Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Herbicide Treatments
2.2. Measurement of Chlorophyll a Fluorescence
2.3. Stomatal Conductance, Chlorophyll Content Index, and Leaf Temperature
2.4. Statistical Analysis
3. Results
3.1. Chlorophyll and Fluorescence Induction Curves
3.2. Chlorophyll and Fluorescence Parameters
3.3. Chlorophyll Content Index, Leaf Temperature, and Stomatal Conductance (gs)
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Manas, F.; Peralta, L.; Raviolo, J.; Garcia, O.H.; Weyers, A.; Ugnia, L.; Gonzalez, C.M.; Larripa, I.; Gorla, N. Genotoxicity of glyphosate assessed by the comet assay and cytogenetictests. Environ. Toxicol. Pharmacol. 2009, 28, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ventrella, A.; Catucci, L.; Agostiano, A. Herbicides affect fluorescence and electron transfer activity of spinach chloroplasts, thylakoid membranes and isolated Photosystem II. Bioelectrochemistry 2010, 79, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.G.; Teicher, H.B.; Streibig, J.C. Linking fluorescence induction curve and biomass in herbicide screening. Pest Manag. Sci. 2003, 59, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.W.; Dahllöf, I. Direct and indirect effects of the herbicides Glyphosate, Bentazone and MCPA on eelgrass (Zostera marina). Aquat. Toxicol. 2007, 82, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Pei, W.; Hui, L.; Roland, G. Chlorophyll fluorescence response to herbicide stress in Alopecurus myosuroides. In Proceedings of the Deutsche Arbeitsbesprechung über Fragen der Unkrautbiologie und Bekämpfung, Braunschweig, Germany, 23–25 February 2016; Volume 452, pp. 57–67. [Google Scholar]
- Vermaas, W.F.; Steinback, K.E.; Arntzen, C.J. Characterization of chloroplast thylakoid polypeptides in the 32-kDa region: Polypeptide extraction and protein phosphorylation affect binding of photosystem II-directed herbicides. Arch. Biochem. Biophys. 1984, 231, 226–232. [Google Scholar] [CrossRef]
- Armel, G.R.; Rardon, P.L.; McComrick, M.C.; Ferry, N.M. Differential Response of Several Carotenoid Biosynthesis Inhibitors in Mixtures with Atrazine. Weed Technol. 2007, 21, 947–953. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Ladle, R.J.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.I.; Brestič, M.; Bussotti, F.; Calatayud, Á.; Dabrowski, P.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Rastogi, A.; Zivcak, M.; Brestič, M.; Daszkowska-Golec, A.; Sitko, K.; Alsharafa, K.Y.; Lotfi, R.; Stypinski, P.; Samborska, I.A.; et al. Prompt chlorophyll fluorescence as a tool for crop phenotyping: An example of barley landraces exposed to various abiotic stress factors. Photosynthetica 2018, 56, 953–961. [Google Scholar] [CrossRef]
- Sujak, A. Interaction between cadmium, zinc and silver-substituted plastocyanin and cytochrome b6f complex—Heavy metals toxicity towards photosynthetic apparatus. Acta Physiol. Plant. 2005, 27, 61–69. [Google Scholar] [CrossRef]
- Sun, L.; Xu, H.; Hao, H.; An, S.; Lu, C.; Wu, R.; Su, W. Effects of bensulfuron-methyl residue on photosynthesis and chlorophyll fluorescence in leaves of cucumber seedlings. PLoS ONE 2019, 14, e0215486. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Tripathi, D.; Yadav, S.; Kalaji, H.; Brestic, M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Hassannejad, S.; Porheidar, S.; Lotfi, R. The effect of nicosulfuron and bentazon on photosynthetic performance of common cocklebur (Xanthium strumarium L.). Environ. Sustain. Indic. 2020, 6, 100026. [Google Scholar] [CrossRef]
- Lotfi, R.; Kalaji, H.M.; Valizadeh, G.R.; Behrozyar, E.K.; Hemati, A.; Gharavi-Kochebagh, P.; Ghassemi, A. Effects of humic acid on photosynthetic efficiency of rapeseed plants growing under different watering conditions. Photosynthetica 2017, 56, 962–970. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Pathom-Aree, W.; Lotfi, R.; Balaji, P.; Elshery, N.; Grska, E.B.; Swiatek, M.; Horaczek, T.; Mojski, J.; Kociel, H.; et al. Effect of Microbial Consortia on Photosynthetic Efficiency of Arabidopsis thaliana under Drought Stress. Chiang Mai J. Sci. 2018, 45, 1–10. [Google Scholar]
- Rastogi, A.; Kovar, M.; He, X.; Zivcak, M.; Kataria, S.; Kalaji, H.; Skalicky, M.; Ibrahimova, U.; Hussain, S.; Mbarki, S.; et al. Special issue in honour of Prof. Reto J. Strasser—JIP-test as a tool to identify salinity tolerance in sweet sorghum genotypes. Photosynthetica 2020, 58, 518–528. [Google Scholar] [CrossRef]
- Rastogi, A.; Stróżecki, M.; Kalaji, H.M.; Łuców, D.; Lamentowicz, M.; Juszczak, R. Impact of warming and reduced precipitation on photosynthetic and remote sensing properties of peatland vegetation. Environ. Exp. Bot. 2019, 160, 71–80. [Google Scholar] [CrossRef]
- Goltsev, V.N.; Kalaji, H.M.; Paunov, M.; Bąba, W.; Horaczek, T.; Mojski, J.; Kociel, H.; Allakhverdiev, S.I. Variable chlorophyll fluorescence and its use for assessing physiological condition of plant photosynthetic apparatus. Russ. J. Plant Physiol. 2016, 63, 869–893. [Google Scholar] [CrossRef]
- Li, R.; Guo, P.-G.; Michael, B.; Stefania, G.; Salvatore, C. Evaluation of Chlorophyll Content and Fluorescence Parameters as Indicators of Drought Tolerance in Barley. Agric. Sci. China 2006, 5, 751–757. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Plant Cell Monographs; Springer Science and Business Media LLC: Basel, Switzerland, 2004; Volume 19, pp. 321–362. [Google Scholar]
- Strasserf, R.J.; Srivastava, A. Polyphasic Chlorophyll a Fluorescence Transient in Plants and Cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants Without Soil. In Circular; California agricultural experiment station: Berkeley, CA, USA, 1950; p. 347. [Google Scholar]
- Dayan, F.E.; Zaccaro, M.L.D.M. Chlorophyll fluorescence as a marker for herbicide mechanisms of action. Pestic. Biochem. Physiol. 2012, 102, 189–197. [Google Scholar] [CrossRef]
- Stirbet, A.; Riznichenko, G.; Rubin, A.B. Govindjee Modeling chlorophyll a fluorescence transient: Relation to photosynthesis. Biochemistry 2014, 79, 291–323. [Google Scholar] [CrossRef]
- Toth, S.Z.; Schansker, G.; Garab, G.; Strasser, R.J. Photosynthetic electron transport activity in heat-treated barley leaves: The role of internal alternative electron donors to photosystem II. Biochim. Biophys. Acta (BBA) Bioenerg. 2007, 1767, 295–305. [Google Scholar] [CrossRef]
- Govindjee, X.C.; Schansker, G.; Van Rensen, J.J. Chloroacetates as inhibitors of photosystem II: Effects on electron acceptor side. J. Photochem. Photobiol. B Boil. 1997, 37, 107–117. [Google Scholar] [CrossRef]
- Hess, F.D. Light-dependent herbicides: An overview. Weed Sci. 2000, 48, 160–170. [Google Scholar]
- Ikeda, Y.; Ohki, S.; Koizumi, K.; Tanaka, A.; Watanabe, H.; Kohno, H.; Van Rensen, J.J.S.; Böger, P.; Wakabayashi, K. Binding site of novel 2-benzylamino-4-methyl-6-trifluoromethyl-1,3,5-triazine herbicides in the D1 protein of Photosystem II. Photosynth. Res. 2003, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.J.; Lim, S.H.; Kim, J.W.; Nah, G.; Fischer, A.; Kim, D.S. Leaf chlorophyll fluorescence discriminates herbicide resistance inEchinochloaspecies. Weed Res. 2016, 56, 424–433. [Google Scholar] [CrossRef]
- Duysens, L.M.N.; Sweers, H.E. Studies on Microalgae and Photosynthetic Bacteria; Japanese Society of Plant Physiologists, University of Tokyo Press: Tokyo, Japan, 1963; pp. 353–372. [Google Scholar]
- Govindjee. Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; pp. 1–41. [Google Scholar]
- Cobb, A.H.; Reade, J.P. Herbicides and Plant Physiology; John Wiley & Sons Ltd.: West Sussex, UK, 2010. [Google Scholar]
- Justin, M.W.; Jordan, M.C.; Gloria, K.M. Abscisic Acid-Induced Reactive Oxygen Species Are Modulated by Flavonols to Control Stomata Aperture. Pant Physiol. 2017, 175, 1807–1825. [Google Scholar]
- Chiang, Y.-J.; Wu, Y.-X.; Chiang, M.-Y.; Wang, C.-Y. Role of Antioxidative System in Paraquat Resistance of Tall Fleabane (Conyza sumatrensis). Weed Sci. 2008, 56, 350–355. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, C.-H. Mechanism for photoinactivation of PSII by methyl viologen at two temperatures in the leaves of rice (Oryza sativa L.). J. Plant Boil. 2003, 46, 10–16. [Google Scholar] [CrossRef]
- Lascano, H.R.; Melchiorre, M.N.; Luna, C.M.; Trippi, V.S. Effect of photo-oxidative stress induced by paraquat in two wheat cultivars with differential tolerance to water stress. Plant Sci. 2003, 164, 841–848. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E. Bioactivity of Herbicides. In Comprehensive Biotechnology; Acadmic press: Cambridge, MA, USA, 2011; pp. 23–35. [Google Scholar]
- Fracheboud, Y.; Jompuk, C.; Ribaut, J.M.; Stamp, P.; Leipner, J. Genetic analysis of cold-tolerance of photosynthesis in maize. Plant Mol. Boil. 2004, 56, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M. Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Sci. 1993, 94, 19–33. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y.; Hua, W.; Last, R.L. A New Light on Photosystem II Maintenance in Oxygenic Photosynthesis. Front. Plant Sci. 2019, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef] [PubMed]
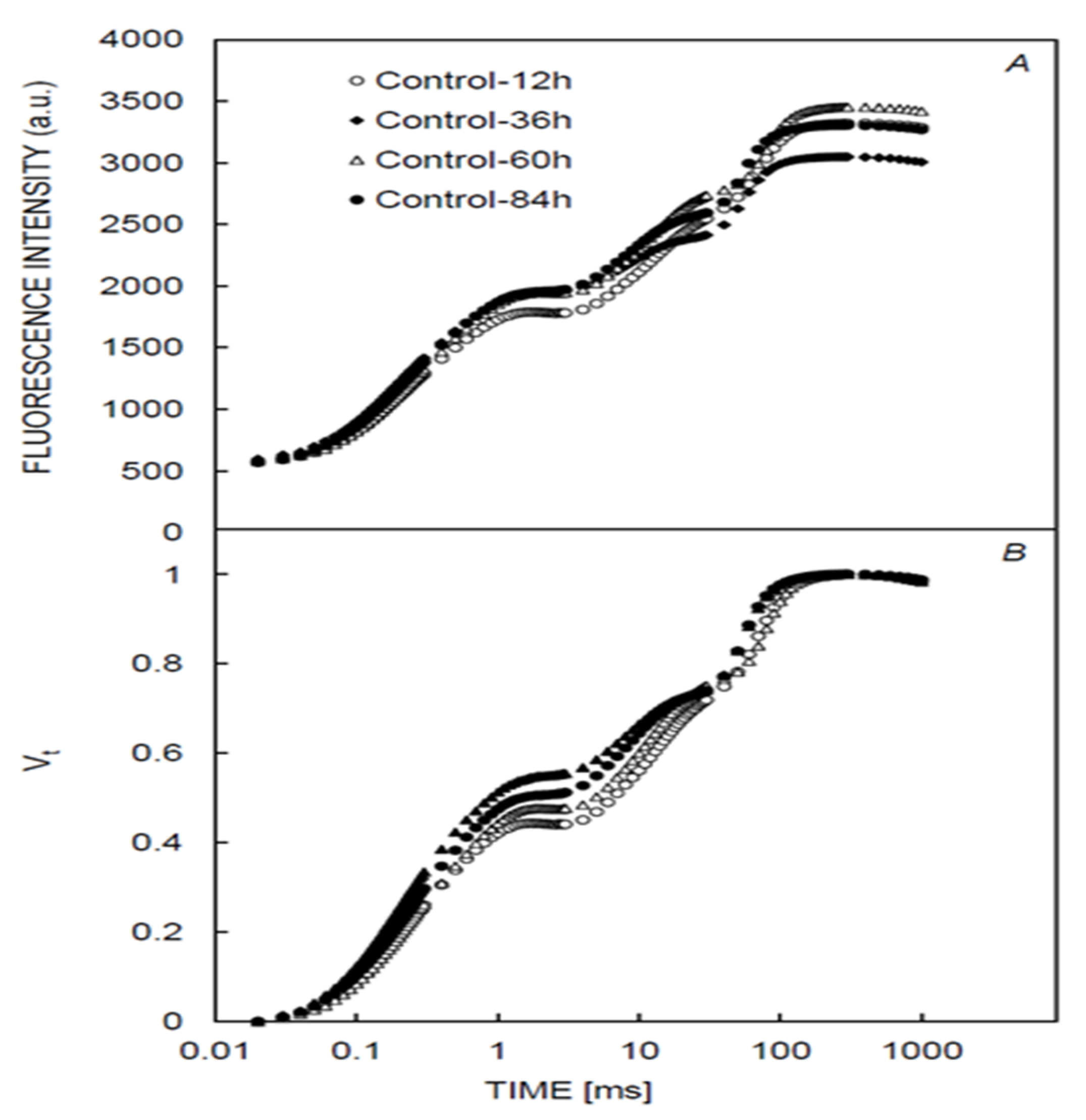
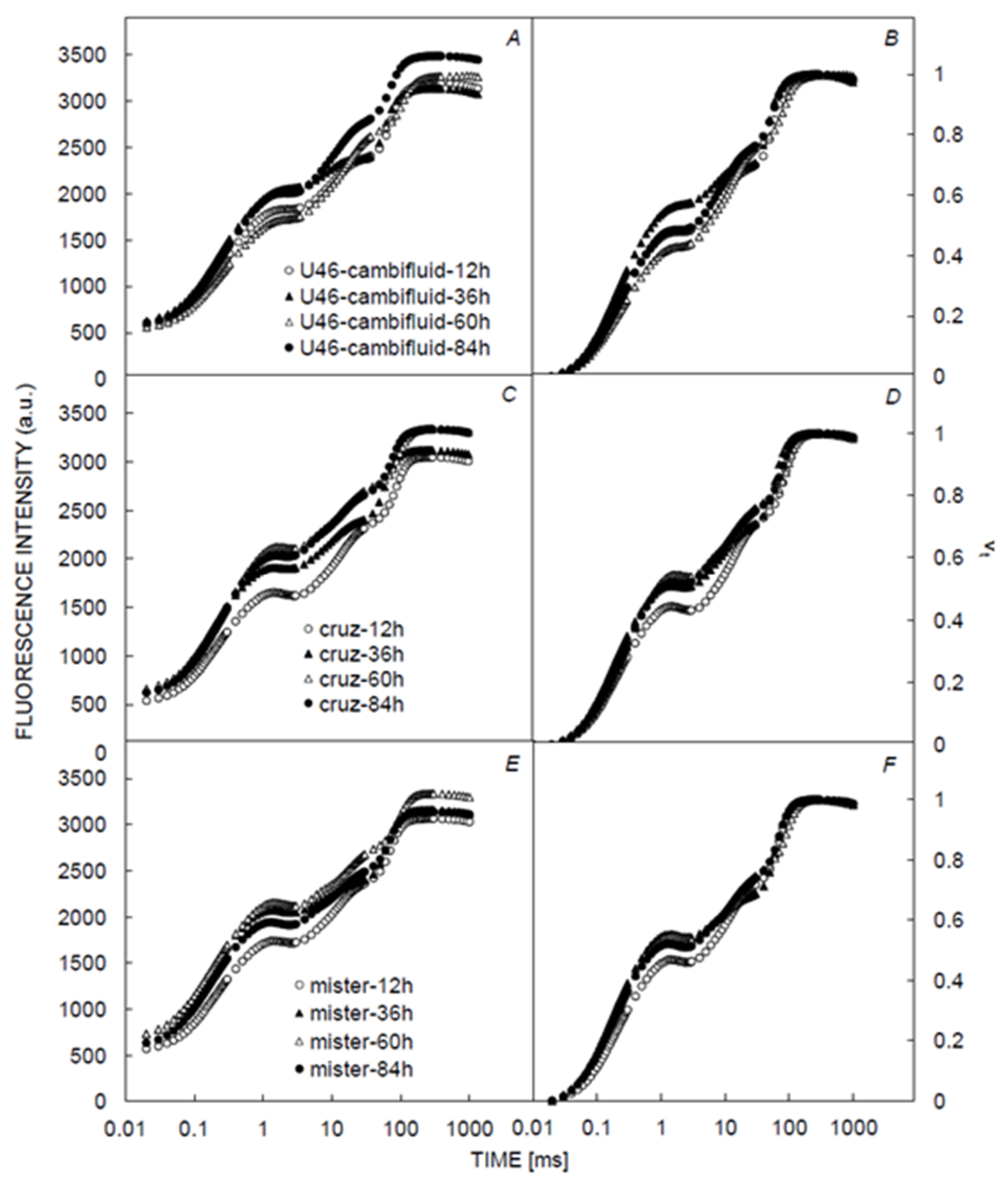
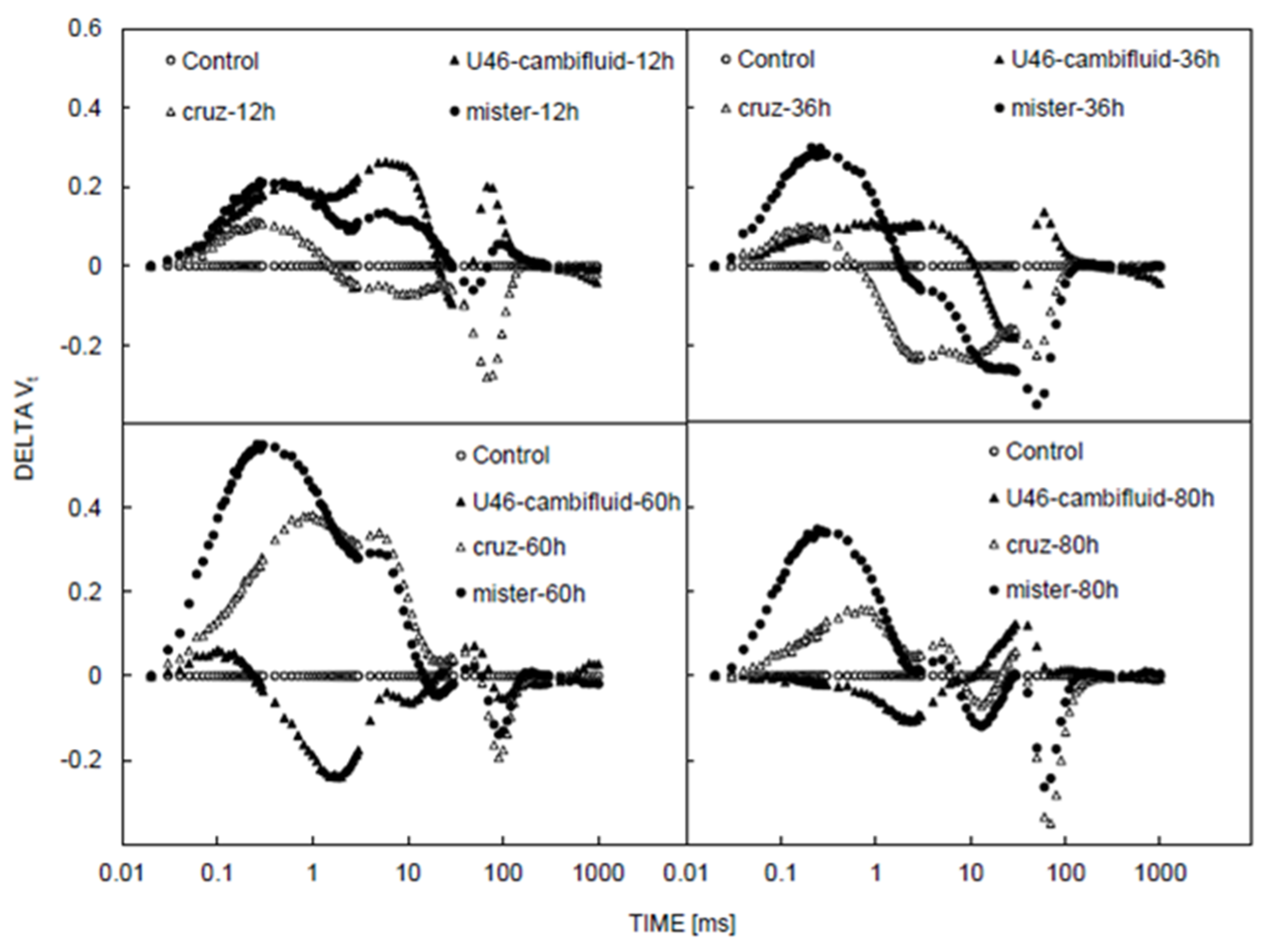
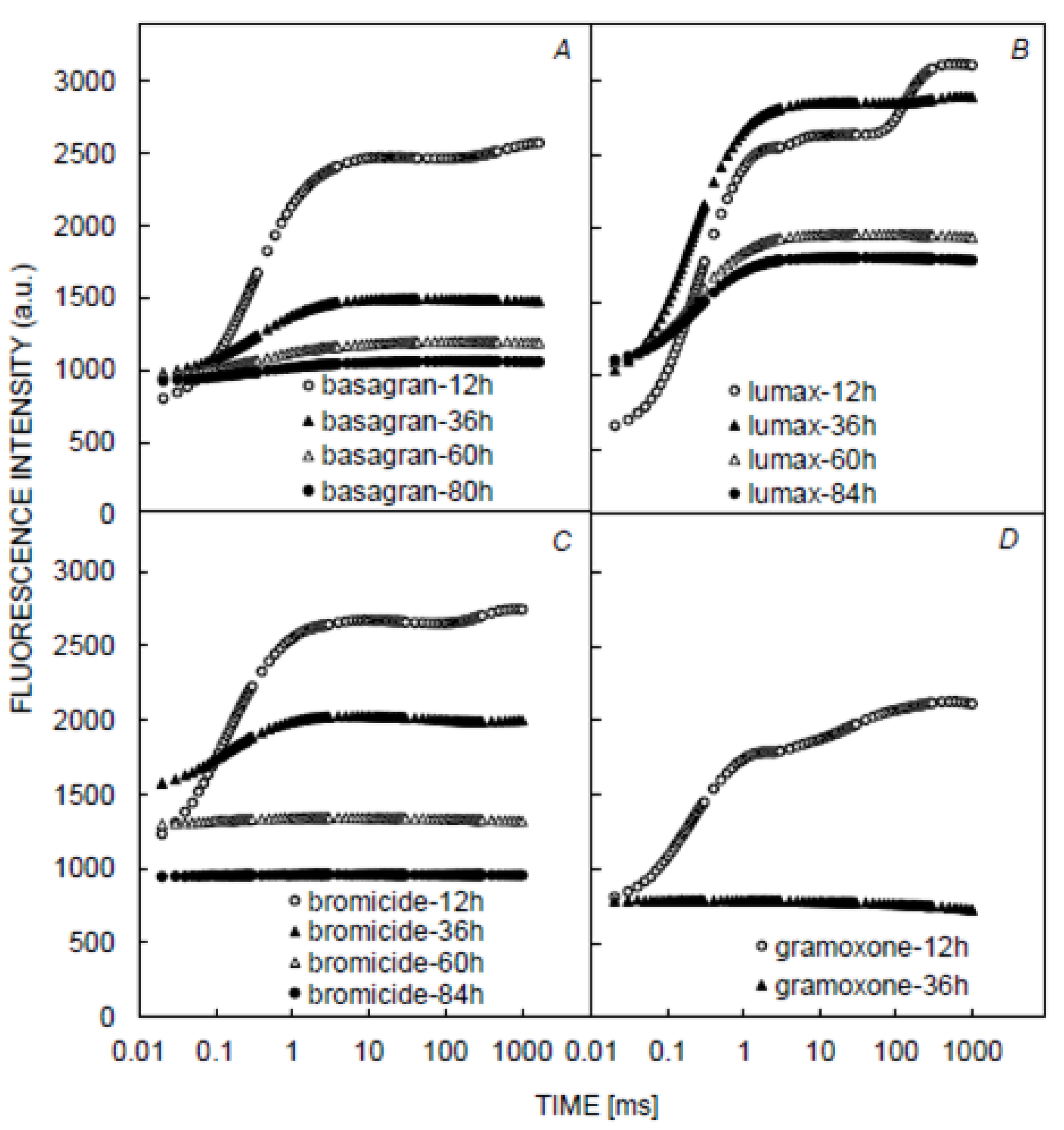
| CCI | LT | gs | Fm | F0 | Fv | Fv/Fm | Fv/Fo | PI | Sm | Tfm | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | |||||||||||
| 12 | 17.8 | 17.5 | 1.45 | 3319 | 492 | 2827 | 0.85 | 5.75 | 2.37 | 22.99 | 300 |
| 36 | 19.3 | 18.2 | 0.52 | 3047 | 511 | 2536 | 0.83 | 4.96 | 1.27 | 16.88 | 270 |
| 60 | 16.7 | 23.7 | 0.59 | 3450 | 486 | 2964 | 0.86 | 6.10 | 2.35 | 22.54 | 290 |
| 84 | 17.7 | 29.7 | 0.15 | 3304 | 484 | 2820 | 0.85 | 5.83 | 1.87 | 17.23 | 290 |
| U.46 CombiFluid | |||||||||||
| 12 | 18.2 | 15.9 | 1.15 | 3188 | 501 | 2687 | 0.84 | 5.36 | 1.80 | 20.10 | 250 |
| 36 | 18.8 | 13.6 | 1.38 | 3135 | 546 | 2589 | 0.83 | 4.74 | 1.10 | 16.07 | 240 |
| 60 | 19.1 | 21.7 | 0.43 | 3271 | 491 | 2780 | 0.85 | 5.66 | 2.47 | 24.17 | 700 |
| 84 | 19.3 | 29.4 | 0.38 | 3884 | 519 | 3365 | 0.85 | 6.48 | 1.93 | 14.50 | 270 |
| Cruz | |||||||||||
| 12 | 16 | 17.1 | 0.88 | 3050 | 464 | 2586 | 0.85 | 5.57 | 2.13 | 26.37 | 290 |
| 36 | 15.5 | 15.9 | 1.75 | 3123 | 572 | 2551 | 0.82 | 4.46 | 1.18 | 19.44 | 230 |
| 60 | 15.4 | 22.3 | 1.28 | 3342 | 585 | 2757 | 0.83 | 4.71 | 1.29 | 23.65 | 290 |
| 84 | 14.1 | 29.2 | 1.23 | 3236 | 536 | 2700 | 0.84 | 5.22 | 1.49 | 20.71 | 290 |
| Mister | |||||||||||
| 12 | 15.9 | 19.7 | 0.34 | 3065 | 492 | 2573 | 0.84 | 5.23 | 1.76 | 21.84 | 270 |
| 36 | 15 | 18.6 | 0.35 | 3157 | 665 | 2492 | 0.79 | 3.75 | 0.80 | 21.03 | 280 |
| 60 | 13.1 | 20.3 | 0.42 | 3334 | 675 | 2659 | 0.80 | 3.94 | 0.92 | 23.47 | 280 |
| 84 | 13.8 | 30.4 | 0.16 | 3139 | 559 | 2580 | 0.82 | 4.62 | 1.17 | 19.53 | 260 |
| Basagran | |||||||||||
| 12 | 14.6 | 17.3 | 0.1667 | 2569 | 741 | 1828 | 0.71 | 2.47 | 0.10 | 20.02 | 900 |
| 36 | 14 | 22.2 | 0.42 | 1493 | 978 | 515 | 0.35 | 0.85 | 0.01 | 0.78 | 100 |
| 60 | 13.7 | 33 | 0.32 | 1196 | 968 | 228 | 0.19 | 0.75 | 0.01 | 1.75 | 100 |
| 84 | 11 | 27.5 | 0.1667 | 1061 | 926 | 135 | 0.13 | 0.72 | 0.00 | 1.48 | 80 |
| Bromicide | |||||||||||
| 12 | 14.2 | 18.2 | 0.28 | 2746 | 1231 | 1515 | 0.55 | 1.23 | 0.02 | 17.95 | 900 |
| 36 | 14.8 | 18.2 | 0.1733 | 2028 | 1583 | 445 | 0.22 | 0.76 | 0.00 | 0.13 | 7 |
| 60 | 14.6 | 21.8 | 0.1513 | 1344 | 1304 | 40 | 0.03 | 0.68 | 0.00 | 0.13 | 5 |
| 84 | 12.9 | 30.2 | 0.1643 | 963 | 949 | 14.67 | 0.02 | 0.68 | 0.00 | 0.13 | 5 |
| Lumax | |||||||||||
| 12 | 13.1 | 19.7 | 0.1497 | 3120 | 552 | 2568 | 0.82 | 4.65 | 0.45 | 28.66 | 700 |
| 36 | 12.2 | 19 | 0.36 | 2898 | 972 | 1926 | 0.67 | 1.98 | 0.03 | 6.33 | 800 |
| 60 | 12.8 | 21.1 | 0.38 | 1962 | 1076 | 886 | 0.45 | 0.95 | 0.01 | 0.45 | 28 |
| 84 | 12.5 | 30.3 | 0.1543 | 1804 | 1092 | 712 | 0.40 | 0.89 | 0.01 | 0.28 | 27 |
| Gramoxone | |||||||||||
| 12 | 17.2 | 19.8 | 0.17 | 2128 | 775 | 1353 | 0.64 | 1.75 | 0.14 | 13.60 | 600 |
| 36 | 3.9 | 23.3 | 0.66 | 786 | 788 | 15 | 0.37 | 0.68 | 0.00 | 0.13 | 4 |
| 60 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0 |
| 84 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0.00 | 0.00 | 0.00 | 0 |
| LSD 5% | 0.9 | 0.493 | 0.124 | 21.4 | 9.05 | 6.16 | 0.032 | 0.773 | 0.008 | 0.152 | 4.17 |
| LSD 1% | 1.2 | 0.659 | 0.166 | 28.6 | 12.09 | 8.23 | 0.043 | 1.03 | 0.001 | 0.205 | 5.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants 2020, 9, 529. https://doi.org/10.3390/plants9040529
Hassannejad S, Lotfi R, Ghafarbi SP, Oukarroum A, Abbasi A, Kalaji HM, Rastogi A. Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants. 2020; 9(4):529. https://doi.org/10.3390/plants9040529
Chicago/Turabian StyleHassannejad, Sirous, Ramin Lotfi, Soheila P Ghafarbi, Abdallah Oukarroum, Amin Abbasi, Hazem M Kalaji, and Anshu Rastogi. 2020. "Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements" Plants 9, no. 4: 529. https://doi.org/10.3390/plants9040529
APA StyleHassannejad, S., Lotfi, R., Ghafarbi, S. P., Oukarroum, A., Abbasi, A., Kalaji, H. M., & Rastogi, A. (2020). Early Identification of Herbicide Modes of Action by the Use of Chlorophyll Fluorescence Measurements. Plants, 9(4), 529. https://doi.org/10.3390/plants9040529







