1. Introduction
Water stress is one of the main stresses that after plant perception leads to an increase in the production of reactive oxygen species (ROS) [
1]. ROS are able to injure the membrane of the cells and increase malondialdehyde (MDA) production [
2]. However, both enzymatic and non-enzymatic systems are used by plants to scavenge over-produced ROS. The main enzymatic antioxidants involved in ROS detoxification processes are catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX) and polyphenol oxidase (PPO) [
3]. Plants produce a wide range of free radical scavenging molecules, including phenolic compounds, vitamins, and terpenoids [
4,
5,
6]. Phenolic compounds are important components involved in plant growth and reproduction, and in the cell defense system against free radicals in conditions of abiotic and biotic stress [
7].
Vitamins could be regarded as compounds of bioregulators or hormone precursors that, in tiny amounts, employ a valuable impact on plant growth and development. Overall, these substances could affect the energy metabolic pathway [
8,
9,
10]. All essential physiological processes, such as photosynthesis, biosynthesis enzymes and secondary metabolites, nutrient and water absorption, and cell division more or less depend on vitamin availability. In the defense of plants in resistance to oxidative stress, vitamins with their antioxidant properties also play an important role as free radical scavengers. Vitamins have favourable effect on enhancing cell division and phytohormone synthesis, for instance cytokines and gibberellins [
11]. Ascorbic acid (AsA) functions as an antioxidant, an enzyme cofactor, and as a pre-cursor for oxalate and tartrate synthesis. AsA is affiliated with chloroplasts in which the effect of oxidative stress on photosynthesis is mitigated. Furthermore, AsA abates the alteration of cell division and works as a primary substrate in the cyclic pathway of hydrogen peroxide enzymatic detoxification [
12].
The common bean (
Phaseolus vulgaris L.) is a grain legume with the world’s largest total production for direct human consumption, approximately 12 million tons per year, mostly in Latin America and Africa [
13]. Beans are a source of protein, carbohydrates, fibre, minerals, and folate [
14]. Environmental stresses, in particular climatic and soil related constraints severely restrict the yields of the common bean [
15,
16,
17]. Fresh water is becoming ever more scarce due to climate change, particularly in arid environments, and causing droughts, which are becoming increasingly serious [
18]. Fresh water scarcity is one of the most noteworthy environmental factors limiting crop production in arid environments [
19,
20,
21,
22]. The common bean is a rapidly growing plant that is very sensitive to soil-water conditions and soil quality, thus, the yield can be significantly decreased by even brief periods of water shortage [
23]. Generally, water stress reduces the yield of the crop, but in a cultivar-dependent manner. Water management is an important factor in common bean production at all stages of plant development as it influences growth, yield, and yield quality. Water stress has been described to decrease many traits in faba beans except for the days to flowering and retention of moisture in the leaves [
24,
25].
Certain water management practices, e.g., irrigation can contribute to the sustained yield in low soil water environments; therefore, recognizing the effects of AsA on managing antioxidant metabolism systems and other physiological and biochemical processes is crucial in developing successful practices to enhance plant tolerance to water stress. The research hypothesis we verified is that ascorbic acid alleviates water stress tolerance of common bean plants and we can point physiological and biochemical determinants of this tolerance. The purpose of this work is also to examine the efficiency of foliar-applied AsA dose for counteracting the drought stress in common bean plants.
3. Discussion
Depending on the soil conditions, environment and variety, the total water requirement for a dry bean crop of 90−100 days may reach 350−500 mm h
−1 [
26]. Our results reported that 88 days were needed to complete the dry bean crop season with irrigation water application of 207.7 and 126 mm ha
−1 season
−1 for the well-watered control and water-stressed treatments, respectively, through sprinkler irrigation systems.
Mostly due to stomata closure and Rubisco inhibition, water stress resulted in a significant reduction of photosynthetic efficiency in plants [
27]. Our results showed that reductions in chlorophyll a, chlorophyll b, chlorophyll a+b, and carotenoids under water stress conditions may be attributed to a mechanism of avoiding damage by reactive oxygen species (ROS) through the drop in leaf photosynthetic pigment content because of water deficits, as was stated by Herbinger et al. [
28]. The carotenoid content decreased in a line with the chlorophyll content. The results of the present research confirmed Efeoğlu et al.’s [
29] conclusion that the carotenoid content in plants was lower under water deficit conditions as a strategy of acclimation. Ascorbic acid (AsA) foliar application significantly increased chlorophyll a, chlorophyll b, carotenoids, and accordingly the total photosynthetic pigments in this study, especially at a dose of 400 mg L
−1.
Previous research showed that exogenous AsA application under water stress increased chlorophyll a, chlorophyll b, and/or total chlorophyll contents [
30,
31]. In the mentioned studies, the increase in chlorophyll contents was mostly due to the protecting role of AsA under oxidative stress conditions. Moreover, photochemical efficiency depended on photosynthetic pigment contents, such as chlorophyll a and b, which influenced the photochemical reactions [
32]. The increase of chlorophyll content affected by AsA application could also enhance the photochemical efficiency of common bean plants [
33]. Our results are in conformity with those published by Hussein and Khursheed [
34] showing that AsA protected photosynthesis and enhanced leaf photosynthetic pigments under drought conditions, controlling the dry matter accumulation.
The increase in carotenoid concentration in the common bean plants, with AsA application under water stress, protected plants against the damaging effect of ROS, which is completely bound to the core complexes of photosystem I and II, and was necessary for chloroplast performance. AsA is considered an efficient antioxidant, that participates in protecting photochemical processes [
35]. Our results confirmed the findings of Helrich [
36] and English [
37], reporting that ascorbic acid application accelerates the scavenging of reactive oxygen species, promotes photosynthesis, and maintains enzyme activity at a stable level.
Assimilation of photosynthetic carbon was significantly reduced by 50% in water stress plants compared to those from the well-watered treatments in the present experiment. Water stress injured photosynthetic machinery at various levels e.g., pigments, stomatal performance, gas exchange, structure, and function of the thylakoid membrane, and electron transport regulated by the CO
2 concentration [
38,
39]. AsA application increased carbonic anhydrase (CA) activity in common bean plants under water stress conditions. The present results are in harmony with those reported by Salama et al. [
39] in
Phaseolus vulgaris under conditions of salinity stress. AsA is a key compound in the activation of Rubisco, phosphoenolpyruvate carboxylase, and carbonic anhydrase under stress conditions [
40]. This may be attributed to AsA-mediated alleviation in the activity of CA, which may mitigate the stress-dependent damage of the plasma membranes.
Plant defense mechanisms against water stress are also associated with peroxidase activity due to the peroxidase (POD) specific role in synthesis of phenols and saponins [
41]. The results of the present study show that the POD activity in common bean leaves was significantly increased under conditions of water stress compared to well-watered plants. Thus, the POD activity significantly increased due to the oxidative stress induced by low water availability, as previously reported by Ramakrishna and Ravishankar [
42]. As far as enzymatic activity was concerned, a significant increase was found in common bean leaves under water stress conditions. Externally-applied ascorbic acid enhanced POD activity, which is in accordance with the previous study on maize where POD and CAT activities have been increased due to exogenous application of ascorbic acid under water stress [
43].
Water stress significantly enhanced the MDA content in common bean leaves. Exogenous application of AsA was effective in decreasing the MDA content under water stress conditions. Recent research by Elkeilsh et al. [
44] confirmed that lipid peroxidation in wheat leaves increased due to water stress. Bor et al. [
45] reported a significant increase in lipid peroxidation in sugar beet grown under water stress. Similar results were reported by Vaidyanathan et al. [
46] in rice and Meloni et al. [
47] in cotton. Malondialdehyde (MDA) is a marker of oxidative stress, which is a product of membrane lipid peroxidation, thus a higher MDA content should correspond to a higher degree of oxidative stress and is used to assess the degree of acclimatization of water-stressed plants [
48]. Moreover, Cao et al. [
49] reported that MDA concentration is good marker of structural integrity of membranes of plants cells exposed to water stress. A 50% higher MDA accumulation was determined in stressed common bean plants in conditions of a present research, indicating that plant cells were damaged by the water deficit, as it was also reported by Keyvan [
48].
Water stress decreases plant growth by affecting various physiological and biochemical processes e.g., secondary metabolite synthesis, nutrient metabolism, respiration, and photosynthesis [
50]. Production of phenols in plants commonly increases under environmental stress conditions [
51,
52]. Our results are in agreement with previous studies by Krol et al. [
53], as they reported an increase in total phenolics, flavonoids, and tannins in common been, under conditions of water stress. In water-stressed plants, the production of flavonoids and other low-molecular-weight antioxidants may be partly correlated with leaf morphological variability and metabolic changes, which avoid oxidative injury [
54]. Therefore, cells create certain responses, e.g., stress protein production, accumulation of organic solutes in different plant parts, and up regulation of antioxidant systems, e.g., antioxidant enzymes [
55]. We found that application of ascorbic acid at a dose of 200 or 400 mg L
−1 via foliar spray significantly increased total phenolics, total flavonoids, and total tannins under water stress conditions. These results confirmed those of Jaleel et al. [
50] and Salama et al. [
39] in
Phaseolus vulgaris under salt stress.
In this study, two processes i.e., DPPH
• radical scavenging and an ABTS
•+ assay were used to examine the antioxidant activity in the leaves of the common bean plants. Phenolic compounds are found to be associated with plant antioxidant activity [
53]. Radical scavenging activity is also referred to phenolic compound structure i.e., the number and orientation of hydroxyl (−OH) groups [
53]. Water stress leads to reactive oxygen species elevation, hence, a higher amount of antioxidants is needed to balance the stress environment and increase the plant adaptation processes [
56]. Under water stress, the expression of several genes that contribute to the synthesis path of phenolic compounds e.g., phenylalanine ammonia-lyase is raised and needs high energy inputs, while these energy-intensive processes are restricted from moderate to severe stress environments [
53]. In this study, significant increases were reported in antioxidant activity i.e. DPPH
• radical scavenging activity and ABTS
•+ scavenging activity of common bean leaves due to ascorbic acid foliar spraying. The application of AsA caused the accumulation of metabolic regulators or bioactive compounds acting as cell component stabilizers which might alleviate the adverse effects of water stress. In addition, the further increase in DPPH
• activity and endogenous AsA was a result of strengthening the antioxidative defense system followed by an increased tolerance of bean plants to water stress. AsA may be engaged in the up-regulation of soluble sugars and proline synthesis to enhance tolerance mechanisms under water stress. The increase in the antioxidant activity was attributed to the increases of secondary metabolites (phenolics and flavonoids) which are considered as a tolerance advantage of water, as well as, other abiotic stresses as reported by and El-Amier et al. [
52], Mostajeran and Rahimi Eichi [
57], and Reddy et al. [
58]. This study recommends the use of AsA at a dose of 200 or 400 mg L
−1 for improving osmoprotectant and antioxidant systems of the common bean, with a positive growth and yield response as a consequence.
In the present study, flavonoids and total phenolics content in common bean extracts are substantially based on the type of solvent, concentrations used, and also solvent polarity. These results were confirmed by Hounsome et al. [
59] and Zhao et al. [
60]. In general, the production of phenol compounds in plants under abiotic stress depended on type of stress, stress intensity, stress duration, plant development stages (usually, germination and plant development are the most sensitive to stress) and plant part type, i.e., whole seedlings or plant parts, namely roots or leaves [
61,
62]. The present results showed that water stress negatively affected common bean growth and yield traits, i.e., plant height, leaf number, leaf area, branch number, and seed yield. This may be attributed to a severe damage of a multitude of molecular, biochemical, and physiological processes, which adversely affected plant growth and development through reductions in cell elongation due to the inhibition of growth-promoting hormones, leading to a decrease in cell turgor and volume [
63,
64,
65,
66]. The response of a crops to water stress is determined by the species, genotype, water stress period and its severity, plant growth stage and its age, and the foliar-applied organic acid [
65]. The exogenous application of AsA at 200 or 400 mg L
−1 significantly enhanced all growth parameters in the light of the present results. Ultimate values of the growth parameters were obtained with the application of 400 mg L
−1 AsA in the conditions of the present research.
In stressed plants, ascorbic acid performs a crucial function in maintaining several metabolic processes [
66]. Mittler [
67] reported that AsA is one of the non-enzymatic antioxidant compounds serving as electron donors to reduce the accumulation of ROS and as a reaction substrate within the enzymatic cycle. These mechanisms are responsible for the differences between experimental treatments presented in this manuscript. It was reported that among a range of morphological, yield and related traits, seed yield determined in contrasting water management conditions (water stress and well watered) is considered the most efficient method for evaluating the integrated water stress tolerance in plants [
68,
69,
70]. In addition, exogenous application of AsA improved plant performance under water stress and increased crop yield in maize (
Zea mays) [
71,
72], flax (
Linum usitatissimum) [
73], and wheat (
Triticum aestivum) [
74].
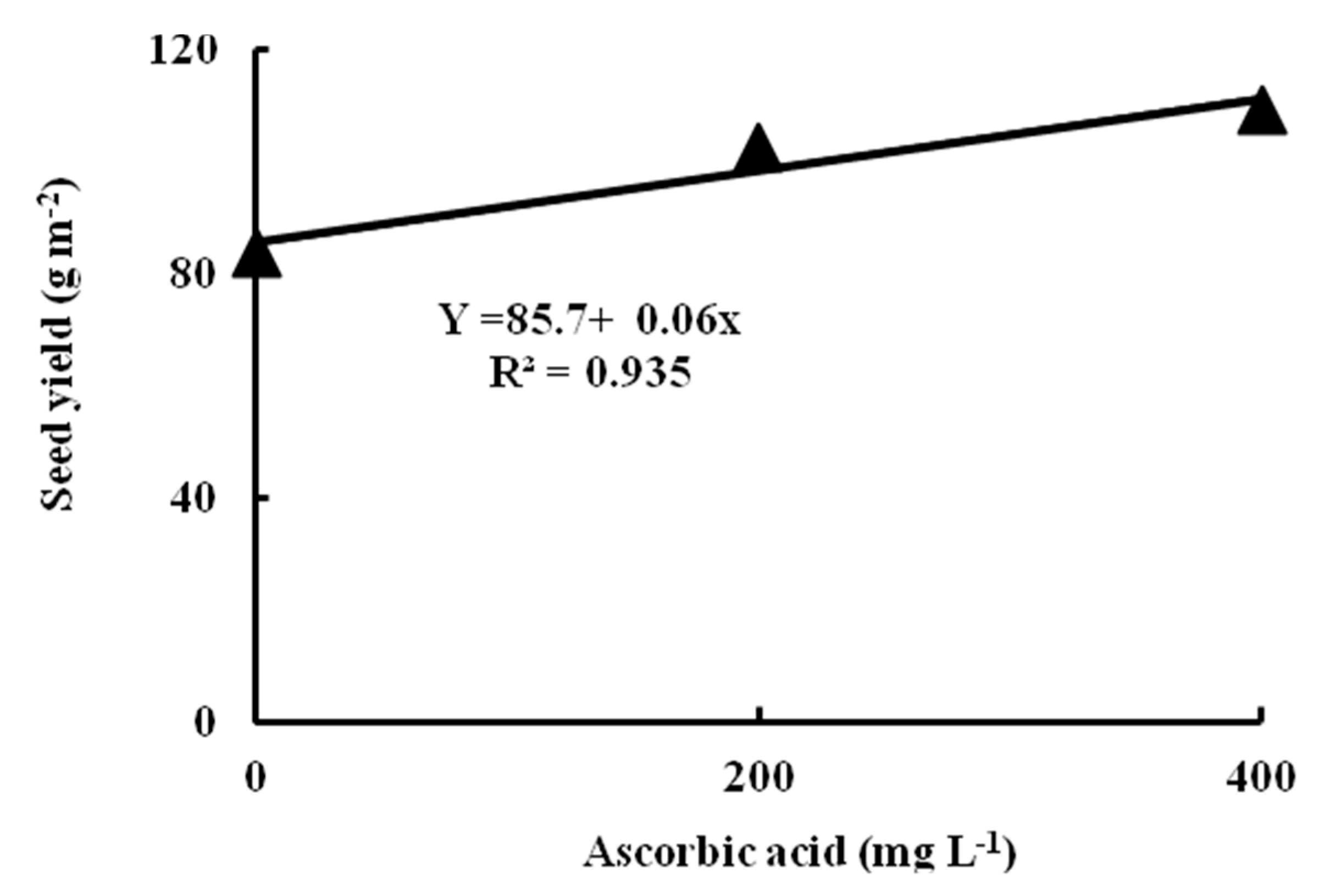
Crop water productivity (WP) which is associated with the ratio of seed yield to water used, in general is inversely proportional to water stress severity. The WP varies with the plant growth stage that is influenced by the length of drought stress and its intensity. Huang et al. [
75] reported that a high WP gave proof of the stomata’s function in sustaining high leaf water content during water stress. A high WP can lead to high photosynthetic activity. However, morphogenetic and metabolic studies are required to better understand the relation between stomatal closure and WUE. The reported WP values reached 3−6 kg ha
−1 mm
−1 [
76]. In addition, Munoz-Perea et al. [
77] reported that the WP values extended from 8.7−10.0 kg ha
−1 mm
−1. Our results showed that WP ranged from 4.63 to 7.86 kg ha
−1 mm
−1. Therefore, using agronomic practices such as an irrigation regime and exogenous application of ascorbic acid can enhance soil moisture conservation and WP would be a key for reducing water use and improving sustainable low-input production systems in the Nubaria region, Egypt and other water shortage environments of the world.
4. Materials and Methods
4.1. Experimental Procedures
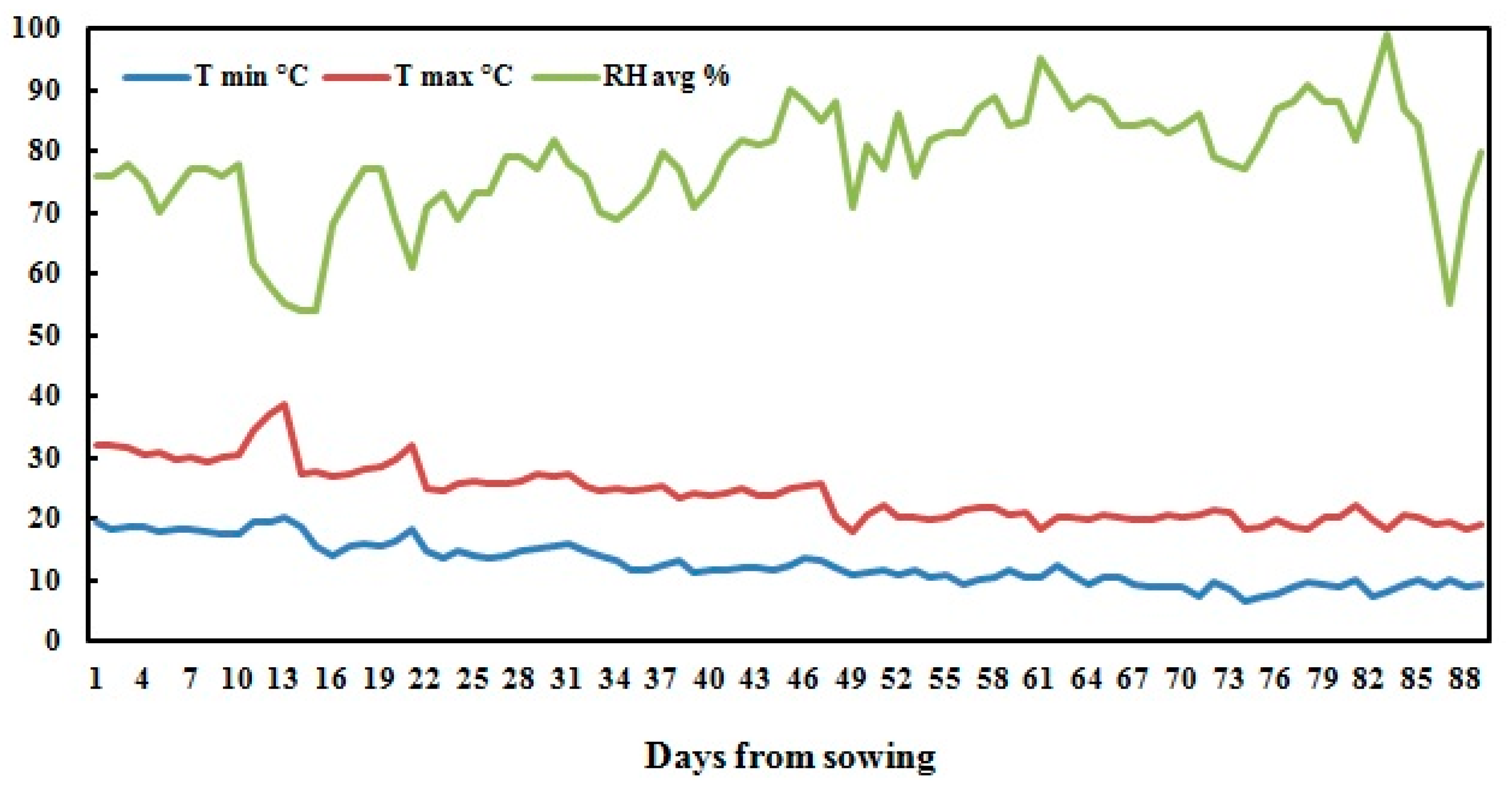
A field experiment was conducted during the 2017 season at the experimental station of National Research Centre, Nubaria, Egypt (30°86’67” N 31°16’67” E) withmean altitude 21 m above sea level). The experimental area is classified as an arid region with cool winters and hot dry summers. Daily temperatures ranged from 6.4−38.8 °C, with an average of 18.1 ± 4.1 °C. The daily relative humidity was 78.3 ± 9.1% in average, and ranged from 54−99%.
Figure 3 shows the climatic data of the experimental site during the growing season. The mechanical analyses of the soil of the experimental site showed 68.9% coarse sand, 17.4% fine sand, 8.4% silt, and 5.3% clay, classified as sandy soil.
The physical and chemical properties of the experimental soil are shown in
Table 1,
Table 2 and
Table 3. Irrigation water was obtained from an irrigation channel passing through the experimental area. The chemical properties of the irrigation water are shown in
Table 9. The experimental field was deeply plowed before planting. First disc harrow, then duck foot cultivator was used for further preparation of the field for planting. A combined driller that facilitated the concurrent application of fertilizer and seeds was used.
Healthy common bean (Phaseolus vulgaris L.) cv. Nebraska seeds were sown on 29 September 2017 and harvested on 26 December 2017. Seeds were obtained from The Horticulture Research Institute, The Agricultural Research Centre, Giza, Egypt, and were sown at the amount of 95 kg ha−1 to achieve the recommended planting density.
Common bean seeds were selected for uniformity by choosing those of equal size and of the same color. The selected seeds were washed with distilled water, sterilized in 1% (v/v) sodium hypochlorite for approximately 2 min, washed thoroughly again with distilled water, and left to dry at room temperature (25 °C) for approximately 1 h. Uniform, air-dried common bean seeds were sown in hills in rows spaced 60 cm apart. The hills were spaced 10−15 cm apart in 3.0 m × 3.5 m plots. Thinning was done to produce two plants per hill. During soil preparation and plant growth, the soil was supplemented with the dose of NPK fertilizer according to the recommendations of the Ministry of Agriculture and Land Reclamation of Egypt for the studied area. These recommendations are 475 kg ha−1 of calcium super-phosphate (15.5% P2O5), 120 kg ha−1 ammonium sulfate (20.5% N), and 60 kg ha−1 potassium sulfate (48% K2O) during seed-bed preparation.
The experiment was conducted using a split-plot design in a randomized complete block design with three replicates. Specifically, irrigation as the first factor was assigned to the main plots. The main plots were then divided into sub-plots (split-plots). Next, dose of ascorbic acid (vitamin C) (Sigma-Aldrich, Saint Louis, MO, USA) as a second factor was allocated to the sub-plots and the number and timing of sprays were based on results from a preliminary pot trial (data not shown). The main plots included two irrigation regimes, namely, (1) 100% of crop evapotranspiration (ETc) throughout the season as a well-watered treatment (WW), and (2) 50% of ETc throughout the season as a water-stressed treatment (WS). The treatment of 50% ETc was applied to start from 19 days after sowing till the end of the growing season. The sub-plots were assigned to three ascorbic acid (AsA) treatments, i.e., (1) 0 mg L−1 AsA, (2) 200 mg L−1 AsA, and (3) 400 mg L−1 AsA. Ascorbic acid was applied to common bean plants as a foliar spraying, twice, at 25 and 40 day after sowing (DAS). All spray treatments were put on during the early morning and before 9:00 a.m., when wind is low, with a hand sprayer at sufficient pressure to keep droplet size small. Spray volume amount of water consisted of approximately 500 m3 per hectare. Plants were sprayed from both sides of the row in order to achieve adequate coverage. Therefore, the experiment consisted of six treatments as combinations of two irrigation regimes and three ascorbic acid doses, namely, T1—100% ETc with 0 mg L−1 AsA (WWAsA1); T2—100% ETc with 200 mg L−1 AsA (WWAsA2); T3—100% ETc with 400 mg L−1 AsA (WWAsA3); T4—50% ETc with 0 mg L−1 AsA (WSAsA1); T5—50% ETc with 200 mg L−1 AsA (WSAsA2); and T6—50% ETc with 400 mg L−1 AsA (WSAsA3). A preliminary experiment was carried out to verify which level of AsA could be used as foliar spraying. We concluded that the doses up to 400 mg L−1 are not harmful and can enhance plant growth. All other agricultural practices for common bean were carried out according to the recommendations of the Egyptian Ministry of Agriculture and Land Reclamation, Egypt.
4.2. Water Requirements and Irrigation Regimes
Crop water requirements (CWR) was determined by estimating crop evapotranspiration (ETc) under standard conditions as follows:
where:
ETc = crop evapotranspiration [mm per day]
ETo = reference crop evapotranspiration [mm per day]
Kc = crop coefficient.
The values of ETc and CWR are equal, whereby ETc refers to the amount of water lost through evapotranspiration and CWR refers to the amount of water that is needed to compensate for the loss. ETc calculated from climatic data by direct integrating the effect of crop characteristics into ETo. The FAO Penman−Monteith method is recommended as the standard for calculating ETo. The Penman−Monteith equation is as follows [
78]:
where:
ETo = reference evapotranspiration [mm per day]
Rn = net radiation at the crop surface ([MJ m−2] per day)
G = soil heat flux density ([MJ m−2] per day)
T = mean daily air temperature at 2 m height [°C]
u2 = wind speed at 2 m height [m s−1]
es = saturation vapour pressure [kPa]
ea = actual vapour pressure [kPa]
es−ea = saturation vapour pressure deficit [kPa]
∆ = slope of saturation vapor pressure curve at temperature T [kPa °C]
γ = psychrometric constant [kPa/ C].
The equation used the standard climatological records of solar radiation (sunshine), air temperature, humidity, and wind speed for daily calculations. The percentage of soil moisture content (θ v) was measured with the profile probe apparatus in sandy soils.
Amount of applied irrigation water (AW) was calculated according to the following equation for the sprinkler irrigation systems:
where:
AW = applied irrigation water depth [mm per day]
Ea = application efficiency equals 75% for sprinkler irrigation system
LR = leaching requirements equals 10% for sprinkler irrigation system.
Irrigation time (IT) for the solid sprinkler system was calculated as follows:
where:
AR = Application rate for sprinkler in [mm h
−1]
where:
Sprinkler discharge in [m3 h–1]
Strip area in [m2].
Crop water productivity (WP) is defined as the relationship between the seed yield and the amount of water elaborated in crop production. WP in kg mm
–1 ha
–1 was calculated as follows:
The irrigation regimes were started on the 18th of October in 2017 at 19 DAS and lasted for 70 days till the end of the season. The application of irrigation regimes through a sprinkler irrigation system were 207.7 and 126 mm ha−1 season−1 for 100% ETc (WW) and 50% ETc (WS), respectively. Plant samples were collected at 46 DAS for the determination of growth performance and biochemical analysis of common bean plants, where irrigation regimes lasted for 28 days. Notably, the common bean received 145.5 and 94.9 mm ha−1 sample−1 for 100% ETc (WW) and 50% ETc (WS), respectively.
4.3. Measurements
Common bean plant samples were collected at 46 DAS during late vegetative growth and before flowering for the determination of growth performance and biochemical analysis. We carefully revmoved 108 plants, i.e., six plants from each plot × two levels of irrigation × three ascorbic acid levels × three replicate plots, from the experimental site, then dipped them in a bucket of water, and plants were shaken gently to remove all adhering soil particles.
Plant height (PH) in cm, leaf number per plant (LNo), leaf area per plant (LA) in cm2, and branch number per plant (BNo) of common bean plants were measured at 46 DAS, while seed yield (SY) in g m−2 and crop water productivity (WP) in kg mm−1 ha−1 of bean plants were measured at final harvest at 88 DAS.
Sample leaf disks were taken on the second fully-expanded not shaded leaf from the top using a cork borer 9 mm in diameter were used for determining chlorophyll, carotenoids, carbonic anhydrase activity (CA), peroxidase activity (POD), malondialdehyde (MDA), 2,2-diphenyl-1-picrylhydrazyl-free radical scavenging assay (DPPH
•), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) cation assay (ABTS
•+). The leaf tissues that oven-dried for 72 h at 70 °C were used for estimating total phenolic (TP), total flavonoids (TF), and total tannins (TT). Common bean leaf chlorophyll a (Chl a), chlorophyll b (Chl b), and carotenoids (Car) concentrations were estimated using 80% (
v/v) acetone extracts and the spectrophotometric method according to Lichtenthaler and Wellburn [
79].
For preparation of enzyme extracts, about 5.0 g of plant material were crushed into a fine powder using liquid nitrogen. The sample was extracted by homogenizing the powder in 10 mL of 50 mM phosphate buffer (pH 7.8) containing 1 mM EDTA and 1% polyvinylpyrrolidine (PVP), with the addition of 1 mM ascorbate in the case of POD assay at 4 °C. The homogenate was centrifuged at 15,000× g for 20 min and the supernatant was used for the enzyme activity assay.
Carbonic anhydrase activity assay (CA; EC 4.2.1.1) were measured in leaves tissue (100 mg FW), which were homogenized with a buffered solution (pH 8.3) that contained 50 mm Veronal H
2SO
4 and 0.2% (
w/v) PVP under ice cold-conditions. The homogenate was centrifuged at 12,000×
g for 2 min and the supernatant was used for the determination of CA activity according to Ohki [
80].
Peroxidase activity (POD; EC1.11.1.7) was assayed by monitoring the increase in absorbance at 430 nm due to the oxidation of pyrogallol [
81]. The reaction mixture consisted of 50 mM potassium phosphate buffer (pH 7.0), 20 mM pyrogallol, 5 mM H
2O
2 and 20 µL of enzyme extract. In total, 1 unit of the enzyme was the amount necessary to decompose 1 µmoL of substrate per minute at 25 °C. Peroxidase activity was expressed as EU g
-1 FW min
−1.
Lipid peroxidation was measured as the amount of malondialdehyde (MDA) determined by the thiobarbituric acid (TBA) reaction [
82]. Frozen samples were homogenized with a pre-chilled mortar and pestle with two volumes of ice-cold 0.1% (
w/v) trichloroacetic acid (TCA) and centrifuged for 15 min at 15,000×
g. Assay mixture containing 1 mL of the supernatant and 2 mL of 0.5% (
w/v) TBA in 20% (
w/v) TCA was heated at 95 °C for 30 min and then rapidly cooled in an ice bath. After centrifugation (10,000×
g for 10 min at 4 °C), the supernatant absorbance was read at 532 nm and the values corresponding to nonspecific absorption (600 nm) were subtracted. Lipid peroxidation products were measured as the content of TBA-reactive substances. MDA content was calculated according to the molar extinction coefficient of 155 mM cm
−1.
ABTS•+ (2,2’-azinobis (3-ethylbenzothiazoline-6-sulfonic acid)), Folin-Ciocalteau reagents, gallic acid, quercetin, DPPH• (2,2-diphenyl-1-picrylhydrazyl), BHT: butyl hydroxytoluene were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
For preparation of common bean methanol extracts to determine secondary metabolites compounds and in vitro antioxidant activity, the dried powder of P. vulgaris leaves (10 g) was submersed separately in 100 mL methanol, for 24 h at room temperature using a shaker. Each mixture was filtered through Whatman No. 1 filter paper and this extraction step was repeated three times. The filtrate was then concentrated to dryness at 40 °C in a rotary evaporator. The crude extracts were stored in a refrigerator until chemical analysis.
The total phenolics (TP) were determined by Folin-Ciocalteu reagent assay using gallic acid as standard according to Singleton and Rossi [
83]. A suitable aliquot (1 mL) of leaf extracts was added to 25 mL volumetric flask, containing 9 mL of distilled water. We added 1 mL of Folin-Ciocalteu’s phenol reagent to the mixture and shaken. After 5 min 10 mL of 7% Na
2CO
3 solution was added to the mixture. The solution was diluted to 25 mL with distilled water and mixed. After incubation for 90 min at room temperature, the absorbance was determined at 750 nm with a spectrophotometer (UV 300, Spectronic-Unicam, Cambridge, UK) against prepared reagent as blank. A total phenolic content in samples was expressed as mg of gallic acid equivalents (GAE) g
−1 dry weight. All samples were analyzed in triplicates.
Total flavonoids (TF) were determined by the aluminum chloride method using quercetin as a standard [
84]. We added 1 mL of plant extracts to 10 mL volumetric flasks, containing 4 mL of distilled water. To the flask 0.3 mL 5% NaNO
2 was added and after 5 min 0.3 mL 10% AlCl
3 was added. At 6 min, 2 mL 1 M NaOH were added and the total volume was made up to 10 mL with distilled water. The solutions were mixed well and the absorbance was measured against prepared reagent blank at 510 nm by using spectrophotometer UV 300. Total flavonoids in the sample were expressed as mg of quercetin equivalents (QE) g
−1 fresh weight. Samples were analyzed in triplicates.
Total tannin (TT) was measured using the Folin-Ciocalteu reagent according to Polshettiwar et al. [
85]. We added 1 mL of plant extracts to 7.5 mL distilled water (dH
2O) then added 0.5 mL of Folin-Ciocalteu reagent and 1 mL of 35% sodium carbonate solution. The volume was made up for 10 mL with distilled water and absorbance was measured against prepared reagent blank at 775 nm by using spectrophotometer UV 300. Total tannins in the sample were expressed as mg of tannic acid equivalent (TE) g
−1 dry weight. All samples were analyzed in triplicates.
Phenolics in mg 100 g
−1 DW and flavonoids in mg 100 g
−1 DW were measured by high-performance liquid chromatography (HPLC). A sample from 100% ETc with 400 mg L
−1 (T3) treatment was used for measuring phenolics and flavonoids. Thus, the dried methanol crude extract (10 mg) of common bean was dissolved in 2 mL methanol HPLC spectral grade by vortex mixing for 15 min. The extract was filtrated through a 0.2 μm Millipore membrane filter. The phenolic and flavonoid compounds were identified by HPLC (Agilent Technologies 1100 series, Waldbronn, Germany), equipped with a quaternary pump (G131A model). The separation was achieved on the ODS reversed-phase column (C18, 25 × 0.46 cm i.d. 5 μm, Amsterdam, Netherlands). The injection volume (35 μL) was carried out with an auto sampling injector. The column temperature was maintained at 35 °C. Gradient phenolic compounds’ separation was carried out with an aqueous formic acid solution 0.1% (A) and methanol (B) as a mobile phase at a flow amount of 0.3 mL min
−1 following the method of Goupy et al. [
86]. In addition, the flavonoid compounds’ separation was carried out with 50 mM H
3PO
4, pH 2.5 (solution A) and acetonitrile (solution B) as a mobile phase at a flow rate of 0.7 mL min
−1 as described by Mattila et al. [
87]. Elutes were monitored using a UV detector set at 280 nm for phenolic acids, and at 330 nm for flavonoids. Chromatographic peaks were identified by comparing the retention times with the respective retention times of known standard reference material. Phenolic acid and flavonoid concentrations were calculated by comparing its peak areas with the peak areas of standards used (with known concentration) based on the data analysis of Hewlett Packard software. Phenolic acid and flavonoid compounds were expressed as µg 100 g
−1 DW.
Determination of DPPH
• (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging activity at 75 µg mL
−1 (DPPH%) was measured spectrophotometrically according to Chu et al. [
88]. We prepared 0.1 mM of DPPH
• in methyl alcohol, and 0.5 mL of this solution was added to 1 mL of plant extract at a concentration (100 µg mL
−1). Methanol was used as blank. The mixture was shaken vigorously and allowed to stand at room temperature. Butyl hydroxytoluene (BHT, Sigma) was used as a positive control; and negative control contained the entire reaction reagent except for the extracts. Then the absorbance was measured at 515 nm against the blank. Lower absorbance of the reaction mixture indicated higher free radical scavenging activity. The capacity to scavenge the DPPH
• radical was calculated using the following equation:
where: (A
c) was the absorbance of the control reaction and (A
s) the absorbance in the presence of the plant extracts.
ABTS
•+ assay [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] enzymatic assay (ABTS%) was generated by oxidation of ABTS
•+ with potassium persulphate [
89]. ABTS
•+ was dissolved in deionized water to 7.4 mM concentration, and potassium persulphate was added to a concentration of 2.6 Mm. The working solution was then prepared by mixing the two stock solutions in equal quantities and allowing them to react for 12−16 h at room temperature in the dark. The solution was then diluted by mixing 1 mL ABTS
•+ solution with 60 mL methanol to obtain an absorbance of 1.1 ± 0.02 units at 734 nm using the spectrophotometer. Fresh ABTS
•+ solution was prepared for each assay. Plant extract of 150 µL at a concentration of 100 µg mL
−1 was allowed to react with 2850 µL of the ABTS
•+ solution for 2 h in a dark condition. Then the absorbance was taken at 734 nm using the spectrophotometer. Results were expressed as in comparison with standard butylhydroxytoluene. A bigger antioxidant capacity of the sample exhibited a smaller production of free radicals.
Percent activity was calculated using the equation:
where: A
0 is the ABTS
•+ absorbance of the control reaction and A
1 is the ABTS
•+ absorbance in the presence of the sample.
4.4. Statical Analysis
All data were subjected to analysis of variance for a split-plot design [
90], after testing for the homogeneity of error variances using Levene test [
91], and testing for normality distribution according to Shapiro and Wilk method [
92]. Statistically significant differences between means were compared at
p ≤ 0.05 using Tukey’s HSD (honestly significant difference) test. The statistical analysis was carried out using GenStat 17th Edition (VSN International Ltd, Hemel Hempstead, UK). Correlation coefficient
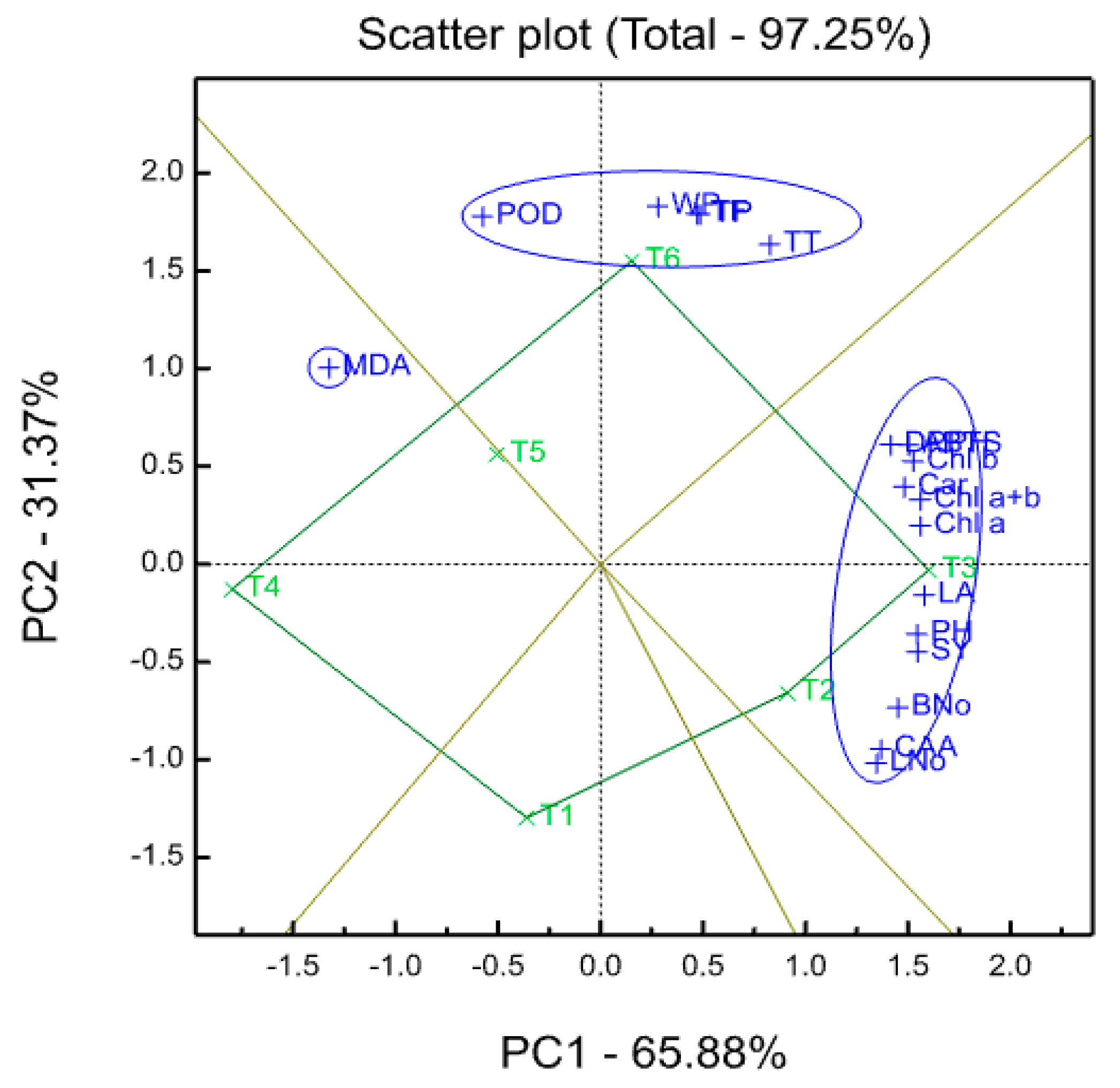
r was calculated to determine the relationship between seed yield and each of the physiological and chemical traits. Hierarchical cluster analysis was performed on the standardized data using a measure of Euclidean distance and Ward minimum variance method as outlined by Ward [
93]. Experimental data were also processed for a principal component analysis (PCA) using GenStat 17th Edition (VSN International Ltd, Hemel Hempstead, UK), in order to evaluate the existing relationships with original variables.