Zinc Hyperaccumulation in Plants: A Review
Abstract
1. Introduction
2. Sources and Bioavailability of Zinc
3. Effect of Zn Excess on Plant Development
3.1. Effect of Zn Excess on Seed Germination
3.2. Effect of Zn Excess on Root Development
3.3. Effect of Zn Excess on Aerial Parts Development
4. Zn Hyperaccumulator Plants
4.1. Morphological Response of Zn Hyperaccumulator Plants
4.2. Physiological and Biochemical Responses in Zn Hyperaccumulator Plants
5. Molecular Mechanisms of Zn Hyperaccumulation
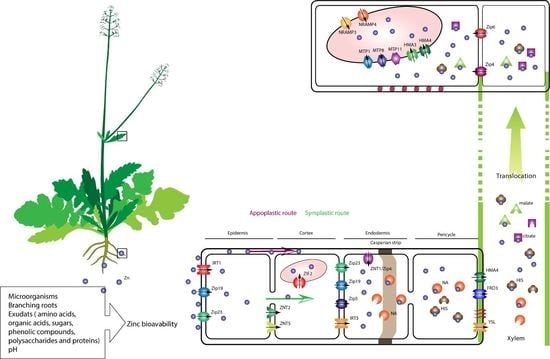
5.1. Zinc Uptake
5.2. Zn Xylem Loading and Transport Processes
5.3. Zinc Sequestration in the Aboveground Part of the Plants
6. Genetic Basis of Zn Hyperaccumulation
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Lehmann, A.; Veresoglou, S.D.; Leifheit, E.F.; Rillig, M.C. Arbuscular mycorrhizal influence on zinc nutrition in crop plants–a meta-analysis. Soil Biol. Biochem. 2014, 69, 123–131. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, C.-X.; Tan, Q.-L.; Zheng, C.-S.; Gui, H.-P.; Zeng, W.-N.; Sun, X.-C.; Zhao, X.-H. Plant nutrition status, yield and quality of satsuma mandarin (Citrus unshiu Marc.) under soil application of Fe-EDDHA and combination with zinc and manganese in calcareous soil. Sci. Hortic. 2014, 174, 46–53. [Google Scholar] [CrossRef]
- Castillo-González, J.; Ojeda-Barrios, D.; Hernández-Rodríguez, A.; González-Franco, A.C.; Robles-Hernández, L.; López-Ochoa, G.R. Zinc Metalloenzymes in Plants. Interciencia 2018, 43, 242–248. [Google Scholar]
- Mousavi, S.R. Zinc in crop production and interaction with phosphorus. Aust. J. Basic and Appl. Sci. 2011, 5, 1503–1509. [Google Scholar]
- Cakmak, I.; Öztürk, L.; Karanlik, S.; Marschner, H.; Ekiz, H. Zinc-efficient wild grasses enhance release of phytosiderophores under zinc deficiency. J. Plant Nutr. 1996, 19, 551–563. [Google Scholar] [CrossRef]
- Cambier, P.; Schvartz, C.; Van Oort, F. Contaminations Métalliques des Agrosystèmes et Écosystèmes Péri-Industriels; Editions Quae: Paris, France, 2009. [Google Scholar]
- Duplay, J.; Semhi, K.; Errais, E.; Imfeld, G.; Babcsanyi, I.; Perrone, T. Copper, zinc, lead and cadmium bioavailability and retention in vineyard soils (Rouffach, France): The impact of cultural practices. Geoderma 2014, 230, 318–328. [Google Scholar] [CrossRef]
- Kwon, M.J.; Boyanov, M.I.; Yang, J.-S.; Lee, S.; Hwang, Y.H.; Lee, J.Y.; Mishra, B.; Kemner, K.M. Transformation of zinc-concentrate in surface and subsurface environments: Implications for assessing zinc mobility/toxicity and choosing an optimal remediation strategy. Environ. Pollut. 2017, 226, 346–355. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Modrzewska, B. Acidity and sorption properties of Zinc-contaminated soil following the application of neutralising substances. J. Ecol. Eng. 2016, 17. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.; dos Reis, A.R. The molecular genetics of zinc uptake and utilization efficiency in crop plants. In Plant Micronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2018; pp. 87–108. [Google Scholar]
- Tiecher, T.L.; Ceretta, C.A.; Tiecher, T.; Ferreira, P.A.; Nicoloso, F.T.; Soriani, H.H.; Rossato, L.V.; Mimmo, T.; Cesco, S.; Lourenzi, C.R. Effects of zinc addition to a copper-contaminated vineyard soil on sorption of Zn by soil and plant physiological responses. Ecotoxicol. Environ. Saf. 2016, 129, 109–119. [Google Scholar] [CrossRef]
- Prasad, M. Essentiality of zinc for human health and sustainable development. In Trace Elements as Contaminants and Nutrients: Consequences in Ecosystems and Human Health; Prasad, M.N.V., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2008; pp. 183–216. [Google Scholar]
- Clemens, S. How metal hyperaccumulating plants can advance Zn biofortification. Plant Soil 2017, 411, 111–120. [Google Scholar] [CrossRef]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. Isrn Ecol. 2011, 2011. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Karyotis, T. Zinc in soils, water and food crops. J. Trace Elem. Med. Biol. 2018, 49, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xing, J.; Ma, S.; He, Y.; Fu, H.; Gao, Y.; Wang, Y.; Wang, Y. Effects of erosion angle on erosion properties of Fe-B alloy in flowing liquid zinc. Metall. Mater. Trans. A 2015, 46, 1900–1907. [Google Scholar] [CrossRef]
- Mateos-Naranjo, E.; Castellanos, E.M.; Perez-Martin, A. Zinc tolerance and accumulation in the halophytic species Juncus acutus. Environ. Exp. Bot. 2014, 100, 114–121. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-F.; Chen, C.-W. Contamination of zinc in sediments at river mouths and channel in northern Kaohsiung Harbor, Taiwan. Int. J. Environ. Sci. Dev. 2012, 3, 517. [Google Scholar] [CrossRef]
- Chahal, D.; Sharma, B.; Singh, P. Distribution of forms of zinc and their association with soil properties and uptake in different soil orders in semi-arid soils of Punjab, India. Commun. Soil Sci. Plant Anal. 2005, 36, 2857–2874. [Google Scholar] [CrossRef]
- Sadeghzadeh, B.; Rengel, Z. Zinc in soils and crop nutrition. In The Molecular and Physiological Basis of Nutrient Use Efficiency in Crops; Hawkesford, M.J., Barraclough, P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; pp. 335–375. [Google Scholar]
- González-Alcaraz, M.N.; van Gestel, C.A. Climate change effects on enchytraeid performance in metal-polluted soils explained from changes in metal bioavailability and bioaccumulation. Environ. Res. 2015, 142, 177–184. [Google Scholar] [CrossRef]
- Adamczyk-Szabela, D.; Markiewicz, J.; Wolf, W.M. Heavy metal uptake by herbs. IV. Influence of soil pH on the content of heavy metals in Valeriana officinalis L. Water Air Soil Pollut. 2015, 226, 106. [Google Scholar] [CrossRef]
- Pinto, E.; Aguiar, A.A.; Ferreira, I.M. Influence of soil chemistry and plant physiology in the phytoremediation of Cu, Mn, and Zn. Crit. Rev. Plant Sci. 2014, 33, 351–373. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.; Khalid, A.; Kanwal, S.; Crowley, D.E. Perspectives of rhizosphere microflora for improving Zn bioavailability and acquisition by higher plants. Int. J. Agric. Biol. 2014, 16, 653–662. [Google Scholar]
- Leitenmaier, B.; Küpper, H. Compartmentation and complexation of metals in hyperaccumulator plants. Front. Plant Sci. 2013, 4, 374. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G. Heavy metal toxicity in plants. In Plants and Heavy Metals; Springer: Berlin, Germany, 2012; pp. 1–25. [Google Scholar]
- Misra, V.; Tiwari, A.; Shukla, B.; Seth, C.S. Effects of soil amendments on the bioavailability of heavy metals from zinc mine tailings. Environ. Monit. Assess. 2009, 155, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wahbi, A.; Ma, L.; Li, B.; Yang, Y. Immobilization of Zn, Cu, and Pb in contaminated soils using phosphate rock and phosphoric acid. J. Hazard. Mater. 2009, 164, 555–564. [Google Scholar] [CrossRef]
- Hafeez, B.; Khanif, Y.; Saleem, M. Role of zinc in plant nutrition-a review. J. Exp. Agric. Int. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of fertilizers with zinc: An excellent investment for humanity and crop production in India. J. Trace Elem. Med. Biol. 2009, 23, 281–289. [Google Scholar] [CrossRef]
- Clemente, R.; Hartley, W.; Riby, P.; Dickinson, N.M.; Lepp, N.W. Trace element mobility in a contaminated soil two years after field-amendment with a greenwaste compost mulch. Environ. Pollut. 2010, 158, 1644–1651. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, J.; Tian, X.; Zhao, A.; Li, M.; Wang, S.; Li, X.; Jia, Z.; Liu, K. Effect of straw amendment on soil Zn availability and ageing of exogenous water-soluble Zn applied to calcareous soil. PLoS ONE 2017, 12, e0169776. [Google Scholar] [CrossRef][Green Version]
- Seshadri, B.; Bolan, N.; Naidu, R. Rhizosphere-induced heavy metal (loid) transformation in relation to bioavailability and remediation. J. Soil Sci. Plant Nutr. 2015, 15, 524–548. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, X.; Zhang, X.; Chen, X.; Tao, K. Effects of key components of S. triqueter root exudates on fractions and bioavailability of pyrene–lead co-contaminated soils. Int. J. Environ. Sci. Technol. 2016, 13, 887–896. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Wang, Y.; Yeh, K.-C. Role of root exudates in metal acquisition and tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Medas, D.; De Giudici, G.; Casu, M.A.; Musu, E.; Gianoncelli, A.; Iadecola, A.; Meneghini, C.; Tamburini, E.; Sprocati, A.R.; Turnau, K. Microscopic processes ruling the bioavailability of Zn to roots of Euphorbia pithyusa L. pioneer plant. Environ. Sci. Technol. 2015, 49, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Tsednee, M.; Yang, S.-C.; Lee, D.-C.; Yeh, K.-C. Root-secreted nicotianamine from Arabidopsis halleri facilitates zinc hypertolerance by regulating zinc bioavailability. Plant Physiol. 2014, 166, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Degryse, F.; Verma, V.; Smolders, E. Mobilization of Cu and Zn by root exudates of dicotyledonous plants in resin-buffered solutions and in soil. Plant soil 2008, 306, 69–84. [Google Scholar] [CrossRef]
- Wei-Hong, X.; Huai, L.; Qi-Fu, M.; Xiong, Z.-T. Root exudates, rhizosphere Zn fractions, and Zn accumulation of ryegrass at different soil Zn levels. Pedosphere 2007, 17, 389–396. [Google Scholar]
- Versieren, L.; Smets, E.; De Schamphelaere, K.; Blust, R.; Smolders, E. Mixture toxicity of copper and zinc to barley at low level effects can be described by the Biotic Ligand Model. Plant Soil 2014, 381, 131–142. [Google Scholar] [CrossRef]
- Hrynkiewicz, K.; Dabrowska, G.; Baum, C.; Niedojadlo, K.; Leinweber, P. Interactive and single effects of ectomycorrhiza formation and Bacillus cereus on metallothionein MT1 expression and phytoextraction of Cd and Zn by willows. Water Air Soil Pollut. 2012, 223, 957–968. [Google Scholar] [CrossRef]
- Ramesh, A.; Sharma, S.K.; Sharma, M.P.; Yadav, N.; Joshi, O.P. Inoculation of zinc solubilizing Bacillus aryabhattai strains for improved growth, mobilization and biofortification of zinc in soybean and wheat cultivated in Vertisols of central India. Appl. Soil Ecol. 2014, 73, 87–96. [Google Scholar] [CrossRef]
- Vogel-Mikuš, K.; Pongrac, P.; Kump, P.; Nečemer, M.; Regvar, M. Colonisation of a Zn, Cd and Pb hyperaccumulator Thlaspi praecox Wulfen with indigenous arbuscular mycorrhizal fungal mixture induces changes in heavy metal and nutrient uptake. Environ. Pollut. 2006, 139, 362–371. [Google Scholar] [CrossRef]
- McCall, K.A.; Huang, C.-c.; Fierke, C.A. Function and mechanism of zinc metalloenzymes. J. Nutr. 2000, 130, 1437S–1446S. [Google Scholar] [CrossRef]
- Robson, A.D. Zinc in Soils and Plants, Proceedings of the International Symposium on ‘Zinc in Soils and Plants’, held at the University of Western Australia, Perth, Australia, 27–28 September 1993; Springer Science & Business Media: Berlin, Germany, 2012; Volume 55. [Google Scholar]
- Kisko, M.; Bouain, N.; Rouached, A.; Choudhary, S.P.; Rouached, H. Molecular mechanisms of phosphate and zinc signalling crosstalk in plants: Phosphate and zinc loading into root xylem in Arabidopsis. Environ. Exp. Bot. 2015, 114, 57–64. [Google Scholar] [CrossRef]
- Samreen, T.; Shah, H.U.; Ullah, S.; Javid, M. Zinc effect on growth rate, chlorophyll, protein and mineral contents of hydroponically grown mungbeans plant (Vigna radiata). Arab. J. Chem. 2017, 10, S1802–S1807. [Google Scholar] [CrossRef]
- Pessarakli, M. Handbook of Photosynthesis; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Jain, A.; Sinilal, B.; Dhandapani, G.; Meagher, R.B.; Sahi, S.V. Effects of deficiency and excess of zinc on morphophysiological traits and spatiotemporal regulation of zinc-responsive genes reveal incidence of cross talk between micro-and macronutrients. Environ. Sci. Technol. 2013, 47, 5327–5335. [Google Scholar] [CrossRef] [PubMed]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Jain, R.; Srivastava, S.; Solomon, S.; Shrivastava, A.; Chandra, A. Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp.). Acta Physiol. Plant. 2010, 32, 979–986. [Google Scholar] [CrossRef]
- Reis, S.; Pavia, I.; Carvalho, A.; Moutinho-Pereira, J.; Correia, C.; Lima-Brito, J. Seed priming with iron and zinc in bread wheat: Effects in germination, mitosis and grain yield. Protoplasma 2018, 255, 1179–1194. [Google Scholar] [CrossRef]
- Gokak, I.; Taranath, T. Seed germination and growth responses of Macrotyloma uniflorum (Lam.) Verdc. exposed to Zinc and Zinc nanoparticles. Int. J. Environ. Sci. 2015, 5, 840. [Google Scholar]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Savochkin, Y.V.; Kuznetsov, V.V. Effects of zinc on Scots pine (Pinus sylvestris L.) seedlings grown in hydroculture. Plant Physiol. Biochem. 2016, 102, 1–9. [Google Scholar] [CrossRef]
- Zhi, Y.; Deng, Z.; Luo, M.; Ding, W.; Hu, Y.; Deng, J.; Li, Y.; Zhao, Y.; Zhang, X.; Wu, W. Influence of Heavy Metals on Seed Germination and Early Seedling Growth in Eruca sativa Mill. Am. J. Plant Sci. 2015, 6, 582. [Google Scholar] [CrossRef]
- Marichali, A.; Dallali, S.; Ouerghemmi, S.; Sebei, H.; Hosni, K. Germination, morpho-physiological and biochemical responses of coriander (Coriandrum sativum L.) to zinc excess. Ind. Crops Prod. 2014, 55, 248–257. [Google Scholar] [CrossRef]
- Marichali, A.; Dallali, S.; Ouerghemmi, S.; Sebei, H.; Casabianca, H.; Hosni, K. Responses of Nigella sativa L. to Zinc excess: Focus on germination, growth, yield and yield components, lipid and terpene metabolism, and total phenolics and antioxidant activities. J. Agric. Food Chem. 2016, 64, 1664–1675. [Google Scholar] [CrossRef] [PubMed]
- Basha, S.A.; Selvaraju, M. Toxic effect of Zinc on growth and nutrient accumulation of cow pea (Vigna unguiculata L.). Int. Lett. Nat. Sci. 2015, 43. [Google Scholar] [CrossRef]
- Nanda, R.; Agrawal, V. Elucidation of zinc and copper induced oxidative stress, DNA damage and activation of defence system during seed germination in Cassia angustifolia Vahl. Environ. Exp. Bot. 2016, 125, 31–41. [Google Scholar] [CrossRef]
- Gupta, S.; Meena, M.; Datta, S. Effect of selected heavy metals (Lead AND Zinc) on seedling growth of Soybean Glycine Max (L.) MERR’. J. Pharm. Pharm. Sci. 2016, 8, 302–305. [Google Scholar]
- Bae, J.; Benoit, D.L.; Watson, A.K. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef]
- Disante, K.B.; Cortina, J.; Vilagrosa, A.; Fuentes, D.; Hernández, E.I.; Ljung, K. Alleviation of Zn toxicity by low water availability. Physiol. Plant. 2014, 150, 412–424. [Google Scholar] [CrossRef]
- Bochicchio, R.; Sofo, A.; Terzano, R.; Gattullo, C.E.; Amato, M.; Scopa, A. Root architecture and morphometric analysis of Arabidopsis thaliana grown in Cd/Cu/Zn-gradient agar dishes: A new screening technique for studying plant response to metals. Plant Physiol. Biochem. 2015, 91, 20–27. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef]
- Küpper, H.; Andresen, E. Mechanisms of metal toxicity in plants. Metallomics 2016, 8, 269–285. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Toxicity of heavy metals and metal-containing nanoparticles on plants. Biochim. Biophys. Acta BBA Proteins Proteom. 2016, 1864, 932–944. [Google Scholar] [CrossRef]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Jia, L.; Chen, H.; Wei, X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat plants. Ecotoxicol. Environ. Saf. 2013, 89, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.-B.; He, X.-J.; Chen, L.; Tang, L.; Gao, S.; Chen, F. Effects of zinc on growth and antioxidant responses in Jatropha curcas seedlings. Int. J. Agric. Biol. 2010, 12, 119–124. [Google Scholar]
- Subba, P.; Mukhopadhyay, M.; Mahato, S.K.; Bhutia, K.D.; Mondal, T.K.; Ghosh, S.K. Zinc stress induces physiological, ultra-structural and biochemical changes in mandarin orange (Citrus reticulata Blanco) seedlings. Physiol. Mol. Biol. Plants 2014, 20, 461–473. [Google Scholar] [CrossRef]
- Liu, D.; Chen, J.; Mahmood, Q.; Li, S.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Lu, K. Effect of Zn toxicity on root morphology, ultrastructure, and the ability to accumulate Zn in Moso bamboo (Phyllostachys pubescens). Environ. Sci. Pollut. Res. 2014, 21, 13615–13624. [Google Scholar] [CrossRef]
- Scheid, D.L.; De Marco, R.; Grolli, A.L.; Da Silva, R.F.; Da Ros, C.O.; Andreazza, R. Growth, tolerance and zinc accumulation in Senna multijuga and Erythrina crista-galli seedlings. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 465–470. [Google Scholar] [CrossRef]
- Sagardoy, R.; Vázquez, S.; Florez-Sarasa, I.; Albacete, A.; Ribas-Carbó, M.; Flexas, J.; Abadía, J.; Morales, F. Stomatal and mesophyll conductances to CO2 are the main limitations to photosynthesis in sugar beet (Beta vulgaris) plants grown with excess zinc. New Phytol. 2010, 187, 145–158. [Google Scholar] [CrossRef]
- Fernàndez, J.; Zacchini, M.; Fleck, I. Photosynthetic and growth responses of Populus clones Eridano and I-214 submitted to elevated Zn concentrations. J. Geochem. Exp. 2012, 123, 77–86. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Anwaar, S.A.; Ali, S.; Ali, S.; Ishaque, W.; Farid, M.; Farooq, M.A.; Najeeb, U.; Abbas, F.; Sharif, M. Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ. Sci. Pollut. Res. 2015, 22, 3441–3450. [Google Scholar] [CrossRef]
- Feigl, G.; Lehotai, N.; Molnár, Á.; Ördög, A.; Rodríguez-Ruiz, M.; Palma, J.M.; Corpas, F.J.; Erdei, L.; Kolbert, Z. Zinc induces distinct changes in the metabolism of reactive oxygen and nitrogen species (ROS and RNS) in the roots of two Brassica species with different sensitivity to zinc stress. Ann. Bot. 2015, 116, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Khan, N.A. Ethylene reverses photosynthetic inhibition by nickel and zinc in mustard through changes in PS II activity, photosynthetic nitrogen use efficiency, and antioxidant metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Vijayarengan, P.; Mahalakshmi, G. Zinc toxicity in tomato plants. World Appl. Sci. J. 2013, 24, 649–653. [Google Scholar]
- Cambrollé, J.; Mancilla-Leytón, J.; Muñoz-Vallés, S.; Luque, T.; Figueroa, M. Zinc tolerance and accumulation in the salt-marsh shrub Halimione portulacoides. Chemosphere 2012, 86, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Monnet, F.; Vaillant, N.; Vernay, P.; Coudret, A.; Sallanon, H.; Hitmi, A. Relationship between PSII activity, CO2 fixation, and Zn, Mn and Mg contents of Lolium perenne under zinc stress. J. Plant Physiol. 2001, 158, 1137–1144. [Google Scholar] [CrossRef]
- Azzarello, E.; Pandolfi, C.; Giordano, C.; Rossi, M.; Mugnai, S.; Mancuso, S. Ultramorphological and physiological modifications induced by high zinc levels in Paulownia tomentosa. Environ. Exp. Bot. 2012, 81, 11–17. [Google Scholar] [CrossRef]
- Todeschini, V.; Lingua, G.; D’agostino, G.; Carniato, F.; Roccotiello, E.; Berta, G. Effects of high zinc concentration on poplar leaves: A morphological and biochemical study. Environ. Exp. Bot. 2011, 71, 50–56. [Google Scholar] [CrossRef]
- Blasco, B.; Graham, N.S.; Broadley, M.R. Antioxidant response and carboxylate metabolism in Brassica rapa exposed to different external Zn, Ca, and Mg supply. J. Plant Physiol. 2015, 176, 16–24. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular mechanism of heavy metal toxicity and tolerance in plants: Central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 2012. [Google Scholar] [CrossRef]
- Tripathi, B.N.; Gaur, J. Relationship between copper-and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 2004, 219, 397–404. [Google Scholar] [CrossRef]
- Al Khateeb, W.; Al-Qwasemeh, H. Cadmium, copper and zinc toxicity effects on growth, proline content and genetic stability of Solanum nigrum L., a crop wild relative for tomato; comparative study. Physiol. Mol. Biol. Plants 2014, 20, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Michael, P.I.; Krishnaswamy, M. The effect of zinc stress combined with high irradiance stress on membrane damage and antioxidative response in bean seedlings. Environ. Exp. Bot. 2011, 74, 171–177. [Google Scholar] [CrossRef]
- Gomes, M.; Duarte, D.; Carneiro, M.; Barreto, L.; Carvalho, M.; Soares, A.; Guilherme, L.; Garcia, Q. Zinc tolerance modulation in Myracrodruon urundeuva plants. Plant Physiol. Biochem. 2013, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Branch, D.; Damghan, I.; Branch, Q.; Qaemshahr, I. Protective role of exogenous nitric oxide against zinc toxicity in Plantago major L. Appl. Ecol. Environ. Res. 2017, 15, 511–524. [Google Scholar]
- Babst-Kostecka, A.; Schat, H.; Saumitou-Laprade, P.; Grodzińska, K.; Bourceaux, A.; Pauwels, M.; Frérot, H. Evolutionary dynamics of quantitative variation in an adaptive trait at the regional scale: The case of zinc hyperaccumulation in Arabidopsis halleri. Mol. Ecol. 2018, 27, 3257–3273. [Google Scholar] [CrossRef]
- Schvartzman, M.S.; Corso, M.; Fataftah, N.; Scheepers, M.; Nouet, C.; Bosman, B.; Carnol, M.; Motte, P.; Verbruggen, N.; Hanikenne, M. Adaptation to high zinc depends on distinct mechanisms in metallicolous populations of Arabidopsis halleri. New Phytol. 2018, 218, 269–282. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Marques, A.P.; Rangel, A.O.; Castro, P.M. Remediation of heavy metal contaminated soils: Phytoremediation as a potentially promising clean-up technology. Crit. Rev. Environ. Sci. Technol. 2009, 39, 622–654. [Google Scholar] [CrossRef]
- Peer, W.A.; Mahmoudian, M.; Freeman, J.L.; Lahner, B.; Richards, E.L.; Reeves, R.D.; Murphy, A.S.; Salt, D.E. Assessment of plants from the Brassicaceae family as genetic models for the study of nickel and zinc hyperaccumulation. New Phytol. 2006, 172, 248–260. [Google Scholar] [CrossRef]
- Baker, A.; Proctor, J.; Van Balgooy, M.; Reeves, R. Hyperaccumulation of nickel by the flora of the ultramafics of Palawan, Republic of the Philippines. In The Vegetation of Ultramafic (Serpentine) Soils’; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept Ltd: hampshire, UK, 1992; pp. 291–304. [Google Scholar]
- Van der Ent, A.; Baker, A.J.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Gupta, N.; Ram, H.; Kumar, B. Mechanism of Zinc absorption in plants: Uptake, transport, translocation and accumulation. Rev. Environ. Sci. Bio Technol. 2016, 15, 89–109. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.; Jaffré, T.; Erskine, P.D.; Echevarria, G.; van der Ent, A. A global database for plants that hyperaccumulate metal and metalloid trace elements. New Phytol. 2018, 218, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.-T.; Qiu, R.-L.; Zeng, X.-W.; Ying, R.-R.; Yu, F.-M.; Zhou, X.-Y. Lead, zinc, cadmium hyperaccumulation and growth stimulation in Arabis paniculata Franch. Environ. Exp. Bot. 2009, 66, 126–134. [Google Scholar] [CrossRef]
- Brooks, R.R. Plants that hyperaccumulate heavy metals. In Plants and the Chemical Elements: Biochemistry, Uptake, Tolerance and Toxicity; Farago, M.E., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1994; pp. 87–105. [Google Scholar]
- Qiu, R.-L.; Thangavel, P.; Hu, P.-J.; Senthilkumar, P.; Ying, R.-R.; Tang, Y.-T. Interaction of cadmium and zinc on accumulation and sub-cellular distribution in leaves of hyperaccumulator Potentilla griffithii. J. Hazard. Mater. 2011, 186, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Gallego, B.; Martos, S.; Cabot, C.; Barceló, J.; Poschenrieder, C. Zinc hyperaccumulation substitutes for defense failures beyond salicylate and jasmonate signaling pathways of Alternaria brassicicola attack in Noccaea caerulescens. Physiol. Plant. 2017, 159, 401–415. [Google Scholar] [CrossRef]
- Kozhevnikova, A.D.; Seregin, I.; Gosti, F.; Schat, H. Zinc accumulation and distribution over tissues in Noccaea caerulescens in nature and in hydroponics: A comparison. Plant Soil 2017, 411, 5–16. [Google Scholar] [CrossRef]
- Li, T.; Yang, X.; Lu, L.; Islam, E.; He, Z. Effects of zinc and cadmium interactions on root morphology and metal translocation in a hyperaccumulating species under hydroponic conditions. J. Hazard. Mater. 2009, 169, 734–741. [Google Scholar] [CrossRef]
- Alford, É.R.; Pilon-Smits, E.A.; Paschke, M.W. Metallophytes—A view from the rhizosphere. Plant Soil 2010, 337, 33–50. [Google Scholar] [CrossRef]
- Belouchrani, A.S.; Mameri, N.; Abdi, N.; Grib, H.; Lounici, H.; Drouiche, N. Phytoremediation of soil contaminated with Zn using Canola (Brassica napus L). Ecol. Eng. 2016, 95, 43–49. [Google Scholar] [CrossRef]
- Bayçu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E.; Moustakas, M. Cadmium-zinc accumulation and photosystem II responses of Noccaea caerulescens to Cd and Zn exposure. Environ. Sci. Pollut. Res. 2017, 24, 2840–2850. [Google Scholar] [CrossRef]
- Zhao, F.; Hamon, R.; McLaughlin, M.J. Root exudates of the hyperaccumulator Thlaspi caerulescens do not enhance metal mobilization. New Phytol. 2001, 151, 613–620. [Google Scholar] [CrossRef]
- Li, T.; Di, Z.; Yang, X.; Sparks, D.L. Effects of dissolved organic matter from the rhizosphere of the hyperaccumulator Sedum alfredii on sorption of zinc and cadmium by different soils. J. Hazard. Mater. 2011, 192, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Dessureault-Rompré, J.; Luster, J.; Schulin, R.; Tercier-Waeber, M.-L.; Nowack, B. Decrease of labile Zn and Cd in the rhizosphere of hyperaccumulating Thlaspi caerulescens with time. Environ. Pollut. 2010, 158, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yin, H.; Li, Y.; Liu, X. Nitric oxide is associated with long-term zinc tolerance in Solanum nigrum. Plant Physiol. 2010, 154, 1319–1334. [Google Scholar] [CrossRef]
- Jin, X.F.; Yang, X.E.; Islam, E.; Liu, D.; Mahmood, Q.; Li, H.; Li, J. Ultrastructural changes, zinc hyperaccumulation and its relation with antioxidants in two ecotypes of Sedum alfredii Hance. Plant Physiol. Biochem. 2008, 46, 997–1006. [Google Scholar] [CrossRef]
- Wójcik, M.; Skórzyńska-Polit, E.; Tukiendorf, A. Organic acids accumulation and antioxidant enzyme activities in Thlaspi caerulescens under Zn and Cd stress. Plant Growth Regul. 2006, 48, 145–155. [Google Scholar] [CrossRef]
- Steffens, J. The heavy metal-binding peptides of plants. Annu. Rev. Plant Biol. 1990, 41, 553–575. [Google Scholar] [CrossRef]
- Sun, Q.; Ye, Z.; Wang, X.; Wong, M.H. Increase of glutathione in mine population of Sedum alfredii: A Zn hyperaccumulator and Pb accumulator. Phytochemistry 2005, 66, 2549–2556. [Google Scholar] [CrossRef]
- Roosens, N.H.; Leplae, R.; Bernard, C.; Verbruggen, N. Variations in plant metallothioneins: The heavy metal hyperaccumulator Thlaspi caerulescens as a study case. Planta 2005, 222, 716. [Google Scholar] [CrossRef]
- Zelko, I.; Lux, A.; Czibula, K. Difference in the root structure of hyperaccumulator Thlaspi caerulescens and non-hyperaccumulator Thlaspi arvense. Int. J. Environ. Pollut. 2008, 33, 123–132. [Google Scholar] [CrossRef]
- Van de Mortel, J.E.; Villanueva, L.A.; Schat, H.; Kwekkeboom, J.; Coughlan, S.; Moerland, P.D.; van Themaat, E.V.L.; Koornneef, M.; Aarts, M.G. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant Physiol. 2006, 142, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Lasat, M.M.; Baker, A.J.; Kochian, L.V. Altered Zn compartmentation in the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens. Plant Physiol. 1998, 118, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.-J.; Qiu, R.-L.; Senthilkumar, P.; Jiang, D.; Chen, Z.-W.; Tang, Y.-T.; Liu, F.-J. Tolerance, accumulation and distribution of zinc and cadmium in hyperaccumulator Potentilla griffithii. Environ. Exp. Bot. 2009, 66, 317–325. [Google Scholar] [CrossRef]
- Merlot, S.; de la Torre, V.S.G.; Hanikenne, M. Physiology and molecular biology of trace element hyperaccumulation. In Agromining: Farming for Metals; Springer: Berlin, Germany, 2018; pp. 93–116. [Google Scholar]
- Shahzad, Z.; Gosti, F.; Frérot, H.; Lacombe, É.; Roosens, N.; Saumitou-Laprade, P.; Berthomieu, P. The five AhMTP1 zinc transporters undergo different evolutionary fates towards adaptive evolution to zinc tolerance in Arabidopsis halleri. PLoS Genet. 2010, 6, e100911. [Google Scholar] [CrossRef] [PubMed]
- Hanikenne, M.; Nouet, C. Metal hyperaccumulation and hypertolerance: A model for plant evolutionary genomics. Curr. Opin. Plant Biol. 2011, 14, 252–259. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Li, C. Absorption and Translocation of Zn Foliar Fertilisers. Master’s Thesis, The University of Queensland, Brisbane, Australia, 2019. [Google Scholar]
- Lin, Y.-F.; Hassan, Z.; Talukdar, S.; Schat, H.; Aarts, M.G. Expression of the ZNT1 zinc transporter from the metal hyperaccumulator Noccaea caerulescens confers enhanced zinc and cadmium tolerance and accumulation to Arabidopsis thaliana. PLoS ONE 2016, 11, e0149750. [Google Scholar] [CrossRef]
- Fasani, E. Plants that hyperaccumulate heavy metals. In Plants and Heavy Metals; Springer: Berlin, Germany, 2012; pp. 55–74. [Google Scholar]
- Talke, I.N.; Hanikenne, M.; Krämer, U. Zinc-dependent global transcriptional control, transcriptional deregulation, and higher gene copy number for genes in metal homeostasis of the hyperaccumulator Arabidopsis halleri. Plant Physiol. 2006, 142, 148–167. [Google Scholar] [CrossRef]
- Caldelas, C.; Weiss, D.J. Zinc homeostasis and isotopic fractionation in plants: A review. Plant Soil 2017, 411, 17–46. [Google Scholar] [CrossRef]
- Shanmugam, V.; Lo, J.-C.; Yeh, K.-C. Control of Zn uptake in Arabidopsis halleri: A balance between Zn and Fe. Front. Plant Sci. 2013, 4, 281. [Google Scholar] [CrossRef]
- Humayan Kabir, A.; Swaraz, A.; Stangoulis, J. Zinc-deficiency resistance and biofortification in plants. J. Plant Nutr. Soil Sci. 2014, 177, 311–319. [Google Scholar] [CrossRef]
- Remy, E.; Cabrito, T.R.; Batista, R.A.; Hussein, M.A.; Teixeira, M.C.; Athanasiadis, A.; Sá-Correia, I.; Duque, P. Intron retention in the 5′ UTR of the novel ZIF2 transporter enhances translation to promote zinc tolerance in Arabidopsis. PLoS Genet. 2014, 10, e1004375. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.A.; Krämer, U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 1553–1567. [Google Scholar] [CrossRef] [PubMed]
- Trampczynska, A.; Küpper, H.; Meyer-Klaucke, W.; Schmidt, H.; Clemens, S. Nicotianamine forms complexes with Zn (II) in vivo. Metallomics 2010, 2, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, K.; Nakanishi, H.; Suzuki, K.; Nishizawa, N.K.; Mori, S. Presence of nicotianamine synthase isozymes and their homologues in the root of graminaceous plants. Soil Sci. Plant Nutr. 1999, 45, 681–691. [Google Scholar] [CrossRef]
- Hammond, J.P.; Bowen, H.C.; White, P.J.; Mills, V.; Pyke, K.A.; Baker, A.J.; Whiting, S.N.; May, S.T.; Broadley, M.R. A comparison of the Thlaspi caerulescens and Thlaspi arvense shoot transcriptomes. New Phytol. 2006, 170, 239–260. [Google Scholar] [CrossRef]
- Cornu, J.Y.; Deinlein, U.; Höreth, S.; Braun, M.; Schmidt, H.; Weber, M.; Persson, D.P.; Husted, S.; Schjoerring, J.K.; Clemens, S. Contrasting effects of nicotianamine synthase knockdown on zinc and nickel tolerance and accumulation in the zinc/cadmium hyperaccumulator Arabidopsis halleri. New Phytol. 2015, 206, 738–750. [Google Scholar] [CrossRef]
- Kozhevnikova, A.D.; Seregin, I.V.; Erlikh, N.T.; Shevyreva, T.A.; Andreev, I.M.; Verweij, R.; Schat, H. Histidine-mediated xylem loading of zinc is a species-wide character in Noccaea caerulescens. New Phytol. 2014, 203, 508–519. [Google Scholar] [CrossRef]
- Milner, M.J.; Kochian, L.V. Investigating heavy-metal hyperaccumulation using Thlaspi caerulescens as a model system. Ann. Bot. 2008, 102, 3–13. [Google Scholar] [CrossRef]
- Frérot, H.; Hautekèete, N.-C.; Decombeix, I.; Bouchet, M.-H.; Créach, A.; Saumitou-Laprade, P.; Piquot, Y.; Pauwels, M. Habitat heterogeneity in the pseudometallophyte Arabidopsis halleri and its structuring effect on natural variation of zinc and cadmium hyperaccumulation. Plant Soil 2018, 423, 157–174. [Google Scholar] [CrossRef]
- Hanikenne, M.; Talke, I.N.; Haydon, M.J.; Lanz, C.; Nolte, A.; Motte, P.; Kroymann, J.; Weigel, D.; Krämer, U. Evolution of metal hyperaccumulation required cis-regulatory changes and triplication of HMA4. Nature 2008, 453, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Verret, F.; Gravot, A.; Auroy, P.; Leonhardt, N.; David, P.; Nussaume, L.; Vavasseur, A.; Richaud, P. Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett. 2004, 576, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Hussain, D.; Haydon, M.J.; Wang, Y.; Wong, E.; Sherson, S.M.; Young, J.; Camakaris, J.; Harper, J.F.; Cobbett, C.S. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. Plant Cell 2004, 16, 1327–1339. [Google Scholar] [CrossRef]
- Lin, Y.F.; Liang, H.M.; Yang, S.Y.; Boch, A.; Clemens, S.; Chen, C.C.; Wu, J.F.; Huang, J.L.; Yeh, K.C. Arabidopsis IRT3 is a zinc-regulated and plasma membrane localized zinc/iron transporter. New Phytol. 2009, 182, 392–404. [Google Scholar] [CrossRef]
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Le Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef]
- Socha, A.L.; Guerinot, M.L. Mn-euvering manganese: The role of transporter gene family members in manganese uptake and mobilization in plants. Front. Plant Sci. 2014, 5, 106. [Google Scholar] [CrossRef]
- Pineau, C.; Loubet, S.; Lefoulon, C.; Chalies, C.; Fizames, C.; Lacombe, B.; Ferrand, M.; Loudet, O.; Berthomieu, P.; Richard, O. Natural variation at the FRD3 MATE transporter locus reveals cross-talk between Fe homeostasis and Zn tolerance in Arabidopsis thaliana. PLoS Genet. 2012, 8, e1003120. [Google Scholar] [CrossRef]
- Kutrowska, A.; Szelag, M. Low-molecular weight organic acids and peptides involved in the long-distance transport of trace metals. Acta Physiol. Plant. 2014, 36, 1957–1968. [Google Scholar] [CrossRef]
- Hassan, Z.; Aarts, M.G. Opportunities and feasibilities for biotechnological improvement of Zn, Cd or Ni tolerance and accumulation in plants. Environ. Exp. Bot. 2011, 72, 53–63. [Google Scholar] [CrossRef]
- Kumar, A.; Aery, N. Impact, metabolism, and toxicity of heavy metals in plants. In Plant Responses to Xenobiotics; Springer: Berlin, Germany, 2016; pp. 141–176. [Google Scholar]
- Song, W.-Y.; Choi, K.S.; Geisler, M.; Park, J.; Vincenzetti, V.; Schellenberg, M.; Kim, S.H.; Lim, Y.P.; Noh, E.W.; Lee, Y. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. Plant Cell 2010, 22, 2237–2252. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mishra, A.; Küpper, H. Protein biochemistry and expression regulation of cadmium/zinc pumping ATPases in the hyperaccumulator plants Arabidopsis halleri and Noccaea caerulescens. Front. Plant Sci. 2017, 8, 835. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.K.; Lu, L.L.; Yang, X.E.; Labavitch, J.M.; Huang, Y.Y.; Brown, P. Stem and leaf sequestration of zinc at the cellular level in the hyperaccumulator Sedum alfredii. New Phytol. 2009, 182, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Cosio, C.; DeSantis, L.; Frey, B.; Diallo, S.; Keller, C. Distribution of cadmium in leaves of Thlaspi caerulescens. J. Exp. Bot. 2005, 56, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ma, X.; Luo, S.; Gao, J.; Yang, X.; Feng, Y. SaZIP4, an uptake transporter of Zn/Cd hyperaccumulator Sedum alfredii Hance. Environ. Exp. Bot. 2018, 155, 107–117. [Google Scholar] [CrossRef]
- Becher, M.; Talke, I.N.; Krall, L.; Krämer, U. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. Plant J. 2004, 37, 251–268. [Google Scholar] [CrossRef]
- Sitko, K.; Rusinowski, S.; Kalaji, H.M.; Szopiński, M.; Małkowski, E. Photosynthetic efficiency as bioindicator of environmental pressure in A. halleri. Plant Physiol. 2017, 175, 290–302. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Choi, H.; Segami, S.; Cho, H.T.; Martinoia, E.; Maeshima, M.; Lee, Y. AtHMA1 contributes to the detoxification of excess Zn (II) in Arabidopsis. Plant J. 2009, 58, 737–753. [Google Scholar] [CrossRef]
- Dräger, D.B.; Desbrosses-Fonrouge, A.G.; Krach, C.; Chardonnens, A.N.; Meyer, R.C.; Saumitou-Laprade, P.; Krämer, U. Two genes encoding Arabidopsis halleri MTP1 metal transport proteins co-segregate with zinc tolerance and account for high MTP1 transcript levels. Plant J. 2004, 39, 425–439. [Google Scholar] [CrossRef]
- Gustin, J.L.; Loureiro, M.E.; Kim, D.; Na, G.; Tikhonova, M.; Salt, D.E. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. Plant J. 2009, 57, 1116–1127. [Google Scholar] [CrossRef]
- Küpper, H.; Kochian, L.V. Transcriptional regulation of metal transport genes and mineral nutrition during acclimatization to cadmium and zinc in the Cd/Zn hyperaccumulator, Thlaspi caerulescens (Ganges population). New Phytol. 2010, 185, 114–129. [Google Scholar] [CrossRef] [PubMed]
- Ishida, J.K.; Caldas, D.G.; Oliveira, L.R.; Frederici, G.C.; Leite, L.M.P.; Mui, T.S. Genome-wide characterization of the NRAMP gene family in Phaseolus vulgaris provides insights into functional implications during common bean development. Genet. Mol. Biol. 2018, 41, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Harada, E.; Vess, C.; Roepenack-Lahaye, E.V.; Clemens, S. Comparative microarray analysis of Arabidopsis thaliana and Arabidopsis halleri roots identifies nicotianamine synthase, a ZIP transporter and other genes as potential metal hyperaccumulation factors. Plant J. 2004, 37, 269–281. [Google Scholar] [CrossRef]
- Oomen, R.J.; Wu, J.; Lelièvre, F.; Blanchet, S.; Richaud, P.; Barbier-Brygoo, H.; Aarts, M.G.; Thomine, S. Functional characterization of NRAMP3 and NRAMP4 from the metal hyperaccumulator Thlaspi caerulescens. New Phytol. 2009, 181, 637–650. [Google Scholar] [CrossRef]
- Stein, R.J.; Höreth, S.; de Melo, J.R.F.; Syllwasschy, L.; Lee, G.; Garbin, M.L.; Clemens, S.; Krämer, U. Relationships between soil and leaf mineral composition are element-specific, environment-dependent and geographically structured in the emerging model Arabidopsis halleri. New Phytol. 2017, 213, 1274–1286. [Google Scholar] [CrossRef]
- Meyer, C.L.; Pauwels, M.; Briset, L.; Godé, C.; Salis, P.; Bourceaux, A.; Souleman, D.; Frérot, H.; Verbruggen, N. Potential preadaptation to anthropogenic pollution: Evidence from a common quantitative trait locus for zinc and cadmium tolerance in metallicolous and nonmetallicolous accessions of Arabidopsis halleri. New Phytol. 2016, 212, 934–943. [Google Scholar] [CrossRef]
- Gonneau, C.; Noret, N.; Gode, C.; Frerot, H.; Sirguey, C.; Sterckeman, T.; Pauwels, M. Demographic history of the trace metal hyperaccumulator Noccaea caerulescens (J. Presl and C. Presl) FK Mey. in Western Europe. Mol. Ecol. 2017, 26, 904–922. [Google Scholar] [CrossRef]
- Nowak, J.; Frérot, H.; Faure, N.; Glorieux, C.; Liné, C.; Pourrut, B.; Pauwels, M. Can zinc pollution promote adaptive evolution in plants? Insights from a one-generation selection experiment. J. Exp. Bot. 2018, 69, 5561–5572. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Aarts, M.G. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- Chao, Y.-E.; Zhang, M.; Tian, S.-K.; Lu, L.-L.; Yang, X.-E. Differential generation of hydrogen peroxide upon exposure to zinc and cadmium in the hyperaccumulating plant species (Sedum alfredii Hance). J. Zhejiang Univ. Sci. B 2008, 9, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Shohag, M.; Feng, Y.; He, Z.; Yang, X. Transcriptome comparison reveals the adaptive evolution of two contrasting ecotypes of Zn/Cd hyperaccumulator Sedum alfredii hance. Front. Plant Sci. 2017, 8, 425. [Google Scholar] [CrossRef] [PubMed]
- Deniau, A.; Pieper, B.; Ten Bookum, W.; Lindhout, P.; Aarts, M.; Schat, H. QTL analysis of cadmium and zinc accumulation in the heavy metal hyperaccumulator Thlaspi caerulescens. Theor. Appl. Genet. 2006, 113, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Willems, G.; Dräger, D.B.; Courbot, M.; Godé, C.; Verbruggen, N.; Saumitou-Laprade, P. The genetic basis of zinc tolerance in the metallophyte Arabidopsis halleri ssp. halleri (Brassicaceae): An analysis of quantitative trait loci. Genetics 2007, 176, 659–674. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Li, Y.; Luo, J.; Yu, J.; Xia, L.; Zhou, C.; Cai, L.; Ma, X. Improvement of the phytoremediation efficiency of Neyraudia reynaudiana for lead-zinc mine-contaminated soil under the interactive effect of earthworms and EDTA. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Basharat, Z.; Novo, L.A.; Yasmin, A. Genome editing weds CRISPR: What is in it for phytoremediation? Plants 2018, 7, 51. [Google Scholar] [CrossRef]
| Species | Family | Hyperaccumulation Criteria | References |
|---|---|---|---|
| Justicia procumbens | Acanthaceae | >10,000 ppm in LDW | [101] |
| Arabidopsis helleri | Brassicaceaa | >10,000 ppm in LDW | [97] |
| Noccaea caerulescens | Brassicaceaa | >10,000 ppm in LDW | [97] |
| Arabis paniculata | Brassicaceae | >10,000 ppm in LDW | [102] |
| Noccaea eburneosa | Brassicaceae | Zn concentration in shoot %DW 1.05 | [103] |
| Noccaea alpestre | Brassicaceae | >10,000 ppm in LDW | [103] |
| Noccaea bulbosum Spruner | Brassicaceae | Zn concentration in shoot %DW 1.05 | [103] |
| Noccaea calaminare | Brassicaceae | >10,000 ppm in LDW | [103] |
| Noccaea limosellifolium | Brassicaceae | Zn concentration in shoot %DW 1.10 | [103] |
| Noccaea praecox | Brassicaceae | >10,000 ppm in LDW | [103] |
| Arabis gemmifera | Brassicaceae | >10,000 ppm in LDW | [101] |
| Noccaeagoesingense | Brassicaceae | >10,000 ppm in LDW | [101] |
| Noccaea brachypetalum | Brassicaceae | Zn concentration in shoot %DW 1.53 | [101] |
| Noccaea cepaeifolium subsp Rotundifolium, | Brassicaceae | Zn concentration in shoot %DW 2.10 | [101] |
| Noccaea stenopterum | Brassicaceae | >10,000 ppm in LDW | [101] |
| Noccaea tatrense | Brassicaceae | >10,000 ppm in LDW | [101] |
| Minuartia verna | Caryophyllaceae | Zn concentration in shoot %DW 1.14 | [101] |
| Polycarpaea synandra | Caryophyllaceae | >3000 ppm (6960 ppm DW) | [101] |
| Sedum alfredii | Crassulaceae | shoot: root ratio >1 | [101] |
| Sedum plumbizincicola | Crassulaceae | >10,000 ppm in LDW | [101] |
| Dichapetalum geloniodes subsp.sumatranum | Dichapetalaceae | >10,000 ppm in LDW | [101] |
| Dichapetalum gelonioides | Dichapetalaceae | >10,000 ppm in LDW | [101] |
| Anthyllis vulneraria | Fabaceae | >10,000 ppm in LDW | [101] |
| Haumaniastrum katanngense | Lamiaceae | Zn concentration in shoot %DW 1.98 | [103] |
| Ficus parietalis | Moraceae | - | [101] |
| Potentilla griffithii | Rosaceae | >10,000 ppm in LDW | [104] |
| Rinorea longiracemosa | Violaceae | - | [101] |
| Viola calaminaria | Violaceae | >10,000 ppm in LDW | [103] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balafrej, H.; Bogusz, D.; Triqui, Z.-E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc Hyperaccumulation in Plants: A Review. Plants 2020, 9, 562. https://doi.org/10.3390/plants9050562
Balafrej H, Bogusz D, Triqui Z-EA, Guedira A, Bendaou N, Smouni A, Fahr M. Zinc Hyperaccumulation in Plants: A Review. Plants. 2020; 9(5):562. https://doi.org/10.3390/plants9050562
Chicago/Turabian StyleBalafrej, Habiba, Didier Bogusz, Zine-El Abidine Triqui, Abdelkarim Guedira, Najib Bendaou, Abdelaziz Smouni, and Mouna Fahr. 2020. "Zinc Hyperaccumulation in Plants: A Review" Plants 9, no. 5: 562. https://doi.org/10.3390/plants9050562
APA StyleBalafrej, H., Bogusz, D., Triqui, Z.-E. A., Guedira, A., Bendaou, N., Smouni, A., & Fahr, M. (2020). Zinc Hyperaccumulation in Plants: A Review. Plants, 9(5), 562. https://doi.org/10.3390/plants9050562