Validation of a Preformulated, Field Deployable, Recombinase Polymerase Amplification Assay for Phytophthora Species
Abstract
1. Introduction
2. Results
2.1. Comparable Results Were Observed between Preformulated and Commercially Available RPA Reactions
2.2. Multiple Platforms Were Effective at Detecting Phytophthora Species by RPA
2.3. Accurate Identification of Phytophthora-Infected Material Was Possible in a Single Blind Sample Evaluation of the Preformulated Assay
2.4. Phytophthora-Infected Fresh Plant Material Can Be Identified in under 15 Min
3. Discussion
4. Materials and Methods
4.1. Reagents and Assay Conditions
4.2. Production of Pure DNA and Crude Plant Extracts
4.3. Initial Evaluation of Preformulated lyophilized Kits Reaction
4.4. Limit of Detection Determination for Preformulated Lyophilized Kits
4.5. Single Blind Multi-Lab Evaluation of Preformulated Lyophilized Kits Reaction
4.6. Fresh Sample Testing
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, F.; Abad, Z.G.; Balci, Y.; Ivors, K. Identification and detection of Phytophthora: Reviewing our progress, identifying our needs. Plant Dis. 2012, 96, 1080–1103. [Google Scholar] [CrossRef] [PubMed]
- Barber, P.; Paap, T.; Burgess, T.; Dunstan, W.; Hardy, G. A diverse range of Phytophthora species are associated with dying urban trees. Urban For. Urban Green. 2013, 12, 569–575. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society: St. Paul, MN, USA, 1996; Volume 90. [Google Scholar]
- Ali-Shtayeh, M.S.; MacDonald, J.D.; Kabashima, J. A method for using commercial ELISA tests to detect zoospores of Phytophthora and Pythium species in irrigation water. Plant Dis. 1991, 75, 305–311. [Google Scholar] [CrossRef]
- Bulluck, R.; Shiel, P.; Berger, P.; Kaplan, D.; Parra, G.; Li, W.; Palm, M. A Comparative analysis of detection techniques used in US regulatory programs to determine Presence of Phytophthora ramorum in Camellia japonica ‘Nucio’s Gem’ in an infested nursery in southern California. Plant Health Prog. 2006, 7, 9. [Google Scholar] [CrossRef]
- Kox, L.F.F.; Van Brouwershaven, I.R.; Van De Vossenberg, B.T.L.H.; Van Den Beld, H.E.; Bonants, P.J.M.; De Gruyter, J. Diagnostic values and utility of immunological, morphological, and molecular methods for in planta detection of Phytophthora ramorum. Phytopathology 2007, 97, 1119–1129. [Google Scholar] [CrossRef]
- O’Brien, P.; Williams, N.; Hardy, G. Detecting Phytophthora. Crit. Rev. Microbiol. 2009, 35, 169–181. [Google Scholar] [CrossRef]
- Timmer, L.W.; Menge, J.A.; Zitko, S.E.; Pond, E.; Miller, S.A.; Johnson, E.L. Comparison of ELISA techniques and standard isolation methods for Phytophthora detection in citrus orchards in Florida and California. Plant Dis. 1993, 77, 791–796. [Google Scholar] [CrossRef]
- Goheen, E.M.; Hansen, E.; Kanaskie, A.; Osterbauer, N.; Parke, J.; Pscheidt, J.; Chastagner, G. Sudden Oak Death and Phytophthora Ramorum: A Guide for Forest Managers, Christmas Tree Growers, and Forest-Tree Nursery Operators in Oregon and Washington; Oregon State University Extension Services: Corvallis, OR, USA, 2006. [Google Scholar]
- MacDonald, J.D.; Stites, J.; Kabashima, J. Comparison of serological and culture plate methods for detecting species of Phytophthora, Pythium, and Rhizoctonia in ornamental plants. Plant Dis. 1990, 74, 655–659. [Google Scholar] [CrossRef]
- Bilodeau, G.; Pelletier, G.; Pelletier, F.; Lévesque, C.A.; Hamelin, R.C. Multiplex real-time polymerase chain reaction (PCR) for detection of Phytophthora ramorum, the causal agent of sudden oak death. Can. J. Plant Pathol. 2009, 31, 195–210. [Google Scholar] [CrossRef]
- Bilodeau, G.J.; Lévesque, C.A.; De Cock, A.W.A.M.; Duchaine, C.; Brière, S.; Uribe, P.; Hamelin, R.C. Molecular detection of Phytophthora ramorum by real-time polymerase chain reaction using TaqMan, SYBR Green, and molecular beacons. Phytopathology 2007, 97, 632–642. [Google Scholar] [CrossRef]
- Garbelotto, M.; Rizzo, D.M.; Hayden, K.; Meija-Chang, M.; Davidson, J.M.; Tjosvold, S. Phytophthora Ramorum and Sudden Oak Death in California: III. Preliminary Studies in Pathogen Genetics. US Dep. Agric. For. Serv. Gen. Tech. PSW-GTR-184, 5th Symp. Calif. Oak Woodlands; Standiford, R., McCreary, D., Eds.; Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture: Albany, CA, USA, 2002; pp. 765–774.
- Hussain, S.; Lees, A.K.; Duncan, J.M.; Cooke, D.E.L. Development of a species-specific and sensitive detection assay for Phytophthora infestans and its application for monitoring of inoculum in tubers and soil. Plant Pathol. 2005, 54, 373–382. [Google Scholar] [CrossRef]
- Martin, F.N.; Tooley, P.W.; Blomquist, C. Molecular detection of Phytophthora ramorum, the causal agent of sudden oak death in California, and two additional species commonly recovered from diseased plant material. Phytopathology 2004, 94, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Schena, L.; Hughes, K.J.D.; Cooke, D.E.L. Detection and quantification of Phytophthora ramorum, P. kernoviae, P. citricola and P. quercina in symptomatic leaves by multiplex real-time PCR. Mol. Plant Pathol. 2006, 7, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Schena, L.; Duncan, J.M.; Cooke, D.E.L. Development and application of a PCR-based “molecular tool box” for the identification of Phytophthora species damaging forests and natural ecosystems. Plant Pathol. 2008, 57, 64–75. [Google Scholar] [CrossRef]
- Winton, L.M.; Hansen, E.M. Molecular diagnosis of Phytophthora lateralis in trees, water, and foliage baits using multiplex polymerase chain reaction. For. Pathol. 2001, 31, 275–283. [Google Scholar] [CrossRef]
- Rojas, J.A.; Miles, T.D.; Coffey, M.D.; Martin, F.N.; Chilvers, M.I. Development and application of qPCR and RPA genus- and species-specific detection of Phytophthora sojae and P. sansomeana root rot pathogens of soybean. Plant Dis. 2017, 101, 1171–1181. [Google Scholar] [CrossRef]
- Miles, T.D.; Martin, F.N.; Coffey, M.D. Development of rapid isothermal amplification assays for detection of Phytophthora spp. in plant tissue. Phytopathology 2015, 105, 265–278. [Google Scholar] [CrossRef]
- Tomlinson, J.; Dickinson, M.; Boonham, N. Rapid detection of Phytophthora ramorum and P. kernoviae by two-minute DNA extraction followed by isothermal amplification and amplicon detection by generic lateral flow device. Phytopathology 2010, 100, 143–149. [Google Scholar] [CrossRef]
- Khan, M.; Li, B.; Yue, J.; Weng, Q.; Chen, Q. Evaluation of different PCR-Based assays and LAMP method for rapid detection of Phytophthora infestans by targeting the Ypt1 gene. Gene Front. Microbiol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Dai, T.-T.; Yang, X.; Hu, T.; Li, Z.; Xu, Y.; Lu, C. A novel LAMP assay for the detection of Phytophthora cinnamomi utilizing a new target gene identified from genome sequences. Plant Dis. 2019. first look. [Google Scholar] [CrossRef]
- Chen, Q.; Li, B.; Liu, P.; Lan, C.; Zhan, Z.; Weng, Q. Development and evaluation of specific PCR and LAMP assays for the rapid detection of Phytophthora melonis. Eur. J. Plant Pathol. 2013, 137, 597–607. [Google Scholar] [CrossRef]
- Li, B.; Liu, P.; Xie, S.; Yin, R.; Weng, Q.; Chen, Q. Specific and sensitive detection of Phytophthora nicotianae by nested PCR and loop-mediated isothermal amplification assays. J. Phytopathol. 2014, 163, 185–193. [Google Scholar] [CrossRef]
- Dai, T.-T.; Lu, C.-C.; Lu, J.; Dong, S.; Ye, W.; Wang, Y.; Zheng, X. Development of a loop-mediated isothermal amplification assay for detection of Phytophthora sojae. FEMS Microbiol. Lett. 2012, 334, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, 1–7. [Google Scholar] [CrossRef]
- Tomlinson, J.A.; Barker, I.; Boonham, N. Faster, simpler, more-specific methods for improved molecular detection of Phytophthora ramorum in the field. Appl. Environ. Microbiol. 2007, 73, 4040–4047. [Google Scholar] [CrossRef]
- Craw, P.; Balachandran, W. Isothermal nucleic acid amplification technologies for point of-care diagnostics: A critical review. Lab Chip 2012, 12, 2469–2486. [Google Scholar] [CrossRef]
- Strayer-Scherer, A.; Jones, J.B.; Paret, M.L. Recombinase polymerase amplification assay for field detection of tomato bacterial spot pathogens. Phytopathology 2019, 109, 690–700. [Google Scholar] [CrossRef]
- Londoño, M.A.; Harmon, C.L.; Polston, J.E. Evaluation of recombinase polymerase amplification for detection of begomoviruses by plant diagnostic clinics. Virol. J. 2016, 13, 48. [Google Scholar] [CrossRef]
- Mekuria, T.A.; Zhang, S.; Eastwell, K.C. Rapid and sensitive detection of Little cherry virus 2 using isothermal reverse transcription-recombinase polymerase amplification. J. Virol. Methods 2014, 205, 24–30. [Google Scholar] [CrossRef]
- Zhang, S.; Ravelonandro, M.; Russell, P.; McOwen, N.; Briard, P.; Bohannon, S.; Vrient, A. Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP® using reverse transcription-recombinase polymerase amplification. J. Virol. Methods 2014, 207, 114–120. [Google Scholar] [CrossRef]
- Burkhardt, A.; Koike, S.T.; Henry, P.; Gordon, T.; Martin, F.N. Detection of Fusarium oxysporum f. species fragariae from infected strawberry plants. Plant Dis. 2018, 103, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, A.; Ramon, M.L.; Smith, B.; Koike, S.T.; Martin, F.N. Development of molecular methods to detect Macrophomina phaseolina from strawberry plants and soil. Phytopathology 2018, 108, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. TrAC Trends Anal. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Lane, C.R.; Hobden, E.; Walker, L.; Barton, V.C.; Inman, A.J.; Hughes, K.J.D.; Barker, I. Evaluation of a rapid diagnostic field test kit for identification of Phytophthora species, including P. ramorum and P. kernoviae at the point of inspection. Plant Pathol. 2007, 56, 828–835. [Google Scholar] [CrossRef]
- Maniatis, T.; Fritsch, E.F.; Sambrook, J. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: Cold Spring Harbor, NY, USA, 1982. [Google Scholar]
- Miles, T.; Martin, F.; Robideau, G.; Bilodeau, G.; Coffey, M. Systematic development of Phytophthora species-specific mitochondrial diagnostic markers for economically important members of the genus. Plant Dis. 2017, 101, 1162–1170. [Google Scholar] [CrossRef]
- Dorrance, A.E.; Berry, S.A.; Anderson, T.R.; Meharg, C. Isolation, storage, pathotype characterization, and evaluation of resistance for Phytophthora sojae in soybean. Plant Health Prog. 2008, 10, 1094. [Google Scholar] [CrossRef]
- Jeffers, S.N.; Martin, S.B. Comparison of two media selective for Phytophthora and Pythium species. Plant Dis. 1986, 70, 1038–1043. [Google Scholar] [CrossRef]
- Si Ammour, M.; Bilodeau, G.J.; Tremblay, D.M.; Van der Heyden, H.; Yaseen, T.; Varvaro, L.; Carisse, O. Development of real-time isothermal amplification assays for on-site detection of Phytophthora infestans in potato leaves. Plant Dis. 2017, 101, 1269–1277. [Google Scholar] [CrossRef]
- Bilodeau, G.J.; Martin, F.; Coffey, M.; Blomquist, C. Development of a multiplex assay for genus and species-specific detection of Phytophthora based on differences in mitochondrial gene order. Phytopathology 2014, 104, 733–748. [Google Scholar] [CrossRef]
- Martin, R.; James, D.; Levesque, C.A. Impacts of molecular diagnostic technologies on plant disease management. Annu. Rev. Phytopathol. 2000, 38, 207–239. [Google Scholar] [CrossRef]
- Glais, L.; Jacquot, E. Detection and characterization of viral species/subspecies using isothermal recombinase polymerase amplification (RPA) assays. Methods Mol. Biol. 2015, 1302, 207–225. [Google Scholar] [PubMed]
- Chimento, A.; Cacciola, S.O.; Garbelotto, M. Detection of mRNA by reverse-transcription PCR as an indicator of viability in Phytophthora ramorum. For. Pathol. 2012, 42, 14–21. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Tomassini, A.; Vannini, A. Detection and quantification of mRNA by reverse transcription real time PCR as indicator of viability of Phytophthora cambivora in soil. Acta Hortic. 2009, 844, 361–366. [Google Scholar] [CrossRef]
- Nocker, A.; Sossa-Fernandez, P.; Burr, M.; Camper, A. Use of propidium monoazide for live/dead distinction in microbial ecology. Appl. Environ. Microbiol. 2007, 73, 5111–5117. [Google Scholar] [CrossRef]
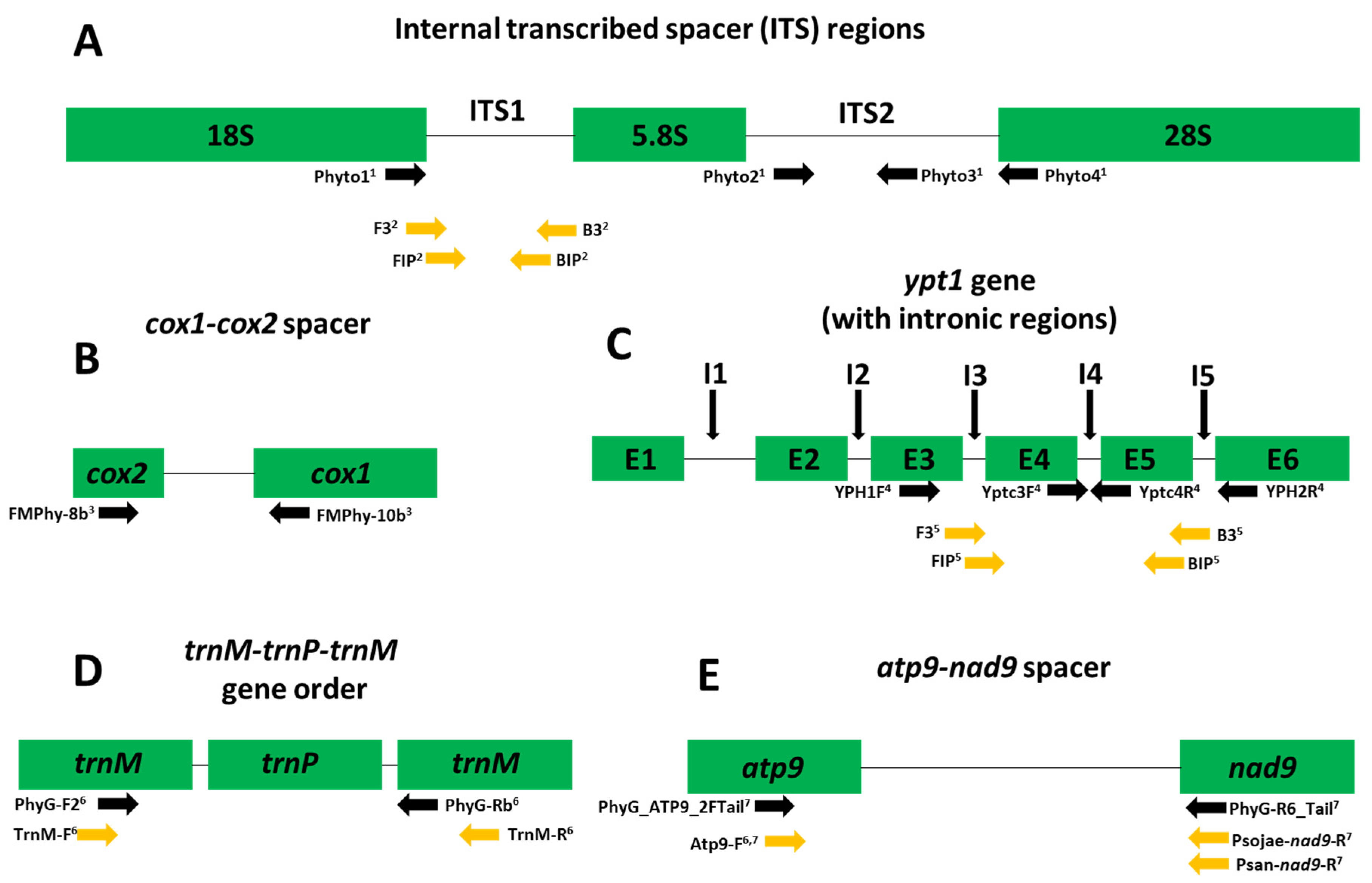

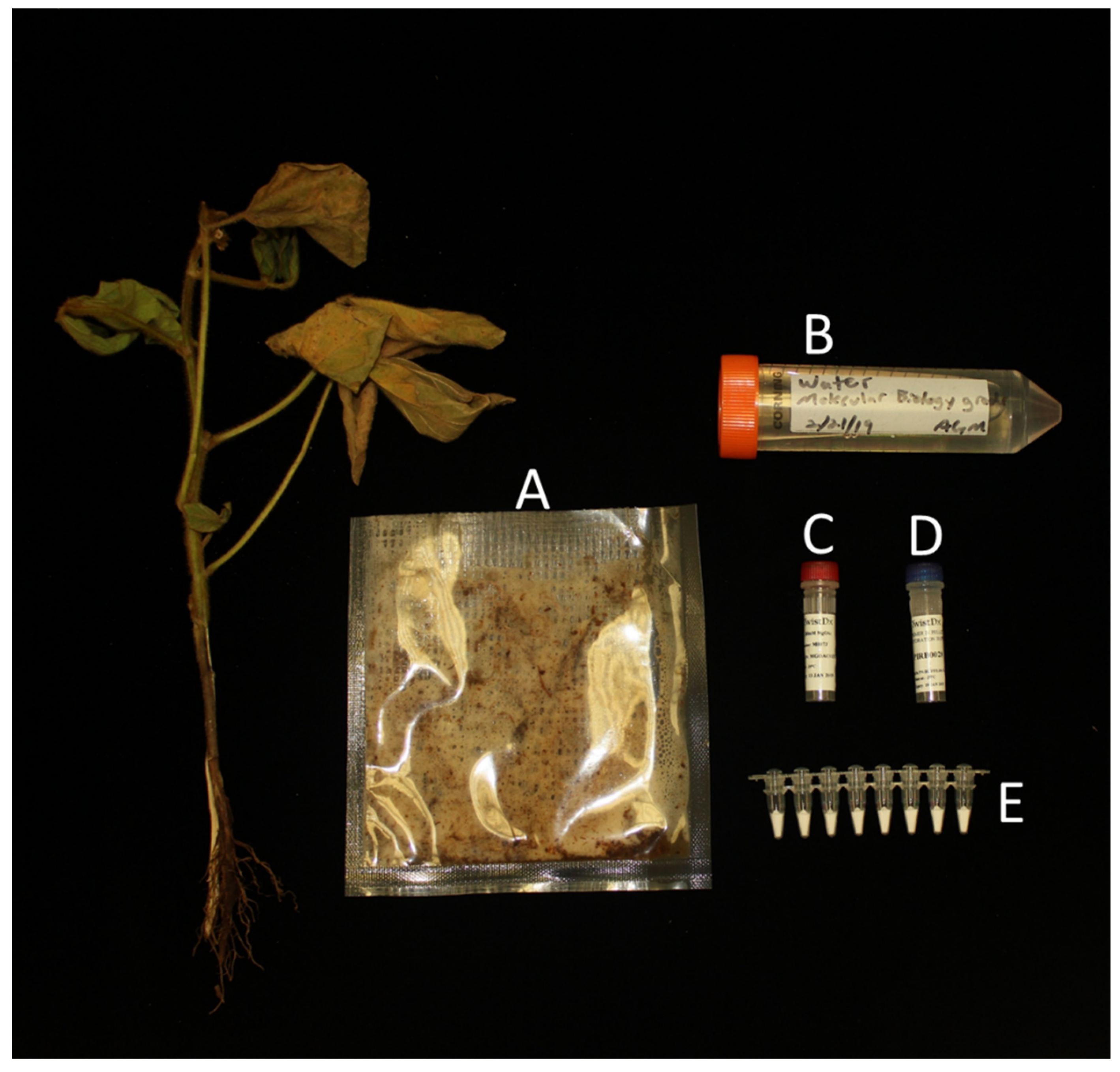
| Sample | Average Time Onset of Amplification with the Commercially Available Reaction a | Average Time Onset of Amplification with the Preformulated Lyophilized Reaction a |
|---|---|---|
| Crude plant extract Phytophthora rubi-infected raspberry cane | 17.4b | 17.6 |
| 8.0b | 8.0 | |
| Crude plant extract infected Phytophthora ramorum-leaf | 15.7 | 15.2 |
| 8.1 | 8.1 | |
| Crude plant extract infected P. ramorum-leaf | 14.8 | 12.3 |
| 8.0 | 8.0 | |
| Purified Pythium splendens DNA (1 ng) | - c | - |
| - | - | |
| Purified Phytophthora cinnamomi DNA (3500 pg) | 6.5 | 5.85 |
| Purified P. cinnamomi DNA (350 pg) | 9.0 | 8.5 |
| Purified P. cinnamomi DNA (35 pg) | 11.4 | 11.0 |
| Purified P. cinnamomi DNA (3.5 pg) | 16.5 | 16.1 |
| Purified P. cinnamomi DNA (0.35 pg) | - | - |
| Sample | Platform | Average Time Onset of Amplification without Plant Extract a | Average Time Onset of Amplification with Plant Extract | ||
|---|---|---|---|---|---|
| Phytophthora Genus (FAM) b | Plant Internal Control (ROX) c | Phytophthora Genus (FAM) | Plant Internal Control (ROX) | ||
| P. ramorum (500 pg/µL) (Positive control) | Axxin T16-ISO | 7.66 | NR d | - e | - |
| Bio-Rad CFX96 | 12.38 | NR | - | - | |
| ViiA7 RT-PCR | 13.01 | NR | - | - | |
| QuantStudio 6 | 6.69 | NR | - | - | |
| Phytophthora-infected citrus | Axxin T16-ISO | - | - | 7.24 | 8.21 |
| Bio-Rad CFX96 | - | - | 9.08 | 10.46 | |
| ViiA7 RT-PCR | - | - | 12.68 | 14.35 | |
| QuantStudio 6 | - | - | 5.55 | 5.34 | |
| Pythium splendens (500 pg/µL) (Negative Control) | Axxin T16-ISO | NR | NR | - | - |
| Bio-Rad CFX96 | NR | NR | - | - | |
| ViiA7 RT-PCR | NR | NR | - | - | |
| QuantStudio 6 | NR | NR | - | - | |
| P. ramorum (0.33 ng/µL) | Axxin T16-ISO | 10.54 | 12.67 | 12.11 | 10.04 |
| Bio-Rad CFX96 | 14.97 | NR | 17.36 | 29.81 | |
| ViiA7 RT-PCR | 12.6 | NR | 13.99 | 8.95 | |
| QuantStudio 6 | 7.53 | NR | 8.11 | 23.21 | |
| P. ramorum (33 pg/µL) | Axxin T16-ISO | 18.90 | NR | 19.71 | 14.22 |
| Bio-Rad CFX96 | 22.66 | NR | NR | 20.45 | |
| ViiA7 RT-PCR | 16.51 | NR | 16.98 | 9.68 | |
| QuantStudio 6 | 9.31 | NR | 11.83 | 22.77 | |
| P. ramorum (3.3 pg/µL) | Axxin T16-ISO | 27.32 | NR | 31.12 | 13.77 |
| Bio-Rad CFX96 | NR | NR | NR | 10.57 | |
| ViiA7 RT-PCR | NR | NR | NR | 9.57 | |
| QuantStudio 6 | 12.99 | NR | 16.01 | 23.09 | |
| P. ramorum (0.33 pg/uL) | Axxin T16-ISO | NR | NR | NR | 10.06 |
| Bio-Rad CFX96 | NR | NR | NR | 15.46 | |
| ViiA7 RT-PCR | NR | NR | NR | 9.28 | |
| QuantStudio 6 | NR | NR | NR | 22.97 | |
| Axxin T16 a | Bio-Rad CFX96 RPA | ViiA7 System RPA | QuantStudio 6 RPA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Pathogen Identified | Phytophthora Genus RPA (FAM) b | Phytophthora Genus qPCR (FAM) | Phytophthora Genus (FAM) | Plant Internal Control (ROX) c | Phytophthora Genus (FAM) | Plant Internal Control (ROX) | Phytophthora Genus (FAM) | Plant Internal Contro (ROX) | |
| Rhamnus californica | Phytophthora cactorum | + d | + | + | + | + | + | + | + | |
| Prunus avium | Pythium sp. | − e | − | − | + | − | + | − | − | |
| Gardenia jasminoides ‘Radicans’ | P. nicotianae | + | + | + | + | + | + | − | + | |
| Gardenia jasminoides ‘Mystery’ | P. nicotianae | + | + | + | + | + | − | + | + | |
| Aucuba japonica ‘Mr. Goldstrike’ | P. citricola | + | + | + | + | + | + | + | + | |
| Asparagus officinalis | Pythium sp. | − | − | − | + | − | + | + | + | |
| Rhus integrifolia | P. nicotianae | + | + | + | + | + | + | + | + | |
| Fragaria x ananassa | P. cactorum | + | + | + | + | + | + | − | + | |
| Rubus sp. | Pythium sp. | − | − | − | + | − | + | − | + | |
| Rubus sp. | P. rubi | + | + | + | + | + | + | + | + | |
| Citrus sp. 1 | P. citrophthora | + | + | + | + | + | + | + | + | |
| Citrus sp. 2 | P. citrophthora | + | + | + | + | + | + | + | + | |
| Citrus sp. 3 | P. citrophthora | + | + | + | + | + | + | + | + | |
| Citrus sp. 4 | P. citrophthora | + | + | + | + | + | + | + | + | |
| Citrus sp. 5 | P. citrophthora | + | + | + | + | + | + | + | + | |
| Myrtus sp. | P. nicotianae | + | + | + | + | + | + | + | + | |
| Pseudotsuga menziesii | P. cambivora | + | + | + | + | + | + | + | + | |
| Hedera sp. | P. tropicalis | + | + | + | + | + | + | + | + | |
| Rhododendron sp. | P. ramorum | + | + | + | + | + | + | + | + | |
| Rhododendron sp. | P. ramorum | + | + | + | + | + | + | − | + | |
| Umbellularia californica | P. ramorum | + | + | + | + | + | + | + | + | |
| Viburnum sp. | P. ramorum | + | + | + | + | + | + | + | + | |
| Rhododendron sp. | P. ramorum | + | + | + | + | + | + | + | + | |
| Water | N/A | − | − | − | − | − | − | − | − | |
| Phytophthora Species a | Host Plant | Platform | Phytophthora Genus (FAM) | Plant Internal Control (ROX) |
|---|---|---|---|---|
| Mean OT b | Mean OT | |||
| P. boehmeriae | Rhododendron sp. | ViiA7 RT-PCR | 6.78 * | NR |
| P. pseudosyringae | Rhododendron sp. | ViiA7 RT-PCR | 10.63 | 5.92 * |
| P. cryptogea | Rhododendron sp. | ViiA7 RT-PCR | 9.52 | 6.96 |
| P. ramorum | Rhododendron sp. | ViiA7 RT-PCR | 9.7 | 7.41 |
| P. infestans | Rhododendron sp. | ViiA7 RT-PCR | 6.20 | 10.74 * |
| P. infestans | Rhododendron sp. | ViiA7 RT-PCR | 8.01 | 9.84 * |
| Leaf only | Rhododendron sp. | ViiA7 RT-PCR | NR c | 8.15 * |
| Water only | Rhododendron sp. | ViiA7 RT-PCR | NR * | NR * |
| P. sojae 1 | Glycine max | Bio-Rad CFX96 | 8.82 | 11.76 |
| P. sojae 2 | Glycine max | Bio-Rad CFX96 | 6.88 | 8.04 |
| P. sojae 3 | Glycine max | Bio-Rad CFX96 | 5.36 | 7.46 |
| P. sojae 4 | Glycine max | Bio-Rad CFX96 | 4.35 | 7.92 |
| P. sojae 5 | Glycine max | Bio-Rad CFX96 | 3.97 | 10.8 |
| P. sojae 6 | Glycine max | Bio-Rad CFX96 | 10.58 | 13.45 |
| P. sojae 7 | Glycine max | Bio-Rad CFX96 | 9.54 | 15.85 |
| P. sojae 8 | Glycine max | Bio-Rad CFX96 | 9.25 | 12.99 |
| P. sojae 9 | Glycine max | Bio-Rad CFX96 | 4.36 | 27.18 |
| P. sojae 10 | Glycine max | Bio-Rad CFX96 | 5.25 | 22.84 |
| Sample a | Host Name | Phytophthora Genus (FAM) | Plant Internal Control (ROX) |
|---|---|---|---|
| Mean OT b | Mean OT b | ||
| Phytophthora ramorum 1 | Rhododendron sp. ‘Cunningham’ | 3.80 | 6.23 |
| P. ramorum 2 | Rhododendron sp. ‘Cunningham’ | 7.83 | 26.9 |
| P. ramorum 3 | Rhododendron sp. ‘Cunningham’ | 5.64 | 5.55 |
| P. ramorum 4 | Rhododendron sp. ‘Cunningham’ | 5.90 | 5.99 |
| P. ramorum 5 | Rhododendron sp. ‘Cunningham’ | 7.07 | 6.32 |
| P. ramorum 6 | Umbellularia californica | 8.39 | 26.9 |
| P. ramorum 7 | Rhododendron sp. ‘Grace Seabrook’ | 10.28 | 5.19 |
| P. ramorum 8 | Rhododendron sp. ‘Grace Seabrook’ | 8.09 | 5.56 |
| P. ramorum 9 | Rhododendron sp. ‘Grace Seabrook’ | 11.14 | 5.12 |
| P. ramorum 10 | Rhododendron sp. ‘Grace Seabrook’ | 12.78 | 6.12 |
| P. ramorum 11 | Rhododendron sp. ‘Grace Seabrook’ | 10.32 | NR c |
| P. ramorum 12 | Rhododendron sp. ‘Taurus’ | 9.05 | 5.57 |
| P. ramorum 13 | Arctostaphylos refugioensis | 11.19 | 6.93 |
| P. ramorum 14 | Rhododendron sp. ‘Rangoon’ | 7.75 | 5.27 |
| Spumella-like flagellate | Fragaria sp. | NR | 27.11 |
| Pythium debaryanum | Fragaria sp. | NR | 24.24 |
| Plasmopara viticola | Vitis vinifera | NR | 18.57 |
| P. multivora | Camellia sinensis | 4.80 | 22.06 |
| P. chlamydospora | Rosa sp. | NR | NR |
| P. brassicae | Brassica oleracea | 5.85 | 1.38 |
| P. syringae 1 | Rhododendron sp. ‘President Roosevelt’ | 5.22 | 6.07 |
| P. syringae 2 | Rhododendron sp. | 4.76 | 5.38 |
| P. syringae 3 | Rhododendron sp. | 5.55 | 6.31 |
| P. syringae 4 | Rhododendron sp. | 5.85 | 5.69 |
| P. syringae 5 | Rhododendron sp. | 5.54 | 6.04 |
| P. syringae 6 | Arctostaphylos densiflora | 7.41 | 25.90 |
| Unknown Isolate 1 | Chiosya ternate | NR | 26.0 |
| Unknown Isolate 2 | Mahonia repens | NR | 9.91 |
| Unknown Isolate 3 | Camellia sasanqua | NR | 24.24 |
| Water control | NA | NR | NR |
| Primers, Probes a | Sequence (5′–3′) | Target |
|---|---|---|
| Primers | ||
| Phytophthora genus specific | ||
| TrnM-F | ATGTAGTTTAATGGTAGAGCGTGGGAATC | tRNA-M |
| TrnM-R | GAACCTACATCTTCAGATTATGAGCCTGATAAG | tRNA-M |
| Plant internal control | ||
| Cox1-IPC-F | CATGCGTGGACCTGGAATGACTATGCATAGA | COX1 |
| Cox1-IPC-R | GGTTGTATTAAAGTTTCGATCGGTTAATAACA | COX1 |
| Probes | ||
| Phytophthora genus specific | ||
| TrnM-P | TAGAGCGTGGGAATCATAATCCTAATGTTG [FAM-dT] A [THF] G [BHQ1-dT] TCAAATCCTACCATCAT [3′-C3SPACER] | tRNA-M |
| Plant internal control | ||
| Cox1-IPC-P | GGTCCGTTCTAGTGACAGCATTCCYACTTTTATTA [ROX- dT] C [THF] C [BHQ2-dT] YCCGGTACTGGC [3′-C3SPACER] | COX1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McCoy, A.G.; Miles, T.D.; Bilodeau, G.J.; Woods, P.; Blomquist, C.; Martin, F.N.; Chilvers, M.I. Validation of a Preformulated, Field Deployable, Recombinase Polymerase Amplification Assay for Phytophthora Species. Plants 2020, 9, 466. https://doi.org/10.3390/plants9040466
McCoy AG, Miles TD, Bilodeau GJ, Woods P, Blomquist C, Martin FN, Chilvers MI. Validation of a Preformulated, Field Deployable, Recombinase Polymerase Amplification Assay for Phytophthora Species. Plants. 2020; 9(4):466. https://doi.org/10.3390/plants9040466
Chicago/Turabian StyleMcCoy, Austin G., Timothy D. Miles, Guillaume J. Bilodeau, Patrick Woods, Cheryl Blomquist, Frank N. Martin, and Martin I. Chilvers. 2020. "Validation of a Preformulated, Field Deployable, Recombinase Polymerase Amplification Assay for Phytophthora Species" Plants 9, no. 4: 466. https://doi.org/10.3390/plants9040466
APA StyleMcCoy, A. G., Miles, T. D., Bilodeau, G. J., Woods, P., Blomquist, C., Martin, F. N., & Chilvers, M. I. (2020). Validation of a Preformulated, Field Deployable, Recombinase Polymerase Amplification Assay for Phytophthora Species. Plants, 9(4), 466. https://doi.org/10.3390/plants9040466





