] [CrossRef]
- Eriksson, M.E.; Israelsson, M.; Olsson, O.; Moritz, T. Increased gibberellin biosynthesis in transgenic trees promotes growth, biomass production and xylem fiber length. Nat. Biotechnol. 2000, 18, 784–788. [Google Scholar] [CrossRef]
- Busov, V.; Meilan, R.; Pearce, D.W.; Rood, S.B.; Ma, C.P.; Tschaplinski, T.J.; Strauss, S.H. Transgenic modification of gai or rgl1 causes dwarfing and alters gibberellins, root growth, and metabolite profiles in Populus. Planta 2006, 224, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, H.T.; Li, J.J.; Wang, B.; Dai, C.; Wang, J.; Liu, K.D. Brassica napus DS-3, encoding a DELLA protein, negatively regulates stem elongation through gibberellin signaling pathway. Theor. Appl. Genet. 2017, 130, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.-Y.; Shang, J.-X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.-P.; Wang, Z.-Y. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Ma, N.; Pei, H.X.; Chen, J.W.; Li, J.; Gao, J.P. A DELLA gene, RhGAI1, is a direct target of EIN3 and mediates ethylene-regulated rose petal cell expansion via repressing the expression of RhCesA2. J. Exp. Bot. 2013, 64, 5075–5084. [Google Scholar] [CrossRef]
- Willige, B.C.; Isono, E.; Richter, R.; Zourelidou, M.; Schwechheimer, C. Gibberellin regulates PIN-FORMED abundance and is required for auxin transport-dependent growth and development in Arabidopsis thaliana. Plant Cell 2011, 23, 2184–2195. [Google Scholar] [CrossRef]
- Oh, E.; Yamaguchi, S.; Hu, J.; Yusuke, J.; Jung, B.; Paik, I.; Lee, H.-S.; Sun, T.-p.; Kamiya, Y.; Choi, G. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 2007, 19, 1192–1208. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin localization and transport in plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef]
- Haywood, V.; Yu, T.-S.; Huang, N.-C.; Lucas, W.J. Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J. 2005, 42, 49–68. [Google Scholar] [CrossRef]
- Zhou, Y.; Underhill, S.J.R. Plasma membrane H-ATPase activity and graft success of breadfruit (Artocarpus altilis) onto interspecific rootstocks of marang (A. odoratissimus) and pedalai (A. sericicarpus). Plant Biol. 2018, 20, 978–985. [Google Scholar] [CrossRef]
- Nicot, N.; Hausman, J.-F.; Hoffmann, L.; Evers, D.l. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef]
- Wang, W.; Vignani, R.; Scali, M.; Cresti, M. A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 2006, 27, 2782–2786. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Thomas, M.; Hetherington, L.; Wang, X.-D.; Offler, C.; Patrick, J. Genotypic differences in seed growth rates of Phaseolus vulgaris L. Factors contributing to cotyledon sink activity and sink size. Aust. J. Plant Physiol. 2000, 27, 119–128. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).