Insights into the SAM Synthetase Gene Family and Its Roles in Tomato Seedlings under Abiotic Stresses and Hormone Treatments
Abstract
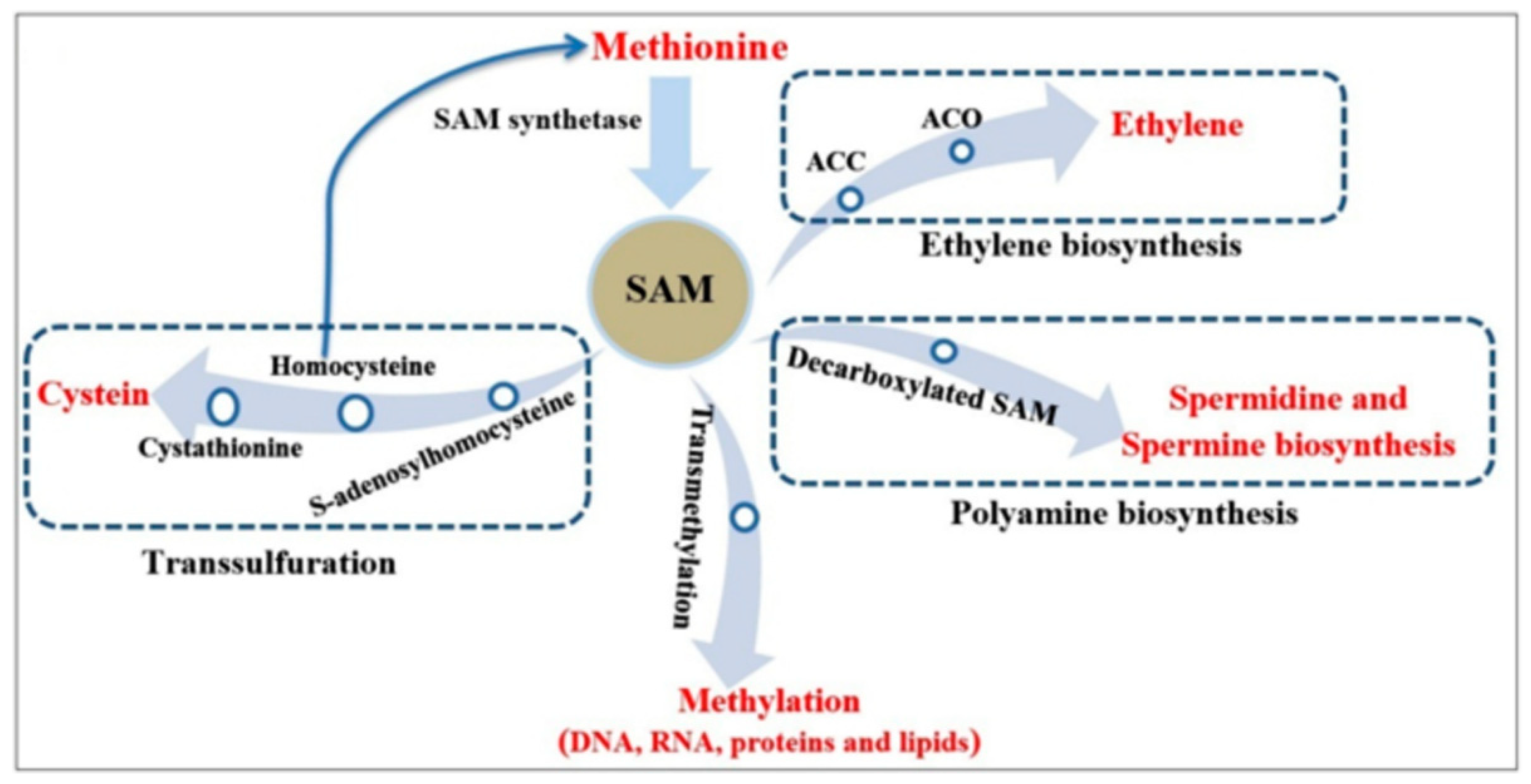
1. Introduction
2. Results
2.1. Physiochemical Properties of SAMS Genes
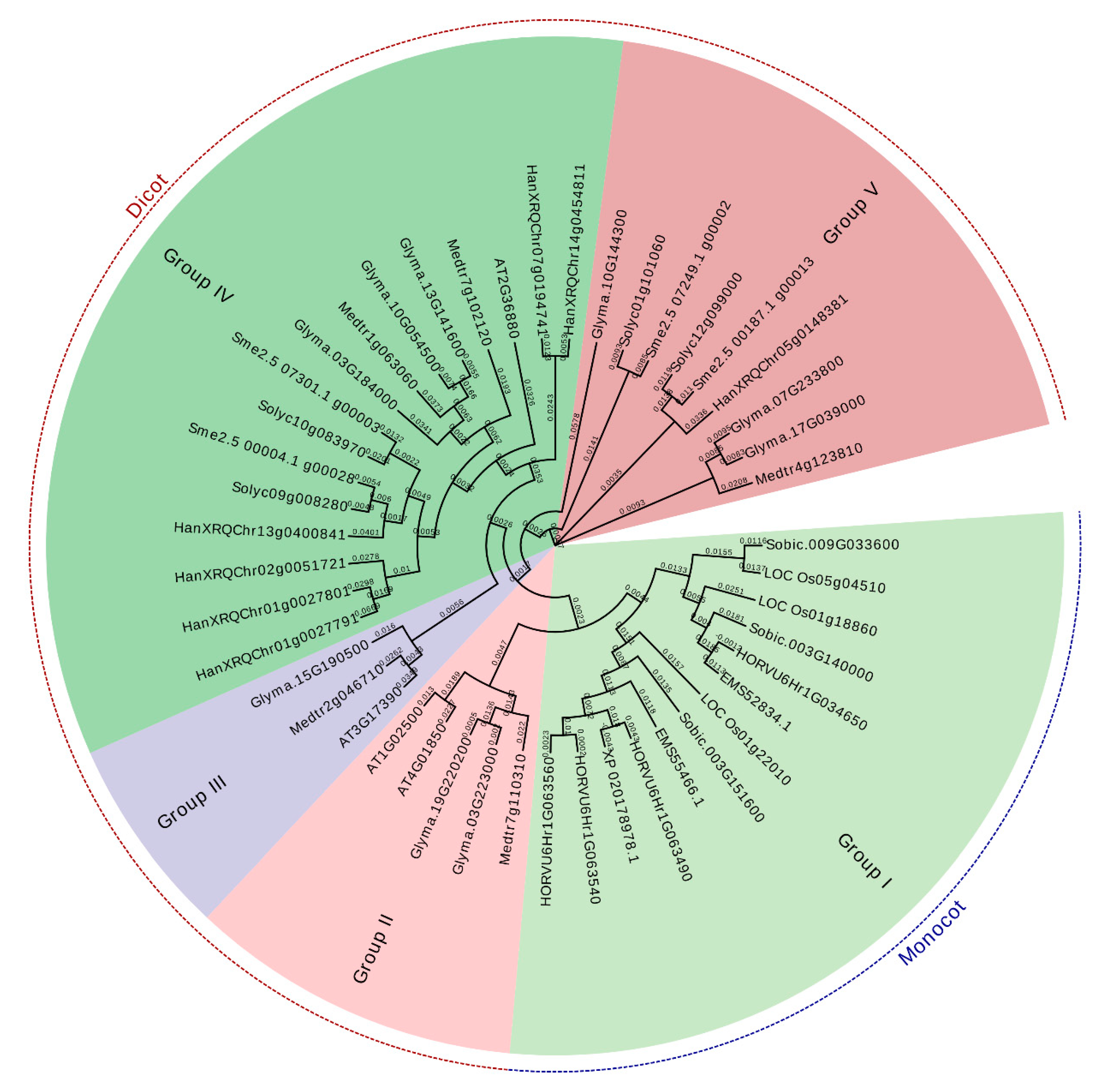
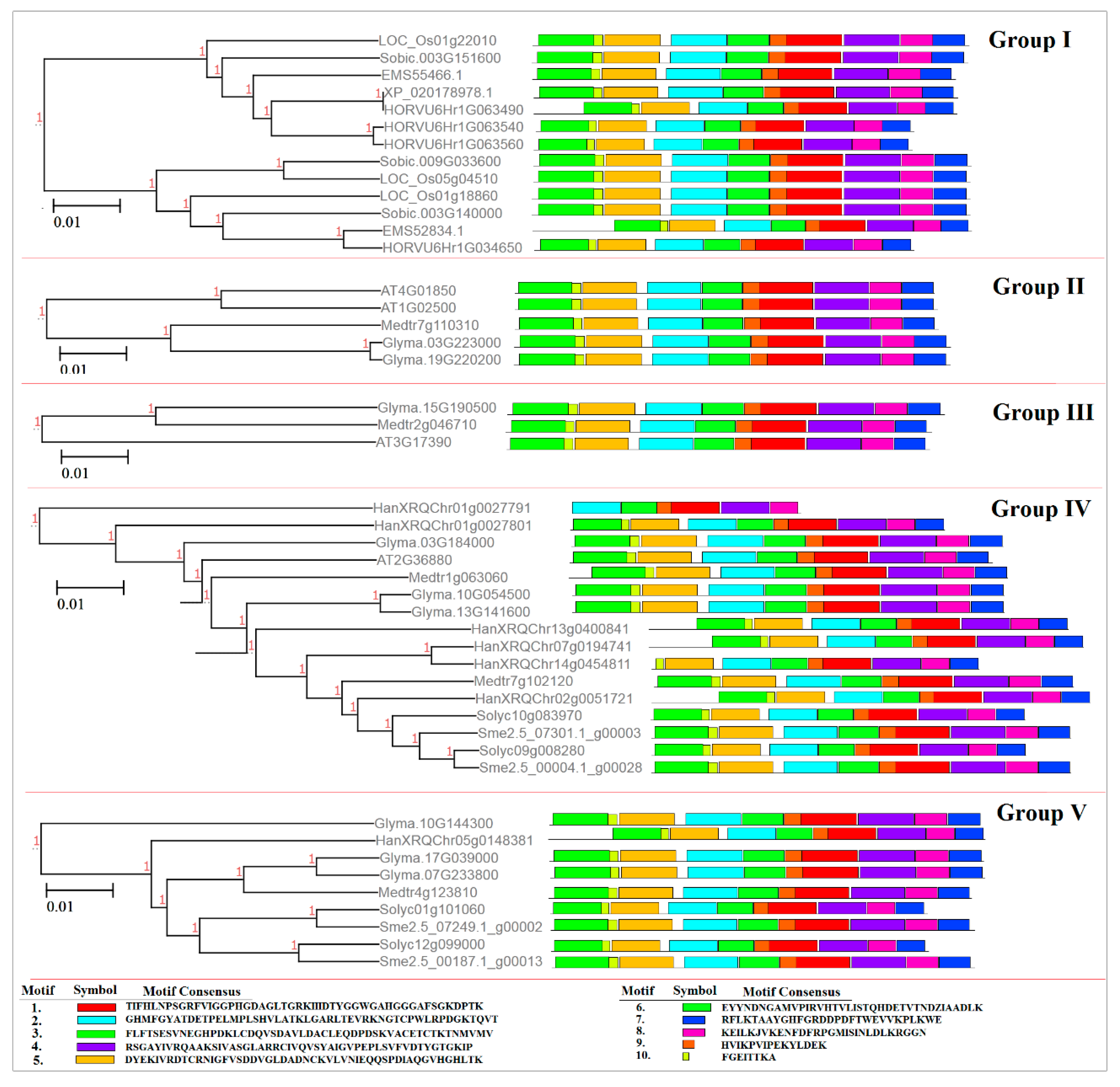
2.2. Phylogenetic and Conserved Motif Analyses of the SAMS Gene Family
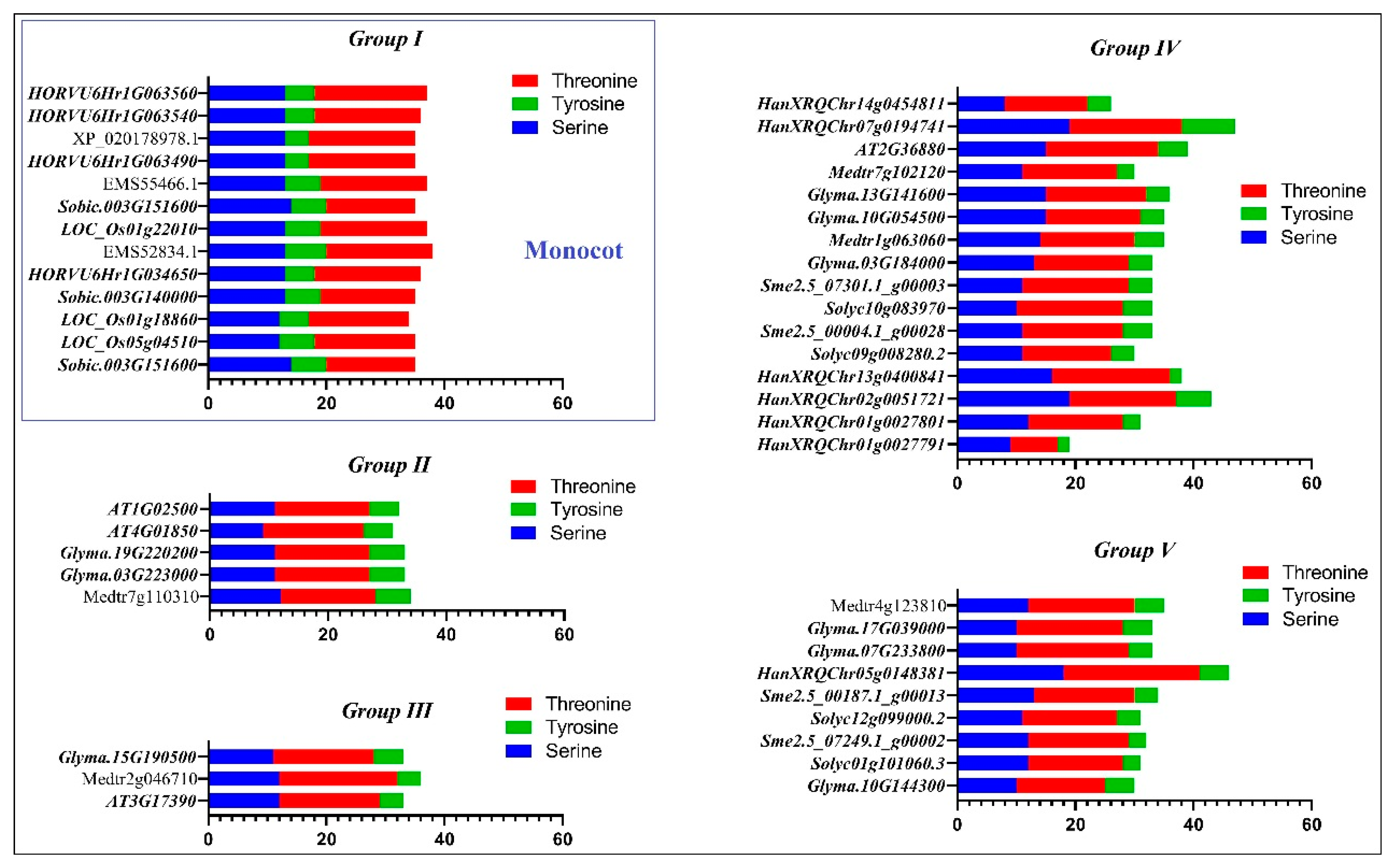
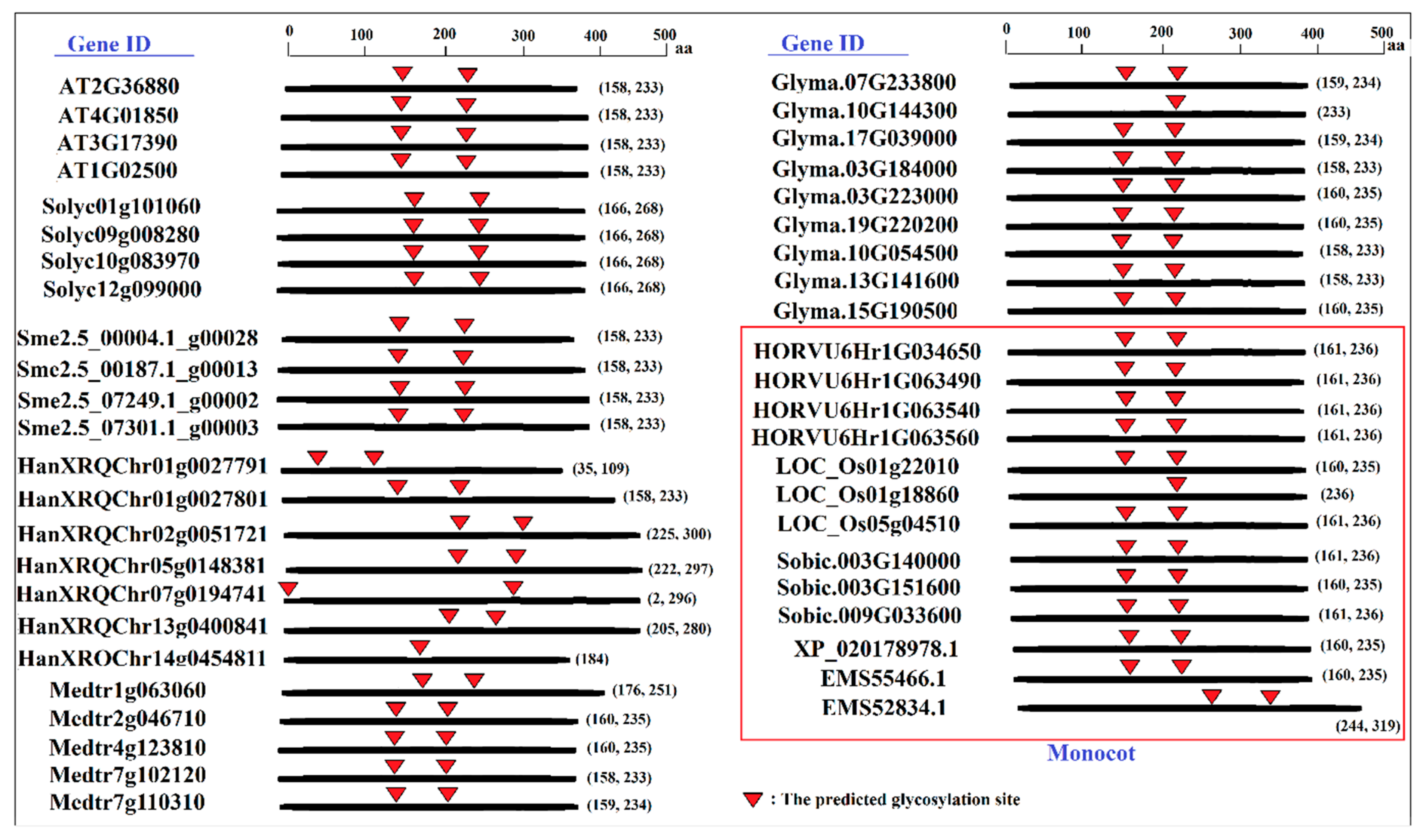
2.3. Prediction of Phosphorylation and Glycosylation Sites
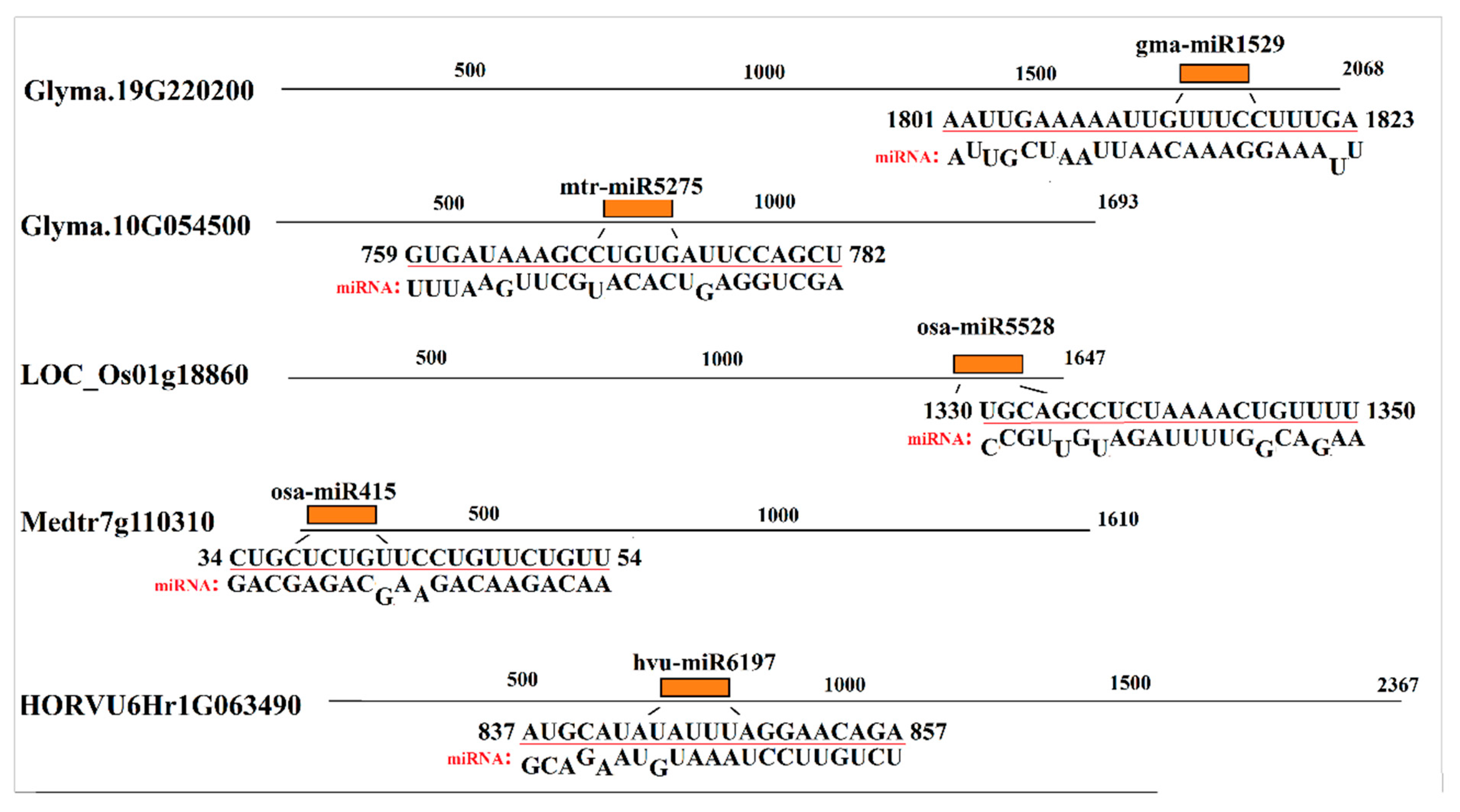
2.4. Prediction of MicroRNA Target Sites
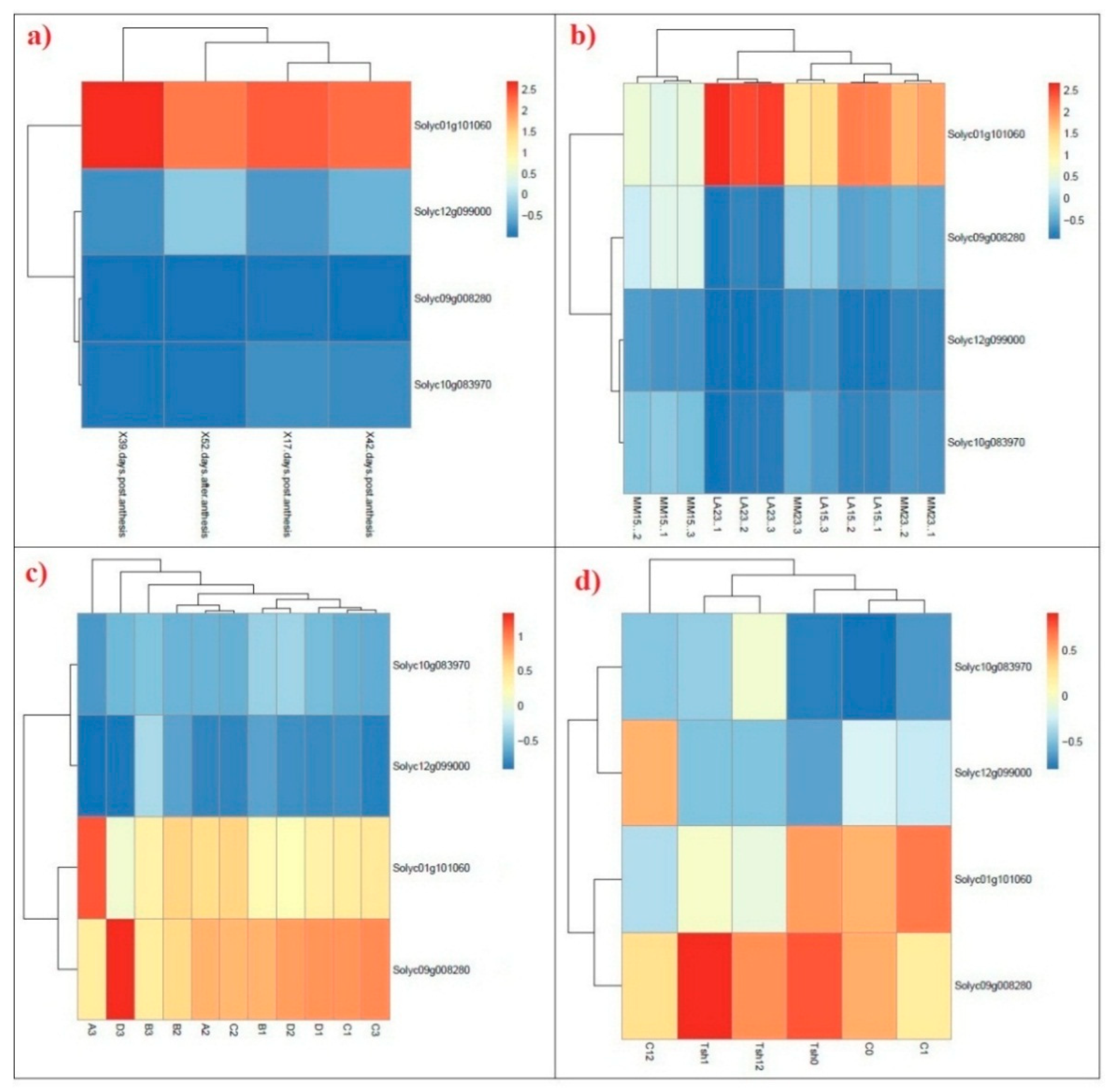
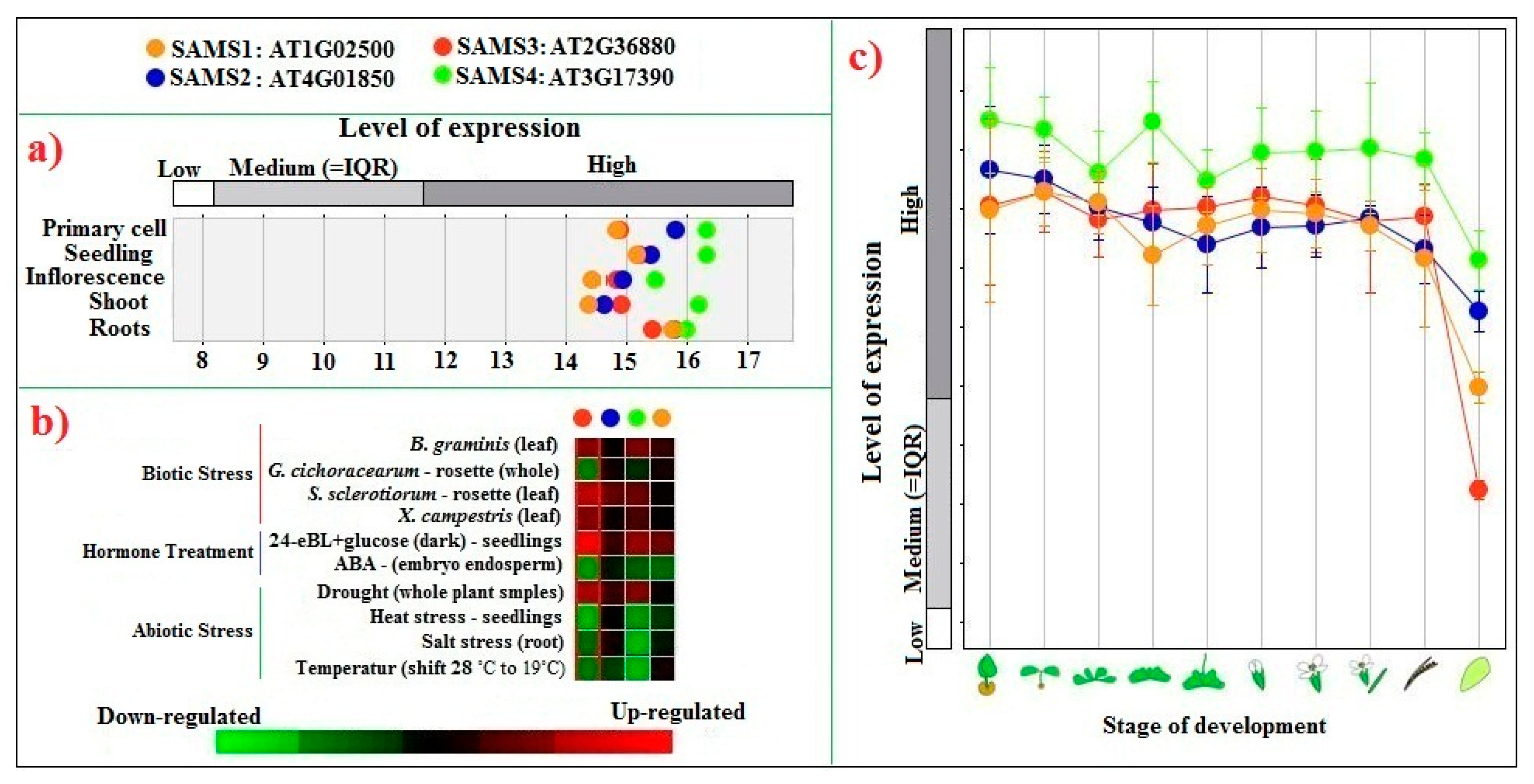
2.5. Transcriptional Profiling of SAMS Genes in Tomato
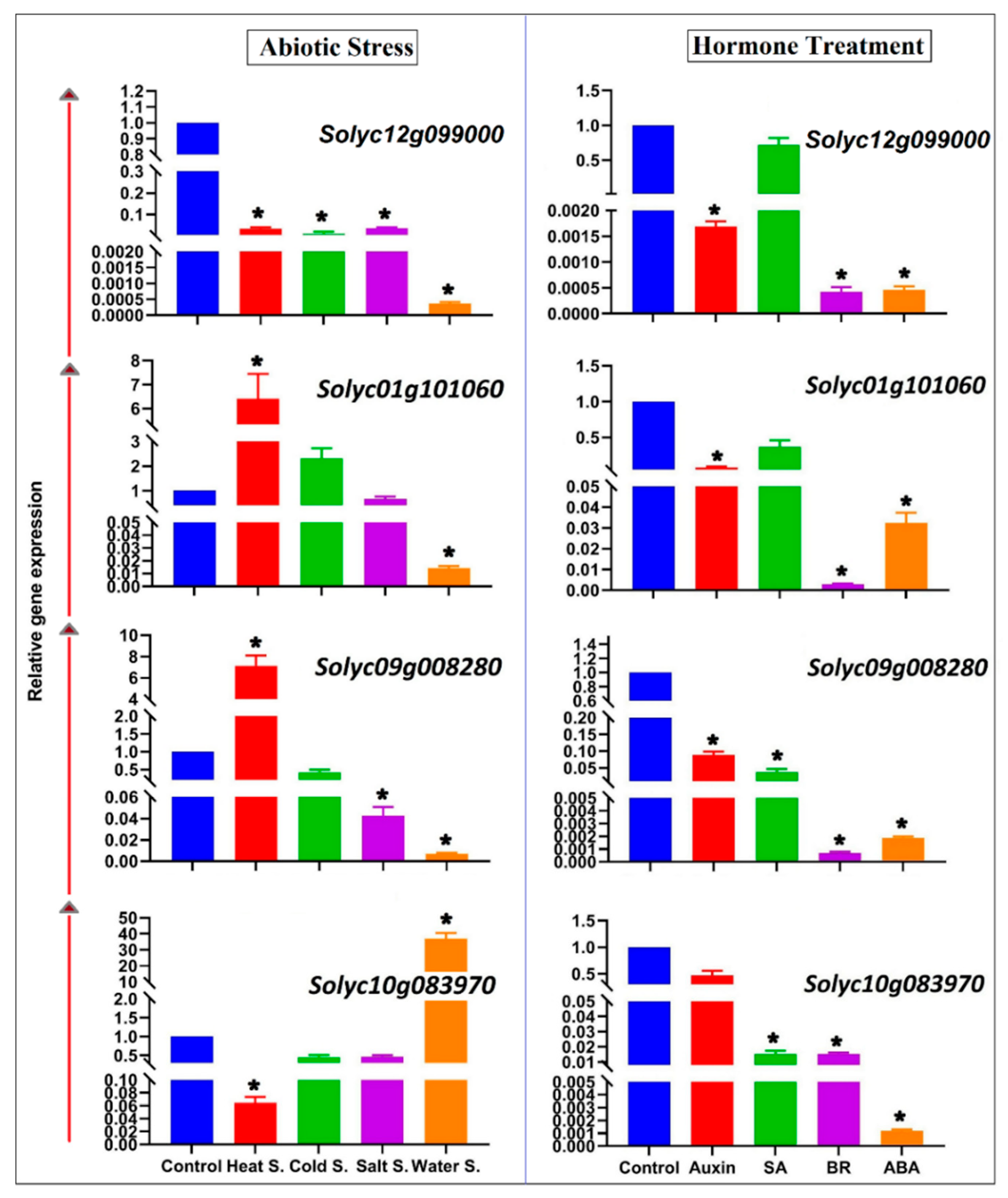
2.6. Expression Patterns of Tomato SAMS Genes Determined via qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Identification and Sequence Analysis of SAMS Genes
4.2. Evolutionary Analysis
4.3. Conserved Motif Recognition
4.4. Prediction of Post-Translational and Post-Transcriptional Modification Sites
4.5. Expression Profile Analysis Using RNA-Seq and Microarray Data
4.6. Plant Growth and Treatments
4.7. RNA Extraction and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, S.C. S-adenosylmethionine. Int. J. Biochem. Cell Biol. 2000, 32, 391–395. [Google Scholar] [CrossRef]
- Martínez-López, N.; Varela-Rey, M.; Ariz, U.; Embade, N.; Vazquez-Chantada, M.; Fernandez-Ramos, D.; Gomez-Santos, L.; Lu, S.C.; Mato, J.M.; Martinez-Chantar, M.L. S-adenosylmethionine and proliferation: New pathways, new targets. Biochem. Soc. Trans. 2008, 36 Pt 5, 848–852. [Google Scholar] [CrossRef]
- Fontecave, M.; Atta, M.; Mulliez, E. S-adenosylmethionine: Nothing goes to waste. Trends Biochem. Sci. 2004, 29, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zou, T.; McCormick, S. S-adenosylmethionine synthetase 3 is important for pollen tube growth. Plant Physiol. 2016, 172, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Sauter, M.; Moffatt, B.; Saechao, M.C.; Hell, R.; Wirtz, M. Methionine salvage and S-adenosylmethionine: Essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 2013, 451, 145–154. [Google Scholar] [CrossRef]
- Wi, S.J.; Kim, W.T.; Park, K.Y. Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 2006, 25, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Li, C.; Tarczynski, M.C. High free-methionine and decreased lignin content result from a mutation in the Arabidopsis S-adenosyl-L-methionine synthetase 3 gene. Plant J. 2002, 29, 371–380. [Google Scholar] [CrossRef]
- Bauerle, M.R.; Schwalm, E.L.; Booker, S.J. Mechanistic diversity of radical S-adenosylmethionine (SAM)-dependent methylation. J. Biol. Chem. 2015, 290, 3995–4002. [Google Scholar] [CrossRef]
- Lin, S.; Shi, Q.; Nix, F.B.; Styblo, M.; Beck, M.A.; Herbin-Davis, K.M.; Hall, L.L.; Simeonsson, J.B.; Thomas, D.J. A NovelS-adenosyl-L-methionine: Arsenic (III) methyltransferase from rat liver cytosol. J. Biol. Chem. 2002, 277, 10795–10803. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef]
- Lee, H.-O.; Wang, L.; Kuo, Y.-M.; Andrews, A.J.; Gupta, S.; Kruger, W.D. S-adenosylhomocysteine hydrolase over-expression does not alter S-adenosylmethionine or S-adenosylhomocysteine levels in CBS deficient mice. Mol. Genet. Metab. Rep. 2018, 15, 15–21. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, Y.; Chen, Y.; Feng, L.; Nie, Z. Homocysteine level and risk of different stroke types: A meta-analysis of prospective observational studies. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ma, F.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Huang, G. Associations between Alzheimer’s disease and blood homocysteine, vitamin B12, and folate: A case-control study. Curr. Alzheimer Res. 2015, 12, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Marco, F.; Cuevas, J.C.; Patron, M.; Ferrando, A.; Carrasco, P.; Tiburcio, A.F.; Altabella, T. Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 2006, 28, 1867–1876. [Google Scholar] [CrossRef]
- Harpaz-Saad, S.; Yoon, G.M.; Mattoo, A.K.; Kieber, J.J. The formation of ACC and competition between polyamines and ethylene for SAM. In Annual Plant Reviews Volume 44; Wiley-Blackwell: Oxford, UK, 2012; pp. 53–81. [Google Scholar]
- Bleecker, A.B.; Kende, H. Ethylene: A gaseous signal molecule in plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Fang, J.; Guo, Z.; Lu, S. Down-regulation of S-adenosylmethionine decarboxylase genes results in reduced plant length, pollen viability, and abiotic stress tolerance. Plant Cell Tissue Organ Cult. 2014, 116, 311–322. [Google Scholar] [CrossRef]
- Wi, S.J.; Kim, S.J.; Kim, W.T.; Park, K.Y. Constitutive S-adenosylmethionine decarboxylase gene expression increases drought tolerance through inhibition of reactive oxygen species accumulation in Arabidopsis. Planta 2014, 239, 979–988. [Google Scholar] [CrossRef]
- Roy, M.; Wu, R. Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci. 2002, 163, 987–992. [Google Scholar] [CrossRef]
- Hazarika, P.; Rajam, M.V. Biotic and abiotic stress tolerance in transgenic tomatoes by constitutive expression of S-adenosylmethionine decarboxylase gene. Physiol. Mol. Biol. Plants 2011, 17, 115–128. [Google Scholar] [CrossRef]
- Espartero, J.; Pintor-Toro, J.A.; Pardo, J.M. Differential accumulation of S-adenosylmethionine synthetase transcripts in response to salt stress. Plant Mol. Biol. 1994, 25, 217–227. [Google Scholar] [CrossRef]
- Li, X.-D.; Xia, B.; Wang, R.; Xu, S.; Jiang, Y.-M.; Yu, F.-B.; Peng, F. Molecular cloning and characterization of S-adenosylmethionine synthetase gene from Lycoris radiata. Mol. Biol. Rep. 2013, 40, 1255–1263. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Yu, F.; Li, J.; Van de Poel, B.; Tan, D.A.N.; Li, J.; Liu, Y.; Li, X.; Dong, M.; Chen, L. FERONIA receptor kinase interacts with S-adenosylmethionine synthetase and suppresses S-adenosylmethionine production and ethylene biosynthesis in A rabidopsis. Plant Cell Environ. 2015, 38, 2566–2574. [Google Scholar] [CrossRef] [PubMed]
- Erb, T.J.; Evans, B.S.; Cho, K.; Warlick, B.P.; Sriram, J.; Wood, B.M.; Imker, H.J.; Sweedler, J.V.; Tabita, F.R.; Gerlt, J.A. A RubisCO-like protein links SAM metabolism with isoprenoid biosynthesis. Nat. Chem. Biol. 2012, 8, 926. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Wang, M.-M.; Yin, Y.-L.; Onac, E.; Zhou, G.-F.; Peng, S.; Xia, X.-J.; Shi, K.; Yu, J.-Q.; Zhou, Y.-H. RNA-seq analysis reveals the role of red light in resistance against Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC Genom. 2015, 16, 120. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Chen, D.; Li, J.; Zhang, Y.; Wang, A. A comparison of the low temperature transcriptomes of two tomato genotypes that differ in freezing tolerance: Solanum lycopersicum and Solanum habrochaites. BMC Plant Biol. 2015, 15, 132. [Google Scholar] [CrossRef]
- Heidari, P.; Ahmadizadeh, M.; Izanlo, F.; Nussbaumer, T. In silico study of the CESA and CSL gene family in Arabidopsis thaliana and Oryza sativa: Focus on post-translation modifications. Plant Gene 2019, 19, 100189. [Google Scholar] [CrossRef]
- Bachmair, A.; Finley, D.; Varshavsky, A. In vivo half-life of a protein is a function of its amino-terminal residue. Science 1986, 234, 179–186. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Ravanel, S.; Block, M.A.; Rippert, P.; Jabrin, S.; Curien, G.; Rébeillé, F.; Douce, R. Methionine metabolism in plants chloroplasts are autonomous for de novo methionine synthesis and can import s-adenosylmethionine from the cytosol. J. Biol. Chem. 2004, 279, 22548–22557. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy. Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Friso, G.; van Wijk, K.J. Update: Post-translational protein modifications in plant metabolism. Plant Physiol. 2015, 169, 1469–1487. [Google Scholar] [CrossRef] [PubMed]
- Silva-Sanchez, C.; Li, H.; Chen, S. Recent advances and challenges in plant phosphoproteomics. Proteomics 2015, 15, 1127–1141. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.N.; Shad Ali, G. Plant serine/arginine-rich proteins: Roles in precursor messenger RNA splicing, plant development, and stress responses. Wiley Interdiscip. Rev. RNA 2011, 2, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Solá, R.J.; Griebenow, K. Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 2009, 98, 1223–1245. [Google Scholar] [CrossRef] [PubMed]
- Schoberer, J.; Shin, Y.-J.; Vavra, U.; Veit, C.; Strasser, R. Analysis of Protein Glycosylation in the ER. In The Plant Endoplasmic Reticulum; Springer: Berlin, Germany, 2018; pp. 205–222. [Google Scholar]
- Ding, J.; Zhou, S.; Guan, J. Finding microRNA targets in plants: Current status and perspectives. Genom. Proteom. Bioinform. 2012, 10, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lei, J.; Ding, L.; Wen, Y.; Ju, H.; Zhang, X. MicroRNA: Function, detection, and bioanalysis. Chem. Rev. 2013, 113, 6207–6233. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, S.; Liu, D.; Duan, Y.; Dong, W. Identification of soybean microRNAs involved in soybean cyst nematode infection by deep sequencing. PLoS ONE 2012, 7, e39650. [Google Scholar] [CrossRef]
- Dong, Z.; Shi, L.; Wang, Y.; Chen, L.; Cai, Z.; Wang, Y.; Jin, J.; Li, X. Identification and dynamic regulation of microRNAs involved in salt stress responses in functional soybean nodules by high-throughput sequencing. Int. J. Mol. Sci. 2013, 14, 2717–2738. [Google Scholar] [CrossRef]
- Devers, E.A.; Branscheid, A.; May, P.; Krajinski, F. Stars and symbiosis: Microrna-and microRNA*-mediated transcript cleavage involved in arbuscular mycorrhizal symbiosis. Plant Physiol. 2011, 156, 1990–2010. [Google Scholar] [CrossRef]
- Cheah, B.H.; Jadhao, S.; Vasudevan, M.; Wickneswari, R.; Nadarajah, K. Identification of functionally important microRNAs from rice inflorescence at heading stage of a qDTY4. 1-QTL bearing Near Isogenic Line under drought conditions. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Guo, W.; Wu, G.; Yan, F.; Lu, Y.; Zheng, H.; Lin, L.; Chen, H.; Chen, J. Identification of novel Oryza sativa miRNAs in deep sequencing-based small RNA libraries of rice infected with Rice stripe virus. PLoS ONE 2012, 7, e46443. [Google Scholar] [CrossRef]
- Unver, T.; Tombuloglu, H. Barley long non-coding RNAs (lncRNA) responsive to excess boron. Genomics 2020, 112, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Hruz, T.; Laule, O.; Szabo, G.; Wessendorp, F.; Bleuler, S.; Oertle, L.; Widmayer, P.; Gruissem, W.; Zimmermann, P. Genevestigator v3: A reference expression database for the meta-analysis of transcriptomes. Adv. Bioinform. 2008, 2008, 420747. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Kumar, G.B.S.; Khan, M.; Doohan, F.M. Brassinosteroid enhances resistance to fusarium diseases of barley. Phytopathology 2013, 103, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Srivastava, S.; Shasany, A.K.; Sharma, A. Identification of miRNAs and their targets involved in the secondary metabolic pathways of Mentha spp. Comput. Biol. Chem. 2016, 64, 154–162. [Google Scholar] [CrossRef]
- Huo, X.; Wang, C.; Teng, Y.; Liu, X. Identification of miRNAs associated with dark-induced senescence in Arabidopsis. BMC Plant Biol. 2015, 15, 266. [Google Scholar] [CrossRef]
- Ahmadizadeh, M.; Heidari, P. Bioinformatics study of transcription factors involved in cold stress. Biharean Biol. 2014, 8, 83–86. [Google Scholar]
- Heidari, P. Comparative Analysis of C-repeat Binding Factors (CBFs) in Tomato and Arabidopsis. Braz. Arch. Biol. Technol. 2019, 62. [Google Scholar] [CrossRef]
- Wang, X.; Oh, M.W.; Komatsu, S. Characterization of S-adenosylmethionine synthetases in soybean under flooding and drought stresses. Biol. Plant. 2016, 60, 269–278. [Google Scholar] [CrossRef]
- Sánchez-Aguayo, I.; Rodríguez-Galán, J.M.; García, R.; Torreblanca, J.; Pardo, J.M. Salt stress enhances xylem development and expression of S-adenosyl-L-methionine synthase in lignifying tissues of tomato plants. Planta 2004, 220, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kumar, V.; Varma, A.; Prasad, R.; Sharma, A.K.; Pal, A.; Arshi, A.; Singh, J. An efficient approach towards the bioremediation of copper, cobalt and nickel contaminated field samples. J. Soils Sediments 2016, 16, 2118–2127. [Google Scholar] [CrossRef]
- Serna, M.; Coll, Y.; Zapata, P.J.; Botella, M.Á.; Pretel, M.T.; Amorós, A. A brassinosteroid analogue prevented the effect of salt stress on ethylene synthesis and polyamines in lettuce plants. Sci. Hortic. (Amsterdam) 2015, 185, 105–112. [Google Scholar] [CrossRef]
- Roje, S. S-Adenosyl-L-methionine: Beyond the universal methyl group donor. Phytochemistry 2006, 67, 1686–1698. [Google Scholar] [CrossRef]
- Das, P.; Banerjee, A.; Roychoudhury, A. Polyamines Ameliorate Oxidative Stress by Regulating Antioxidant Systems and Interacting with Plant Growth Regulators. Mol. Plant Abiotic Stress Biol. Biotechnol. 2019, 135–143. [Google Scholar] [CrossRef]
- Guo, Z.; Tan, J.; Zhuo, C.; Wang, C.; Xiang, B.; Wang, Z. Abscisic acid, H2O2 and nitric oxide interactions mediated cold-induced S-adenosylmethionine synthetase in Medicago sativa subsp. falcata that confers cold tolerance through up-regulating polyamine oxidation. Plant Biotechnol. J. 2014, 12, 601–612. [Google Scholar] [CrossRef]
- Qi, Y.-C.; Wang, F.-F.; Zhang, H.; Liu, W.-Q. Overexpression of suadea salsa S-adenosylmethionine synthetase gene promotes salt tolerance in transgenic tobacco. Acta Physiol. Plant. 2010, 32, 263–269. [Google Scholar] [CrossRef]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Humana Press: Totowa, NJ, USA, 2005; pp. 571–607. [Google Scholar]
- Savojardo, C.; Martelli, P.L.; Fariselli, P.; Profiti, G.; Casadio, R. BUSCA: An integrative web server to predict subcellular localization of proteins. Nucleic Acids Res. 2018, 46, W459–W466. [Google Scholar] [CrossRef]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.-H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Brunak, S. Prediction of glycosylation across the human proteome and the correlation to protein function. Pac. Symp. Biocomput. 2002, 310–322. [Google Scholar]
- Dai, X.; Zhuang, Z.; Zhao, P.X. psRNATarget: A plant small RNA target analysis server (2017 release). Nucleic Acids Res. 2018, 46, W49–W54. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Organism | Locus ID | Length (aa) | MW (KDa) | pl | Instability Index | Aliphatic Index | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| Arabidopsis | AT2G36880 | 390 | 42.497 | 5.76 | stable | 83.74 | −0.236 | Cytoplasm |
| AT4G01850 | 393 | 43.255 | 5.67 | stable | 79.34 | −0.353 | Cytoplasm | |
| AT3G17390 | 393 | 42.796 | 5.51 | stable | 85.04 | −0.255 | Cytoplasm | |
| AT1G02500 | 393 | 43.158 | 5.51 | stable | 80.33 | −0.306 | Cytoplasm | |
| Tomato | Solyc01g101060.3 | 393 | 43.301 | 5.51 | stable | 82.80 | −0.335 | Cytoplasm |
| Solyc09g008280.2 | 390 | 42.652 | 5.76 | stable | 82.95 | −0.283 | Cytoplasm | |
| Solyc10g083970.1 | 390 | 42.660 | 6.12 | stable | 80.97 | −0.313 | Cytoplasm | |
| Solyc12g099000.2 | 393 | 43.082 | 5.41 | stable | 84.05 | −0.313 | Cytoplasm | |
| Eggplant | Sme2.5_00004.1_g00028.1 | 390 | 42.624 | 5.76 | stable | 81.97 | −0.290 | Cytoplasm |
| Sme2.5_00187.1_g00013.1 | 393 | 43.122 | 5.68 | stable | 84.30 | −0.307 | Cytoplasm | |
| Sme2.5_07249.1_g00002.1 | 394 | 43.367 | 5.49 | stable | 82.34 | −0.331 | Chloroplast | |
| Sme2.5_07301.1_g00003.1 | 390 | 42.627 | 6.08 | stable | 81.23 | −0.305 | Cytoplasm | |
| Sunflower | HanXRQChr01g0027791 | 238 | 26.223 | 9.13 | stable | 92.52 | −0.153 | Cytoplasm |
| HanXRQChr01g0027801 | 390 | 42.969 | 5.97 | stable | 81.97 | −0.346 | Cytoplasm | |
| HanXRQChr02g0051721 | 457 | 49.894 | 5.93 | stable | 84.03 | −0.227 | Chloroplast | |
| HanXRQChr05g0148381 | 455 | 49.847 | 5.42 | stable | 83.76 | −0.257 | Chloroplast | |
| HanXRQChr07g0194741 | 453 | 49.887 | 5.97 | stable | 83.05 | −0.237 | Chloroplast | |
| HanXRQChr13g0400841 | 437 | 48.132 | 6.22 | stable | 83.62 | −0.291 | Chloroplast | |
| HanXRQChr14g0454811 | 341 | 37.276 | 6.42 | stable | 84.34 | −0.297 | Cytoplasm | |
| Triticum urartu | XP_020178978.1 | 394 | 42.765 | 5.52 | stable | 85.86 | −0.203 | Cytoplasm |
| EMS55466.1 | 394 | 42.788 | 5.61 | stable | 84.39 | −0.212 | Cytoplasm | |
| EMS52834.1 | 479 | 52.800 | 6.51 | stable | 81.98 | −0.290 | Chloroplast | |
| Barley | HORVU6Hr1G034650 | 396 | 43.158 | 5.33 | stable | 81.72 | −0.253 | Cytoplasm |
| HORVU6Hr1G063490 | 394 | 42.766 | 5.52 | stable | 85.86 | −0.203 | Chloroplast | |
| HORVU6Hr1G063540 | 394 | 42.814 | 5.49 | stable | 83.40 | −0.219 | Cytoplasm | |
| HORVU6Hr1G063560 | 394 | 42.828 | 5.58 | stable | 83.40 | −0.220 | Cytoplasm | |
| Rice | LOC_Os01g22010 | 394 | 42.901 | 5.68 | stable | 81.14 | −0.264 | Cytoplasm |
| LOC_Os01g18860 | 396 | 43.310 | 5.22 | stable | 82.70 | −0.274 | Cytoplasm | |
| LOC_Os05g04510 | 396 | 43.220 | 5.74 | stable | 82.45 | −0.289 | Cytoplasm | |
| Sorghum | Sobic.003G140000 | 396 | 43.257 | 5.32 | stable | 83.69 | −0.284 | Cytoplasm |
| Sobic.003G151600 | 394 | 42.785 | 5.50 | stable | 82.13 | −0.237 | Cytoplasm | |
| Sobic.009G033600 | 396 | 42.976 | 5.57 | stable | 83.21 | −0.260 | Cytoplasm | |
| Medicago truncatula | Medtr1g063060 | 408 | 44.904 | 6.24 | stable | 81.67 | −0.294 | Cytoplasm |
| Medtr2g046710 | 396 | 43.174 | 5.77 | stable | 83.43 | −0.287 | Cytoplasm | |
| Medtr4g123810 | 393 | 42.916 | 5.67 | stable | 85.29 | −0.254 | Cytoplasm | |
| Medtr7g102120 | 390 | 42.654 | 6.36 | stable | 82.46 | −0.300 | Cytoplasm | |
| Medtr7g110310 | 394 | 43.279 | 5.59 | stable | 80.86 | −0.327 | Cytoplasm | |
| Soybean | Glyma.07G233800 | 392 | 43.055 | 5.65 | stable | 82.53 | −0.330 | Cytoplasm |
| Glyma.10G144300 | 389 | 42.620 | 5.41 | stable | 85.42 | −0.259 | Cytoplasm | |
| Glyma.17G039000 | 392 | 42.981 | 5.43 | stable | 83.52 | −0.328 | Cytoplasm | |
| Glyma.03G184000 | 390 | 42.727 | 6.13 | stable | 81.46 | −0.298 | Cytoplasm | |
| Glyma.03G223000 | 394 | 43.224 | 5.57 | stable | 80.13 | −0.337 | Cytoplasm | |
| Glyma.19G220200 | 394 | 43.250 | 5.57 | stable | 79.87 | −0.345 | Cytoplasm | |
| Glyma.10G054500 | 390 | 42.608 | 5.86 | stable | 82.46 | −0.283 | Cytoplasm | |
| Glyma.13G141600 | 390 | 42.586 | 5.82 | stable | 82.46 | −0.276 | Cytoplasm | |
| Glyma.15G190500 | 395 | 43.053 | 5.50 | stable | 82.89 | −0.304 | Cytoplasm |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, P.; Mazloomi, F.; Nussbaumer, T.; Barcaccia, G. Insights into the SAM Synthetase Gene Family and Its Roles in Tomato Seedlings under Abiotic Stresses and Hormone Treatments. Plants 2020, 9, 586. https://doi.org/10.3390/plants9050586
Heidari P, Mazloomi F, Nussbaumer T, Barcaccia G. Insights into the SAM Synthetase Gene Family and Its Roles in Tomato Seedlings under Abiotic Stresses and Hormone Treatments. Plants. 2020; 9(5):586. https://doi.org/10.3390/plants9050586
Chicago/Turabian StyleHeidari, Parviz, Faezeeh Mazloomi, Thomas Nussbaumer, and Gianni Barcaccia. 2020. "Insights into the SAM Synthetase Gene Family and Its Roles in Tomato Seedlings under Abiotic Stresses and Hormone Treatments" Plants 9, no. 5: 586. https://doi.org/10.3390/plants9050586
APA StyleHeidari, P., Mazloomi, F., Nussbaumer, T., & Barcaccia, G. (2020). Insights into the SAM Synthetase Gene Family and Its Roles in Tomato Seedlings under Abiotic Stresses and Hormone Treatments. Plants, 9(5), 586. https://doi.org/10.3390/plants9050586







