Is the Tolerance of Commercial Peach Cultivars to Brown Rot Caused by Monilinia laxa Modulated by its Antioxidant Content?
Abstract
1. Introduction
2. Results
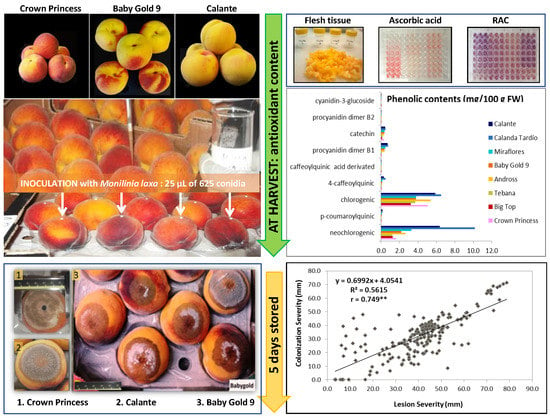
2.1. Physicochemical Parameters and Biochemical Composition
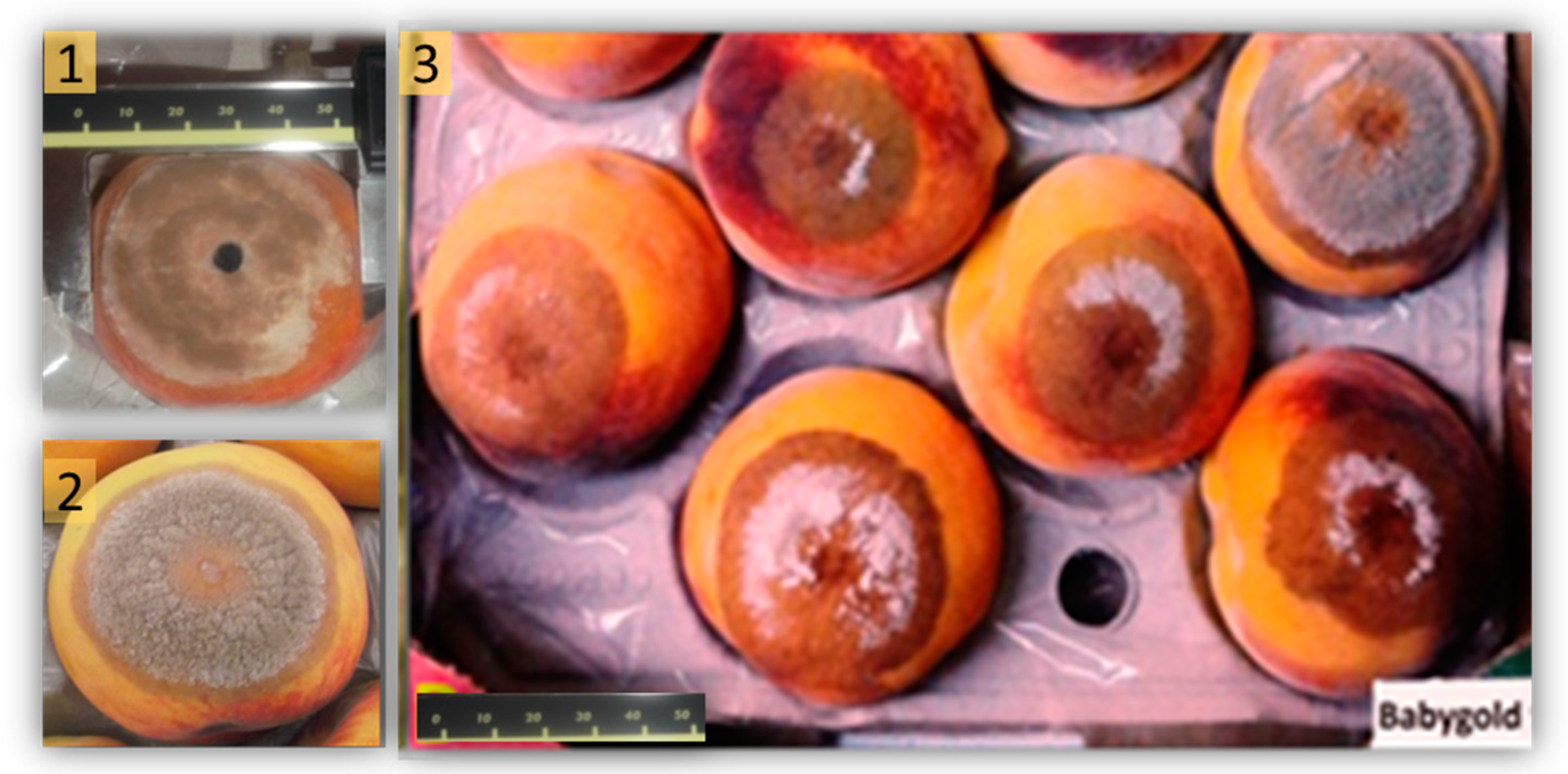
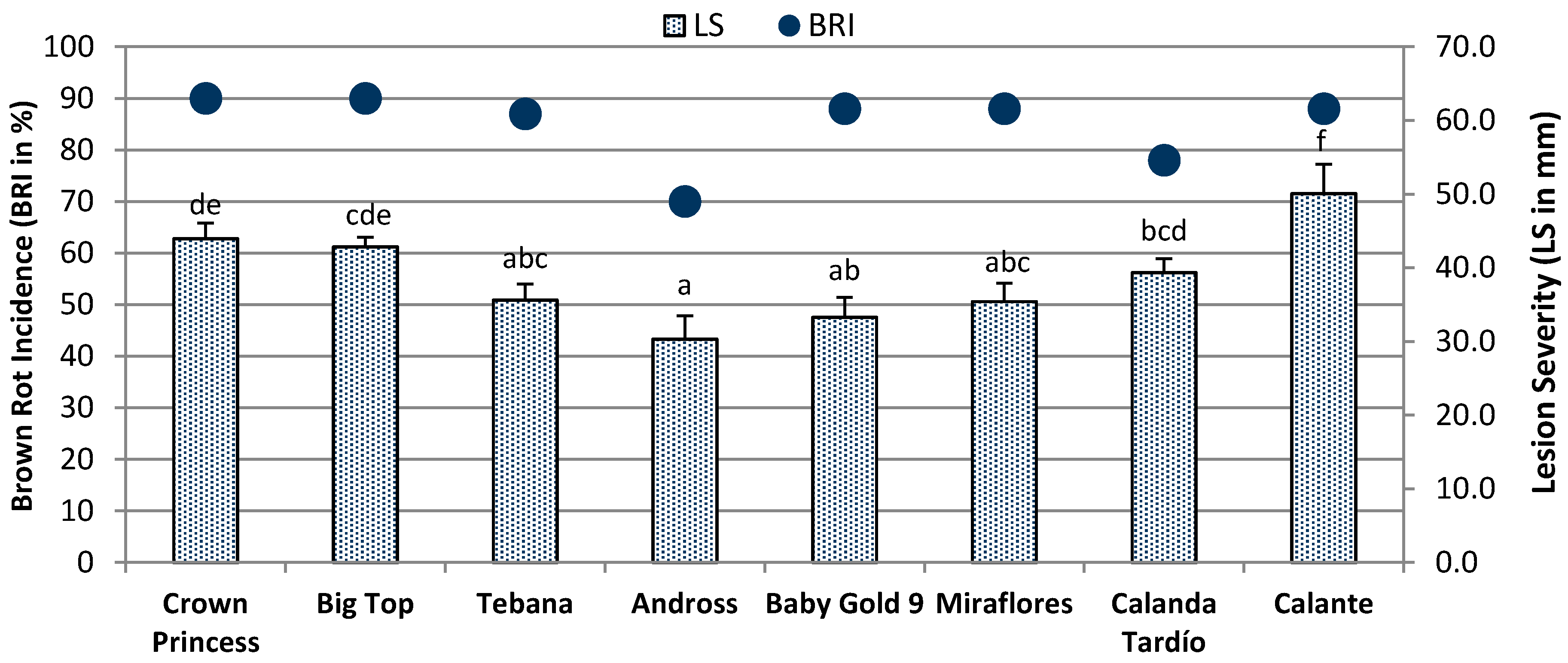
2.2. Fruit Susceptibility
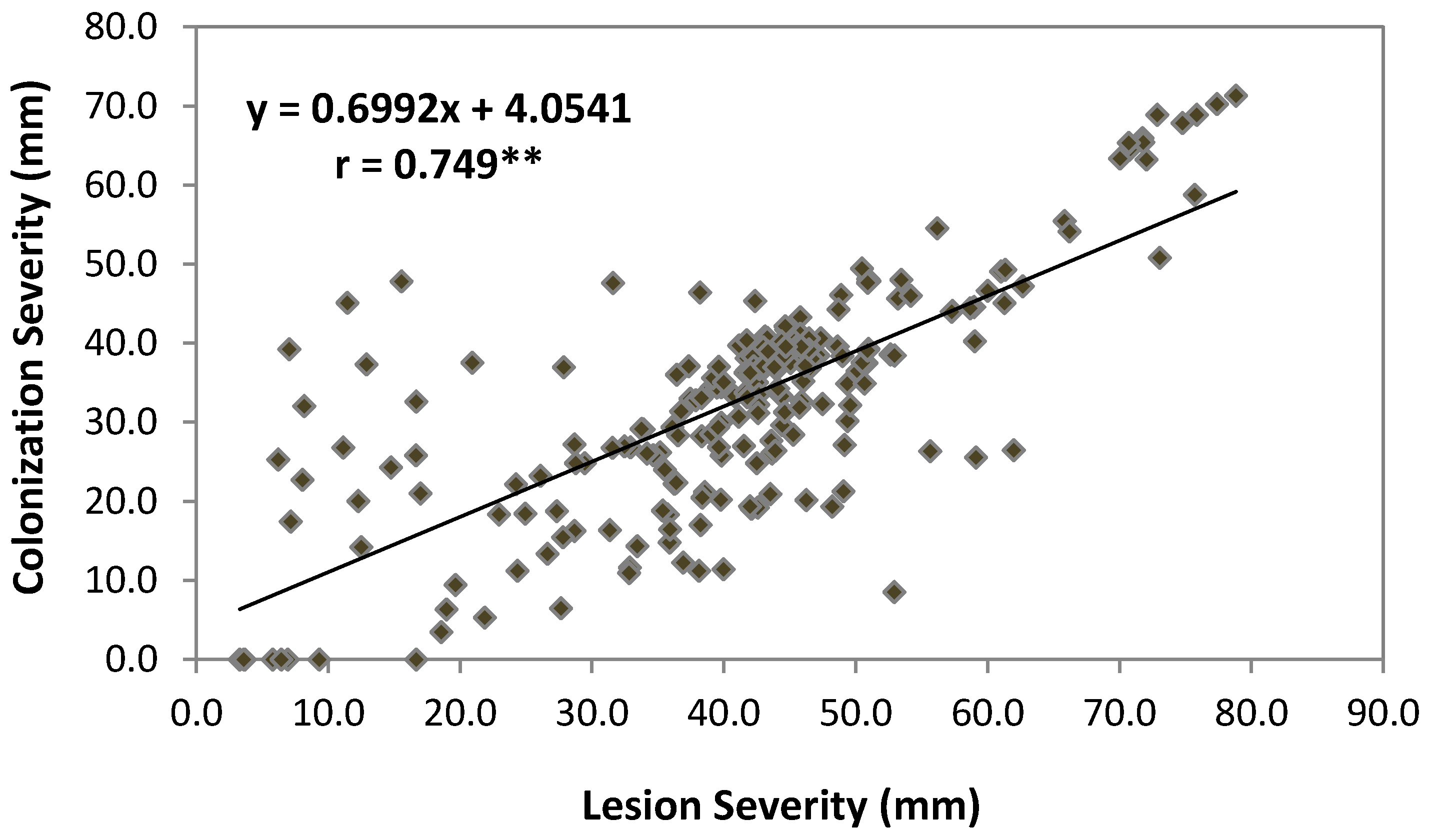
2.3. Pearson’s Correlation
3. Discussion
4. Materials and Methods
4.1. Peach Cultivars
4.2. Physicochemical and Biochemical Quality Parameters
4.3. In Vivo Assay: Inoculum, Inoculation, and Brown Rot Evaluation
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 April 2020).
- Egüen, B.; Melgarejo, P.; De Cal, A. Sensitivity of Monilinia fructicola from Spanish peach orchards to thiophanate-methyl, iprodione, and cyproconazole: Fitness analysis and competitiveness. Eur. J. Plant Pathol. 2015, 141, 789–801. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Moreno, M.A.; Giménez, R.; Gogorcena, Y. Optimizing protocols to evaluate brown rot (Monilinia laxa) susceptibility in peach and nectarine fruits. Australas. Plant Pathol. 2017, 46, 183–189. [Google Scholar] [CrossRef]
- Villarino, M.; Egüen, B.; Lamarca, N.; De Cal, A. Occurrence of Monilinia laxa and M. fructigena after introduction of M. fructicola in peach orchards in Spain. Eur. J. Plant Pathol. 2013, 137, 835–845. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Peach brown rot: Still in search of an ideal management option. Agriculture 2018, 8, 125. [Google Scholar] [CrossRef]
- Casals, C.; Teixidó, N.; Vi, I.; Cambray, J.; Usall, J. Control of Monilinia spp. on stone fruit by curing treatments. Part II: The effect of host and Monilinia spp. variables on curing efficacy. Postharvest Biol. Technol. 2010, 56, 26–30. [Google Scholar] [CrossRef]
- Villarino, M.; Larena, I.; Martínez, F.; Melgarejo, P.; De Cal, A. Analysis of genetic diversity in Monilinia fructicola from the Ebro Valley in Spain using ISSR and RAPD markers. Eur. J. Plant Pathol. 2012, 132, 511–524. [Google Scholar] [CrossRef]
- Usall, J.; Casals, C.; Sisquella, M.; Palou, L.; De Cal, A. Alternative technologies to control postharvest diseases of stone fruits. Stewart Postharvest Rev. 2015, 4, 2. [Google Scholar]
- Liu, J.; Suia, Y.; Wisniewskia, M.; Droby, S.; Tianb, S.; Norell, J.; Hershkovitz, V. Effect of heat treatment on inhibition of Monilinia fructicola and induction of disease resistance in peach fruit. Postharvest Biol. Technol. 2012, 65, 61–68. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Schnabel, G. Field strains of Monilinia fructicola resistant to both MBC and DMI fungicides isolated from stone fruit orchards in the eastern United States. Plant Dis. 2013, 97, 1063–1068. [Google Scholar] [CrossRef]
- Elmer, P.A.G.; Gaunt, R. The biological characteristics of dicarboximide resistant isolates of Monilinia fructicola from New Zealand stone orchards. Plant Pathol. 1994, 43, 130–137. [Google Scholar] [CrossRef]
- Holb, I.; Schnabel, G. Differential effect of triazoles on mycelial growth and disease measurements of Monilinia fructicola isolates with reduces sensitivity to DMI fungicides. Crop Prot. 2007, 26, 753–759. [Google Scholar] [CrossRef]
- Egüen, B.; Melgarejo, P.; De Cal, A. The effect of fungicide resistance on the structure of Monilinia laxa populations in Spanish peach and nectarine orchards. Eur. J. Plant Pathol. 2016, 145, 815–827. [Google Scholar] [CrossRef]
- Mari, M.; Di Francesco, A.; Bertolini, P. Control of fruit postharvest diseases: Old issues and innovative approaches. Stewart Postharvest Rev. 2014, 1, 1–4. [Google Scholar]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest biocontrol ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biol. Technol. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Feliciano, A.; Feliciano, A.; Ogawa, J. Monilinia fructicola resistance in the peach cultivar Bolinha. Phytopathology 1987, 77, 776–780. [Google Scholar] [CrossRef]
- Gradziel, T.M.; Wang, D. Evaluation of brown rot resistance and its relation to enzymatic browning in clingstone peach germplasm. J. Am. Soc. Hortic. Sci. 1993, 118, 675–679. [Google Scholar] [CrossRef]
- Bostock, R.; Wilcox, S.; Wang, G.; Adaskaveg, J. Suppression of Monilinia fructicola cutinase production by peach fruit surface phenolic acids. Physiol. Mol. Plant Pathol. 1999, 54, 37–50. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.; Cardinali, A. Role of phenolics in the resistance mechanism of plants against fungal pathogens and insects. In Phytochemistry: Advances in Research; Imperato, F., Ed.; Research Signpost: Kerala, India, 2006; pp. 23–67. [Google Scholar]
- Mari, M.; Casalini, L.; Baraldi, E.; Bertolini, P. Susceptibility of apricot and peach fruit to Monilinia laxa during phenological stages. Postharvest Biol. Technol. 2003, 30, 105–109. [Google Scholar] [CrossRef]
- Lee, M.; Bostock, R.M. Fruit exocarp phenols in relation to quiescence and development of Monilinia fructicola infections in Prunus spp.: A role for cellular redox? Phytopathology 2007, 97, 269–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomidis, T.; Tsipouridis, C.; Darara, V. Seasonal variation of nutrient elements in peach fruits (cv. May Crest) and its correlation with development of Brown rot (Monilinia laxa). Sci. Hortic. 2007, 111, 300–303. [Google Scholar] [CrossRef]
- Villarino, M.; Sandín-España, P.; Melgarejo, P.; De Cal, A. High chlorogenic and neochlorogenic acid levels in immature peaches reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. J. Agric. Food Chem. 2011, 59, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Guidarelli, M.; Zubini, P.; Nanni, V.; Bonghi, C.; Rasori, A.; Bertolini, P. Gene expression analysis of peach fruit at different growth stages and with different susceptibility to Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 503–513. [Google Scholar] [CrossRef]
- Oliveira Lino, L.; Pacheco, I.; Mercier, V.; Faoro, F.; Bassi, D.; Bornard, I.; Quilot-Turion, B. Brown rot strikes Prunus fruit: An ancient fight almost always lost. J. Agric. Food Chem. 2016, 64, 4029–4047. [Google Scholar] [CrossRef] [PubMed]
- Botanga, C.J.; Bethke, G.; Chen, Z.; Gallie, D.R.; Fiehn, O.; Glazebrook, J. Metabolite profiling of Arabidopsis inoculated with Alternaria brassicicola reveals that ascorbate reduces disease severity. Mol. Plant Microbe Interact. 2012, 25, 1628–1638. [Google Scholar] [CrossRef]
- Wang, S.D.; Zhu, F.; Yuan, S.; Yang, H.; Xu, F.; Shang, J.; Xu, M.Y.; Jia, S.D.; Zhang, Z.W.; Wang, J.H.; et al. The roles of ascorbic acid and glutathione in symptom alleviation to SA-deficient plants infected with RNA viruses. Planta 2011, 234, 171–181. [Google Scholar] [CrossRef]
- Romero-Romero, M.T.; López-Delgado, H.A. Ameliorative effects of hydrogen peroxide, ascorbate and dehydroascorbate in Solanum tuberosum infected by Phytoplasma. Am. J. Potato Res. 2009, 86, 218–226. [Google Scholar] [CrossRef]
- Holb, I. Brown rot blight of pome and stone fruits: Symptom, disease cycle, host resistance, and biological control. Int. J. Hortic. Sci. 2008, 14, 15–21. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Rubio, M.; Sánchez-Pérez, R. Aplicación de herramientas biotecnológicas en la mejora genética de frutales del género Prunus. ITEA 2005, 101, 319–332. [Google Scholar]
- Rungjindamai, N.; Jeffries, P. Epidemiology and management of brown rot on stone fruit caused by Monilinia laxa. Eur. J. Plant Pathol. 2014, 140, 1–17. [Google Scholar] [CrossRef]
- Martínez-García, P.J.; Parfitt, D.; Bostock, R.; Fresnedo-Ramírez, J.; Vazquez-Lobo, A.; Ogundiwin, E.A.; Gradziel, T.M.; Crisosto, C.H. Application of genomic and quantitative genetic tools to identify candidateresistance genes for brown rot resistance in peach. PLoS ONE 2013, 8, e78634. [Google Scholar] [CrossRef]
- Pacheco, I.; Bassi, D.; Eduardo, I.; Ciacciulli, A.; Pirona, R.; Rossini, L. QTL mapping for brown rot (Monilinia fructigena) resistance in an intraspecific peach (Prunus persica L. Batsch) F1 progeny. Tree Genet. Genomes 2014, 10, 1223–1242. [Google Scholar] [CrossRef]
- Obi, V.I.; Barriuso, J.J.; Gogorcena, Y. Effects of pH and titratable acidity on the growth and development of Monilinia laxa (Aderh. & Ruhl.) in vitro and in vivo. Eur. J. Plant Pathol. 2018, 151, 781–790. [Google Scholar]
- Bostock, R. Brown Rot Disease Assay and Phenotypic Data Collection. Available online: https://www.rosbreed.org/node/679 (accessed on 20 April 2020).
- Dirlewanger, E.; Moing, A.; Rothan, C.; Svanella, L.; Pronier, V.; Guye, A.; Plomion, C.; Monet, R. Mapping QTLs controlling fruit quality in peach (Prunus persica (L.) Batsch). Theor. Appl. Genet. 1999, 98, 18–31. [Google Scholar] [CrossRef]
- Abidi, W.; Cantín, C.; Jiménez, S.; Giménez, R.; Moreno, M.; Gogorcena, Y. Influence of antioxidant compounds, total sugars and genetic background on the chilling injury susceptibility of a non-melting peach (Prunus persica (L.) Batsch) progeny. J. Sci. Food Agric. 2015, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Font i Forcada, C.; Oraguzie, N.; Igartua, E.; Moreno, M.A.; Gogorcena, Y. Population structure and marker-trait associations for pomological traits in peach and nectarine cultivars. Tree Genet. Genomes 2013, 9, 331–349. [Google Scholar] [CrossRef]
- Saidani, F.; Giménez, R.; Aubert, C.; Chalot, G.; Betrán, J.A.; Gogorcena, Y. Phenolic, sugar and acid profiles and the antioxidant composition in the peel and pulp of peach fruits. J. Food Compos. Anal. 2017, 62, 126–133. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverria, G. Differential effect of cultivar and harvest date on nectarine colour, quality and consumer acceptance. Sci. Hortic. 2009, 26, 753–759. [Google Scholar] [CrossRef]
- Montevecchi, G.; Vasile Simone, G.; Masino, F.; Bignami, C.; Antonelli, A. Physical and chemical characterization of Pescabivona, a Sicilian white flesh peach cultivar [Prunus persica (L.) Batsch]. Food Res. Int. 2012, 45, 123–131. [Google Scholar] [CrossRef]
- Aubert, C.; Bony, P.; Chalot, G.; Landry, P.; Lurol, S. Effects of storage temperature, storage duration, and subsequent ripening on the physicochemical characteristics, volatile compounds, and phytochemicals of western red nectarine (Prunus persica L. Batsch). J. Agric. Food Chem. 2014, 62, 4707–4724. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Gil, M.I.; Cremin, P.; Waterhouse, A.L.; Hess-Pierce, B.; Kader, A.A. HPLC–DAD–ESIMS analysis of phenolic compounds in nectarines, peaches, and plums. J. Agric. Food Chem. 2001, 49, 4748–4760. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, W.; Yin, X.; Su, M.; Sun, C.; Li, X.; Chen, K. Phenolic composition and antioxidant properties of different peach [Prunus persica (L.) Batsch] cultivars in China. Int. J. Mol. Sci. 2015, 16, 5762–5778. [Google Scholar] [CrossRef] [PubMed]
- Saidani, F. Peach Fruit Quality: Nutritional and Sensory Aspects. Master’s Thesis, IAMZ-UdL, Zaragoza, Spain, 2016; 102p. [Google Scholar]
- Obi, V.I.; Barriuso, J.J.; Usall, J.; Gogorcena, Y. Breeding strategies for identifying superior peach genotypes resistant to brown rot. Sci. Hortic. 2019, 246, 1028–1036. [Google Scholar] [CrossRef]
- Wang, G.; Michailides, T.; Hammock, B.; Lee, Y.; Bostock, R. Molecular cloning, characterization and expression of a redox-responsive cutinase from Monilinia fructicola (Wint.) honey. Fungal Genet. Biol. 2002, 35, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Venkidasamy, B.; Upadhyaya, C.P.; Packiaraj, G.; Rajakumar, G.; Thiruvengadam, M. Alleviation of Phytophthora infestans mediated necrotic stress in the transgenic potato (Solanum tuberosum L.) with enhanced ascorbic acid accumulation. Plants 2019, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Okamura, M. An improved method for determination of L-ascorbic acid and L-dehydroascorbic acid in blood plasma. Clin. Chim. Acta 1980, 103, 259–268. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
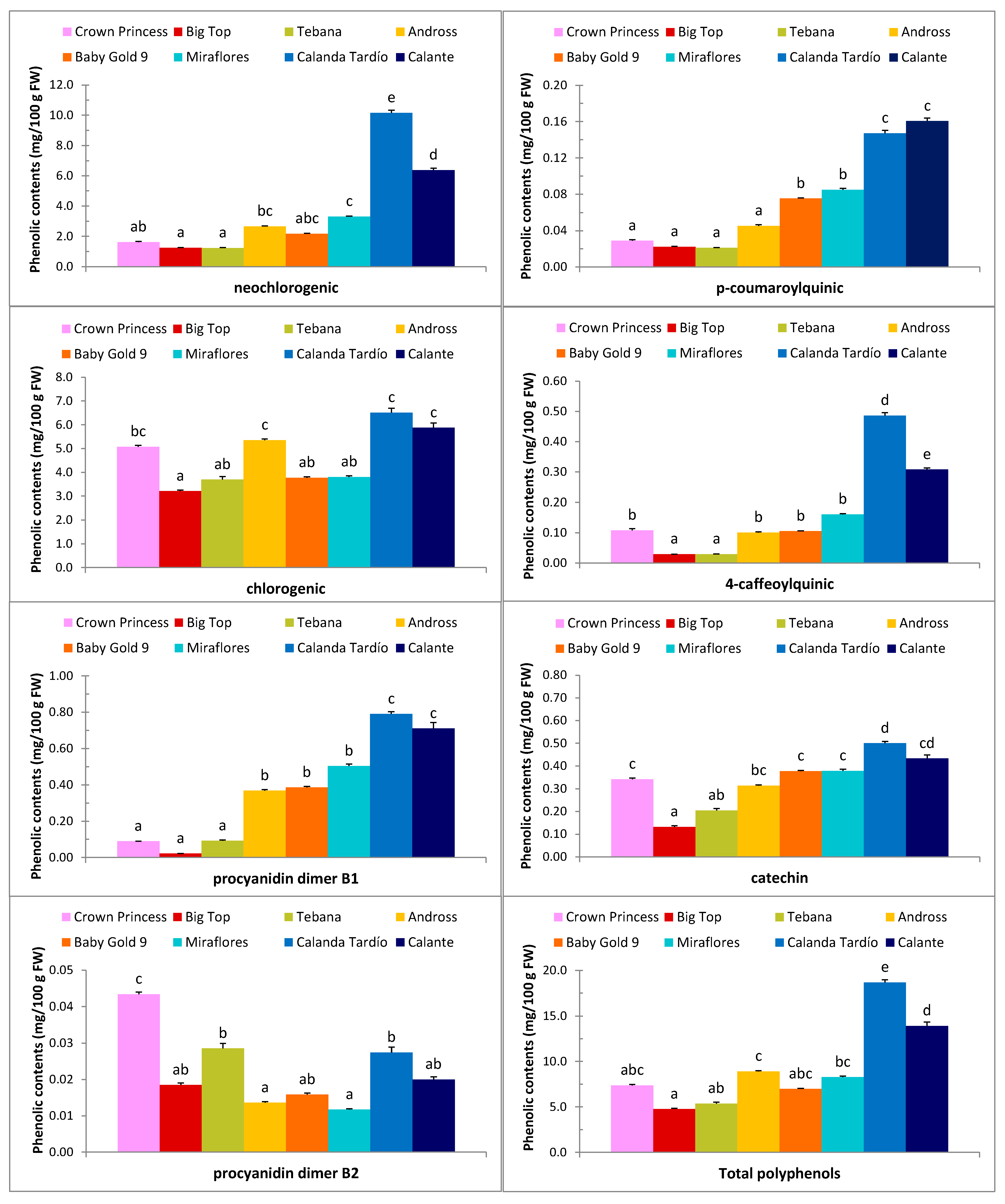
| Cultivars | Origin | Harvest Date | pH | TA | FF1 | SSC1 | AsA* | RAC* | NCGA* | CGA* | TPP* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (N) | (°Brix) | ||||||||||
| Crown Princess | USA | 19-June a | 3.82 a | 0.56 b | 26.17 a | 10.85 a | 5.18 a | 54.60 bc | 1.63 ab | 5.08 bc | 7.37 abc |
| Big Top | USA | 03-July b | 4.15 b | 0.42 a | 31.95 b | 12.25 ab | 4.09 a | 20.01 a | 1.25 a | 3.21 a | 4.77 a |
| Tebana | Italy | 03-July b | 4.12 b | 0.43 a | 33.04 bc | 10.58 a | 9.11 bc | 38.43 ab | 1.24 a | 3.70 ab | 5.37 ab |
| Andross | USA | 02-Aug c | 4.31 b | 0.35 a | 31.56 b | 14.15 bc | 14.91 d | 88.08 d | 2.66 bc | 5.36 c | 8.91 c |
| Baby Gold 9 | USA | 21-Aug d | 4.17 b | 0.41 a | 36.41 b | 13.68 bc | 8.75 b | 75.36 cd | 2.17 abc | 3.77 ab | 6.99 abc |
| Miraflores | Spain | 10-Sep e | 3.79 a | 0.61 b | 35.49 bc | 13.43 bc | 10.88 c | 89.82 d | 3.31 c | 3.80 ab | 8.28 bc |
| Calanda Tardío | Spain | 07-Oct f | 3.68 a | 0.75 c | 59.84 e | 15.53 c | 8.89 b | 186.45 e | 10.16 e | 6.52 c | 18.67 e |
| Calante | Spain | 07-Oct f | 3.65 a | 0.72 c | 46.18 d | 14.55 bc | 10.72 bc | 168.63 e | 6.37 d | 5.88 c | 13.92 d |
| Trait | LS | %C | CS | AsA | RAC | NCGA | CGA | TPP |
|---|---|---|---|---|---|---|---|---|
| %BRI | 0.471** | 0.832** | 0.682** | −0.537** | ns | ns | ns | ns |
| LS | 0.597** | 0.749** | −0.579** | ns | ns | ns | ns | |
| %C | 0.830** | −0.652** | ns | ns | ns | ns | ||
| CS | −0.590** | ns | ns | ns | ns | |||
| AsA | ns | ns | ns | ns | ||||
| RAC | 0.876** | 0.704** | 0.967** | |||||
| NCGA | 0.707** | 0.970** | ||||||
| CGA | 0.861** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obi, V.I.; Montenegro, J.; Barriuso, J.J.; Saidani, F.; Aubert, C.; Gogorcena, Y. Is the Tolerance of Commercial Peach Cultivars to Brown Rot Caused by Monilinia laxa Modulated by its Antioxidant Content? Plants 2020, 9, 589. https://doi.org/10.3390/plants9050589
Obi VI, Montenegro J, Barriuso JJ, Saidani F, Aubert C, Gogorcena Y. Is the Tolerance of Commercial Peach Cultivars to Brown Rot Caused by Monilinia laxa Modulated by its Antioxidant Content? Plants. 2020; 9(5):589. https://doi.org/10.3390/plants9050589
Chicago/Turabian StyleObi, Vitus I., Joaquín Montenegro, Juan J. Barriuso, Fayza Saidani, Christophe Aubert, and Yolanda Gogorcena. 2020. "Is the Tolerance of Commercial Peach Cultivars to Brown Rot Caused by Monilinia laxa Modulated by its Antioxidant Content?" Plants 9, no. 5: 589. https://doi.org/10.3390/plants9050589
APA StyleObi, V. I., Montenegro, J., Barriuso, J. J., Saidani, F., Aubert, C., & Gogorcena, Y. (2020). Is the Tolerance of Commercial Peach Cultivars to Brown Rot Caused by Monilinia laxa Modulated by its Antioxidant Content? Plants, 9(5), 589. https://doi.org/10.3390/plants9050589