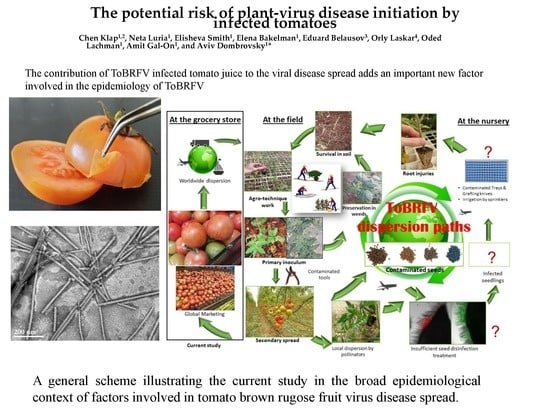
The Potential Risk of Plant-Virus Disease Initiation by Infected Tomatoes
Abstract
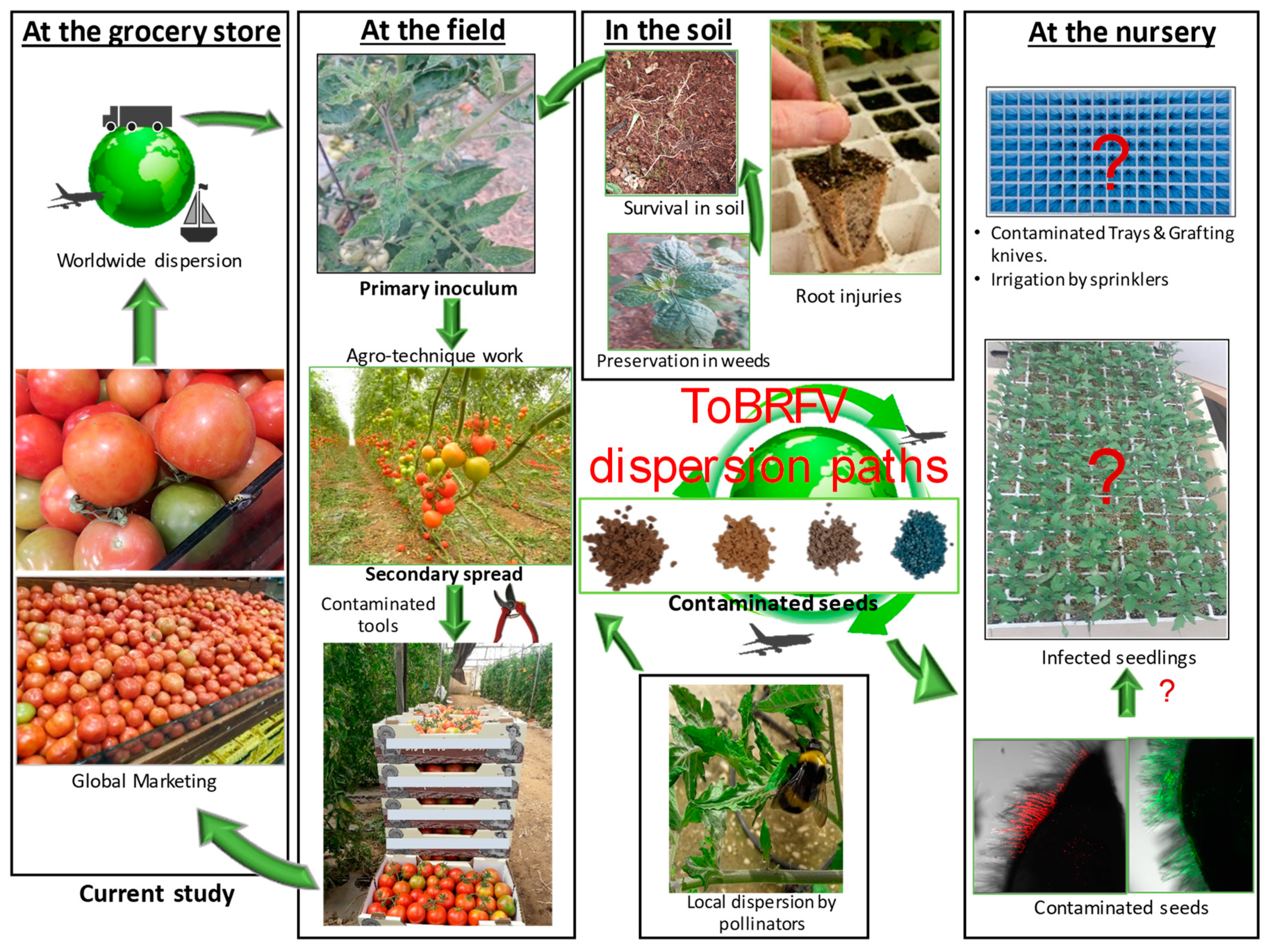
1. Introduction
2. Results
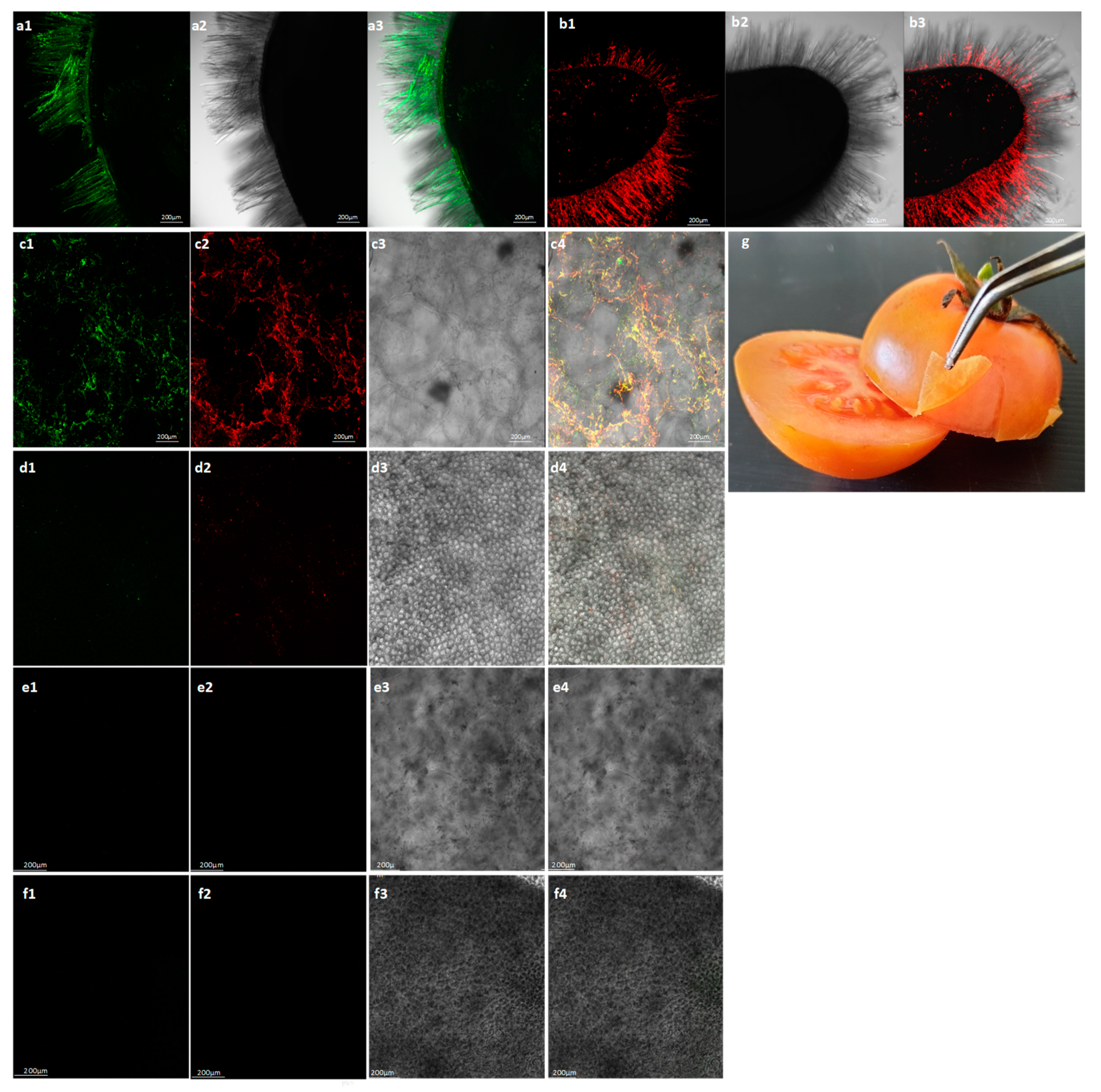
2.1. Locally Distributed Tomatoes Showing Viral-Like Disease Symptoms Were Co-Infected by ToBRFV and PepMV
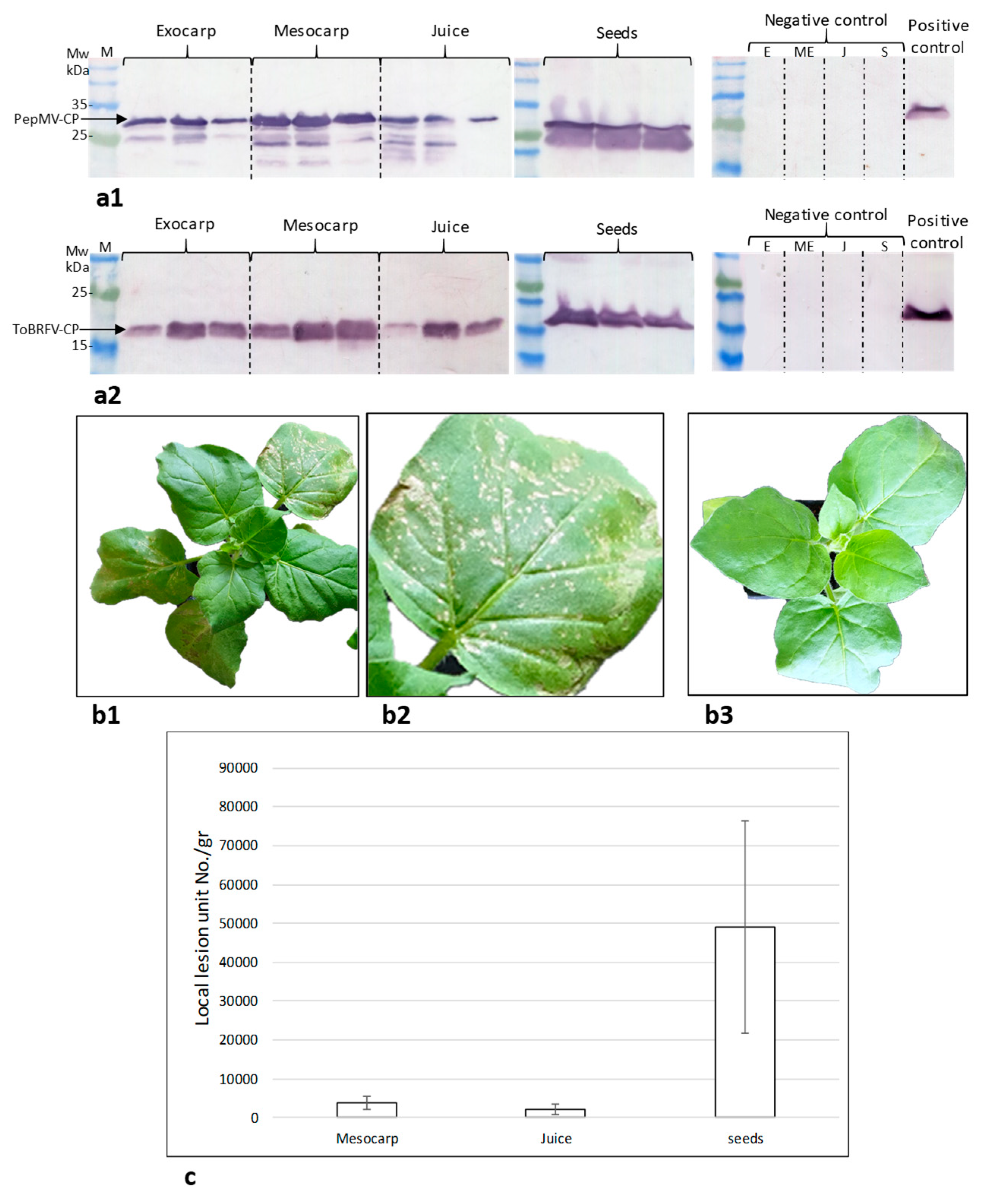
2.2. Assessment of the Tomato Fruit ToBRFV Infectivity Potential
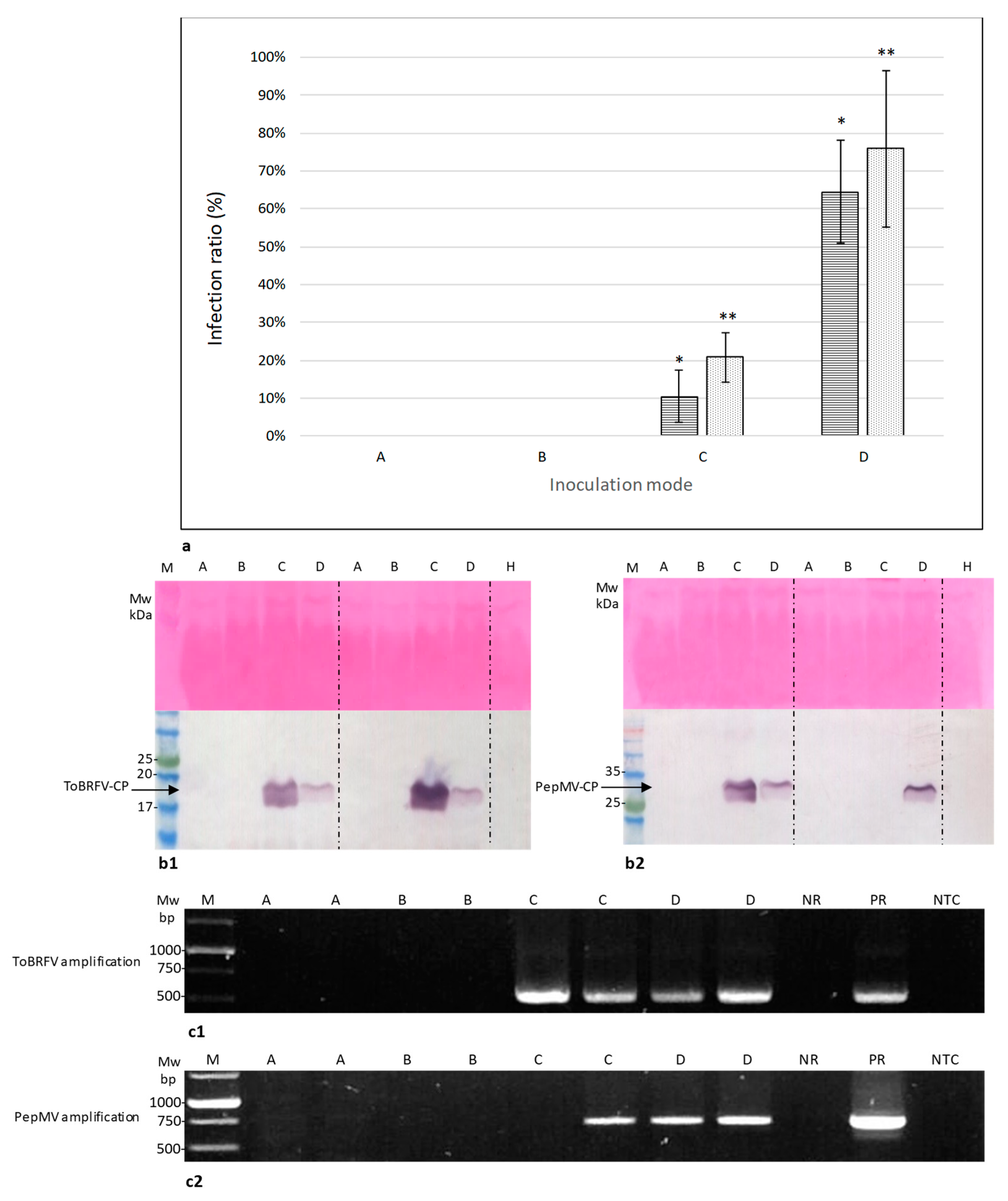
2.3. Symptomatic Co-Infected Tomato Fruits Could Constitute a Source of Tomato Plant Viral Infection
3. Discussion
4. Materials and Methods
4.1. Tomatoes and Tomato Plant Samples Subjected to Analyses
4.2. Viral RNA Extraction, Reverse Transcription (RT) and PCR Amplification
4.3. Western Blot Analysis
4.4. Indirect Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. Transmission Electron Microscopy (TEM)
4.6. In Situ Immunofluorescence Labelling of ToBRFV and PepMV
4.7. Biological Assays for Fruit Infectivity Potential
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salem, N.; Mansour, A.; Ciuffo, M.; Falk, B.; Turina, M. A new tobamovirus infecting tomato crops in Jordan. Arch. Virol. 2016, 161, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef] [PubMed]
- Menzel, W.; Knierim, D.; Winter, S.; Hamacher, J.; Heupel, M. First report of tomato brown rugose fruit virus infecting tomato in Germany. New Dis. Rep. 2019. [Google Scholar] [CrossRef]
- Panno, S.; Caruso, A.; Davino, S. First report of tomato brown rugose fruit virus on tomato crops in Italy. Plant Dis. 2019, 103. [Google Scholar] [CrossRef]
- Skelton, A.; Buxton-Kirk, A.; Ward, R.; Harju, V.; Frew, L.; Fowkes, A. First report of Tomato brown rugose fruit virus in tomato in the United Kingdom. New Dis. Rep. 2019, 40, 12. [Google Scholar] [CrossRef]
- Cambrón-Crisantos, J.M.; Rodríguez-Mendoza, J.; Valencia-Luna, J.B.; Rangel, S.A.; de Jesús García-Ávila, C.; López-Buenfil, J.A. First report of Tomato brown rugose fruit virus (ToBRFV) in Michoacan, Mexico. Mex. J. Phytopathol. 2018, 37, 185–192. [Google Scholar]
- Ling, K.-S.; Tian, T.; Gurung, S.; Salati, R.; Gilliard, A. First report of tomato brown rugose fruit virus infecting greenhouse tomato in the US. Plant Dis. 2019. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, H.; Han, S.; Geng, C.; Tian, Y.; Li, X. First report of Tomato brown rugose fruit virus infecting tomato in China. Plant Dis. 2019. [Google Scholar] [CrossRef]
- Smith, E.; Dombrovsky, A. Aspects in Tobamovirus Management in Intensive Agriculture. Plant Pathol. Manag. Plant Dis. IntechOpen 2019. [Google Scholar] [CrossRef]
- Wright, D.; Mumford, R. Pepino mosaic Potexvirus (PepMV): First records in tomato in the United Kingdom. Plant Disease Notice No. 89. Growth Horm. Igf Res. 1999, 11, S147. [Google Scholar]
- Van der Vlugt, R.A.A.; Stijger, C.C.M.M.; Verhoeven, J.T.J.; Lesemann, D.E. First report of Pepino mosaic virus on tomato. Plant Dis. 2000, 84, 103. [Google Scholar] [CrossRef]
- Lesemann, D.E.; Dalchow, J.; Winter, S.; Pfeilstetter, E. Occurrence of Pepino mosaic virus in European tomato crops: Identification, etiology and epidemiology. Mitteilungen-Biologischen Bundesanstalt Fur Land Und Forstwirtschaft 2000, 376, 323. [Google Scholar]
- French, C.; Bouthillier, M.; Bernardy, M.; Ferguson, G.; Sabourin, M.; Johnson, R.; Masters, C.; Godkin, S.; Mumford, R. First report of Pepino mosaic virus in Canada and the United States. Plant Dis. 2001, 85, 1121. [Google Scholar] [CrossRef] [PubMed]
- Jordá, C.; Perez, A.L.; Martínez-Culebras, P.; Abad, P.; Lacasa, A.; Guerrero, M. First report of Pepino mosaic virus on tomato in Spain. Plant Dis. 2001, 85, 1292. [Google Scholar] [CrossRef] [PubMed]
- Mumford, R.; Metcalfe, E. The partial sequencing of the genomic RNA of a UK isolate of Pepino mosaic virus and the comparison of the coat protein sequence with other isolates from Europe and Peru. Arch. Virol. 2001, 146, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Roggero, P.; Masenga, V.; Lenzi, R.; Coghe, F.; Ena, S.; Winter, S. First report of Pepino mosaic virus in tomato in Italy. Plant Pathol. 2001, 50, 798. [Google Scholar] [CrossRef]
- Jones, R.A.C.; Koening, R.; Lesemann, D.E. Pepino mosaic virus, a new potexvirus from pepino (Solanum muricatum). Ann. Appl. Biol. 1980, 94, 61–68. [Google Scholar] [CrossRef]
- Mumford, R.A.; Jones, R.A.C. Pepino Mosaic Virus; AAB Descriptions of Plant Viruses 411 No 11 Wellesbourne, UK: Association of Applied Biologists. 2005. Available online: http://www.dpvweb.net/dpv/showadpv.php?dpvno=411 (accessed on 12 May 2020).
- Hanssen, I.; Paeleman, A.; Vandewoestijne, E.; Van Bergen, L.; Bragard, C.; Lievens, B.; Vanachter, A.C.R.C.; Thomma, B.P.H.J. Pepino mosaic virus isolates and differential symptomatology in tomato. Plant Pathol. 2009, 58, 450–460. [Google Scholar] [CrossRef]
- Sempere, R.N.; Gómez-Aix, C.; Ruíz-Ramón, F.; Gómez, P.; Hasiów-Jaroszewska, B.; Sánchez-Pina, M.A. Pepino mosaic virus RNA-dependent RNA polymerase pol domain is a hypersensitive response-like elicitor shared by necrotic and mild isolates. Phytopathology 2016, 106, 395–406. [Google Scholar] [CrossRef]
- Ling, K.S. Molecular characterization of two Pepino mosaic virus variants from imported tomato seed reveals high levels of sequence identity between Chilean and US isolates. Virus Genes 2007, 34, 1–8. [Google Scholar] [CrossRef]
- Maroon-Lango, C.; Guaragna, M.; Jordan, R.; Hammond, J.; Bandla, M.; Marquardt, S. Two unique US isolates of Pepino mosaic virus from a limited source of pooled tomato tissue are distinct from a third (European-like) US isolate. Arch. Virol. 2005, 150, 1187–1201. [Google Scholar] [CrossRef]
- Gómez, P.; Sempere, R.; Elena, S.F.; Aranda, M.A. Mixed infections of Pepino mosaic virus strains modulate the evolutionary dynamics of this emergent virus. J. Virol. 2009, 83, 12378–12387. [Google Scholar] [CrossRef] [PubMed]
- Hasiów-Jaroszewska, B.; Pospieszny, H.; Borodynko, N. New necrotic isolates of Pepino mosaic virus representing the CH2 genotype. J. Phytopathol. 2009, 157, 494–496. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Thomma, B.P. Pepino mosaic virus: A successful pathogen that rapidly evolved from emerging to endemic in tomato crops. Mol. Plant Pathol. 2010, 11, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Hasiów-Jaroszewska, B.; Borodynko, N. Characterization of the necrosis determinant of the European genotype of pepino mosaic virus by site-specific mutagenesis of an infectious cDNA clone. Arch. Virol. 2012, 157, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Hasiów-Jaroszewska, B.; Borodynko, N.; Jackowiak, P.; Figlerowicz, M.; Pospieszny, H. Single mutation converts mild pathotype of the Pepino mosaic virus into necrotic one. Virus Res. 2011, 159, 57–61. [Google Scholar] [CrossRef]
- Hasiów-Jaroszewska, B.; Paeleman, A.; Ortega-Parra, N.; Borodynko, N.; Minicka, J.; Czerwoniec, A.; Thomma, B.P.; Hanssen, I.M. Ratio of mutated versus wild-type coat protein sequences in P epino mosaic virus determines the nature and severity of yellowing symptoms on tomato plants. Mol. Plant Pathol. 2013, 14, 923–933. [Google Scholar] [CrossRef]
- Duff-Farrier, C.R.; Bailey, A.M.; Boonham, N.; Foster, G.D. A pathogenicity determinant maps to the N-terminal coat protein region of the Pepino mosaic virus genome. Mol. Plant Pathol. 2015, 16, 308–315. [Google Scholar] [CrossRef]
- Chewachong, G.M.; Miller, S.A.; Blakeslee, J.J.; Francis, D.M.; Morris, T.J.; Qu, F. Generation of an attenuated, cross-protective Pepino mosaic virus variant through alignment-guided mutagenesis of the viral capsid protein. Phytopathology 2015, 105, 126–134. [Google Scholar] [CrossRef]
- Lacasa, A.; Guerrero, M.; Hita, I.; Martinez, M.; ALcázar, A.; Cano, A.; Jordá, C.; Bielza, P.; Contreras, J. Implication of bumble bees (Bombus spp.) on Pepino mosaic virus (PepMV) spread on tomato crops. Boletín de Sanidad Vegetal Plagas (España) 2003, 29, 393–403. [Google Scholar]
- Levitzky, N.; Smith, E.; Lachman, O.; Luria, N.; Mizrahi, Y.; Bakelman, H.; Sela, N.; Laskar, O.; Milrot, E.; Dombrovsky, A. The bumblebee Bombus terrestris carries a primary inoculum of Tomato brown rugose fruit virus contributing to disease spread in tomatoes. PLoS ONE 2019, 14, e0210871. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Mumford, R.; Blystad, D.R.; Cortez, I.; Hasiow-Jaroszewska, B.; Hristova, D.; Pagán, I.; Pereira, A.M.; Peters, J.; Pospieszny, H.; et al. Seed transmission of Pepino mosaic virus in tomato. Eur. J. Plant Pathol. 2010, 126, 145–152. [Google Scholar] [CrossRef]
- Cordoba-Selle, M.D.C.; Garcia-Randez, A.; Alfaro-Fernandez, A.; Jorda-Gutierrez, C. Seed transmission of Pepino mosaic virus and efficacy of tomato seed disinfection treatments. Plant Dis. 2007, 91, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Smith, E. Seed Transmission of Tobamoviruses: Aspects of Global Disease Distribution. Adv. Seed Biol. Tech. 2017. [Google Scholar] [CrossRef]
- EPPO. European and Mediterranean Plant Protection Organization (EPPO) Global Database; EPPO: Paris, France, 2019. [Google Scholar]
- Turco, S.; Golyaev, V.; Seguin, J.; Gilli, C.; Farinelli, L.; Boller, T.; Schumpp, O.; Pooggin, M.M. Small RNA-omics for virome reconstruction and antiviral defense characterization in mixed infections of cultivated Solanum plants. Mol. Plant-Microbe Interact. 2018, 31, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Pagán, I.; del Carmen Córdoba-Sellés, M.; Martínez-Priego, L.; Fraile, A.; Malpica, J.M.; Jordá, C.; García-Arena, F. Genetic structure of the population of Pepino mosaic virus infecting tomato crops in Spain. Phytopathology 2006, 96, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Davino, S.; Davino, M.; Bellardi, M.G.; Agosteo, G.E. Pepino mosaic virus and Tomato chlorosis virus causing mixed infection in protected tomato crops in Sicily. Phytopathol. Mediterr. 2008, 47, 35–41. [Google Scholar]
- Gómez, P.; Sempere, R.; Amari, K.; Gómez-Aix, C.; Aranda, M. Epidemics of Tomato torrado virus, Pepino mosaic virus and Tomato chlorosis virus in tomato crops: Do mixed infections contribute to torrado disease epidemiology? Ann. Appl. Biol. 2010, 156, 401–410. [Google Scholar] [CrossRef]
- Iacono, G.; Hernandez-Llopis, D.; Alfaro-Fernandez, A.; Davino, M.; Font, M.; Panno, S.; Galipenso, L.; Rubio, L.; Davino, S. First report of Southern tomato virus in tomato crops in Italy. New Dis Rep. 2015, 32, 27. [Google Scholar] [CrossRef]
- Koenig, R. Indirect ELISA Methods for the Broad Specificity Detect. Plant Viruses. J. Gen Virol. 1981, 55, 53–62. [Google Scholar] [CrossRef]
- Reingold, V.; Lachman, O.; Belausov, E.; Koren, A.; Mor, N.; Dombrovsky, A. Epidemiological study of Cucumber green mottle mosaic virus in greenhouses enables reduction of disease damage in cucurbit production. Ann. Appl. Biol. 2016, 168, 29–40. [Google Scholar] [CrossRef]

| ELISA | ToBRFV | PepMV | |||||
|---|---|---|---|---|---|---|---|
| Exp. 1 a,b (n = 24) | Exp. 2 (n = 15) | Exp. 3 (n = 15) | Exp. 4 (n = 21) | Exp. 1 a,b (n = 24) | Exp. 2 (n = 15) | Exp. 3 (n = 15) | |
| O.D. range | 0.067–2.776 | 0.265–0.936 | 0.246–0.929 | 0.121–1.214 | 0.874–3.44 | 0.844–1.856 | 0.607–1.801 |
| NR | 0.019 | 0.022–0.032 | 0.022–0.032 | 0.032–0.051 | 0.0314 | 0.006–0.021 | 0.006–0.021 |
| Infection ratio (%) | 100 | 100 | 100 | 100 | 79 | 100 | 100 |
| Treat. | ToBRFV a | PepMV b | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exp. 1 (n = 96) | Exp. 2 (n = 60) | Exp. 3 (n = 60) | Exp. 4 (n = 84) | Exp. 1 (n = 96) | Exp. 2 (n = 60) | Exp. 3 (n = 60) | ||||||||
| Infect. Rate (%) | ELISA O.D. Range | Infect. Rate (%) | ELISA O.D. Range | Infect. Rate (%) | ELISA O.D. Range | Infect. Rate (%) | ELISA O.D. Range | Infect. Rate (%) | ELISA O.D. Range | Infect. Rate (%) | ELISA O.D. Range | Infect. Rate (%) | ELISA O.D. Range | |
| A | 0 | 0.01–0.03 | 0 | 0.01–0.02 | 0 | 0.02–0.07 | 0 | 0.01–0.03 | 0 | 0.01–0.03 | 0 | 0.01–0.02 | 0 | 0.04–0.08 |
| B | 0 | 0.01–0.03 | 0 | 0.01–0.02 | 0 | 0–0.07 | 0 | 0.01–0.03 | 0 | 0.01–0.03 | 0 | 0.01–0.03 | 0 | 0.05–0.06 |
| C | 29 | 0.04–0.95 | 13 | 0.07–1.67 | 20 | 0.32–1.4 | 5 | 0.31 | 5 | 0.08 | 20 | 0.08–0.44 | 7 | 0.52 |
| D | 88 | 0.1–1.09 | 47 | 0.25–1.17 | 93 | 0.11–0.83 | 67 | 0.09–0.66 | 67 | 0.1–2.26 | 47 | 0.32–0.84 | 80 | 0.13–1.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klap, C.; Luria, N.; Smith, E.; Bakelman, E.; Belausov, E.; Laskar, O.; Lachman, O.; Gal-On, A.; Dombrovsky, A. The Potential Risk of Plant-Virus Disease Initiation by Infected Tomatoes. Plants 2020, 9, 623. https://doi.org/10.3390/plants9050623
Klap C, Luria N, Smith E, Bakelman E, Belausov E, Laskar O, Lachman O, Gal-On A, Dombrovsky A. The Potential Risk of Plant-Virus Disease Initiation by Infected Tomatoes. Plants. 2020; 9(5):623. https://doi.org/10.3390/plants9050623
Chicago/Turabian StyleKlap, Chen, Neta Luria, Elisheva Smith, Elena Bakelman, Eduard Belausov, Orly Laskar, Oded Lachman, Amit Gal-On, and Aviv Dombrovsky. 2020. "The Potential Risk of Plant-Virus Disease Initiation by Infected Tomatoes" Plants 9, no. 5: 623. https://doi.org/10.3390/plants9050623
APA StyleKlap, C., Luria, N., Smith, E., Bakelman, E., Belausov, E., Laskar, O., Lachman, O., Gal-On, A., & Dombrovsky, A. (2020). The Potential Risk of Plant-Virus Disease Initiation by Infected Tomatoes. Plants, 9(5), 623. https://doi.org/10.3390/plants9050623