Separation of New Coumarin Glycosides from Toddalia asiatica Using Offline Two-Dimensional High-Performance Liquid Chromatography
Abstract
1. Introduction
2. Results and Discussion
2.1. Construction of a Two-Dimensional HPLC System
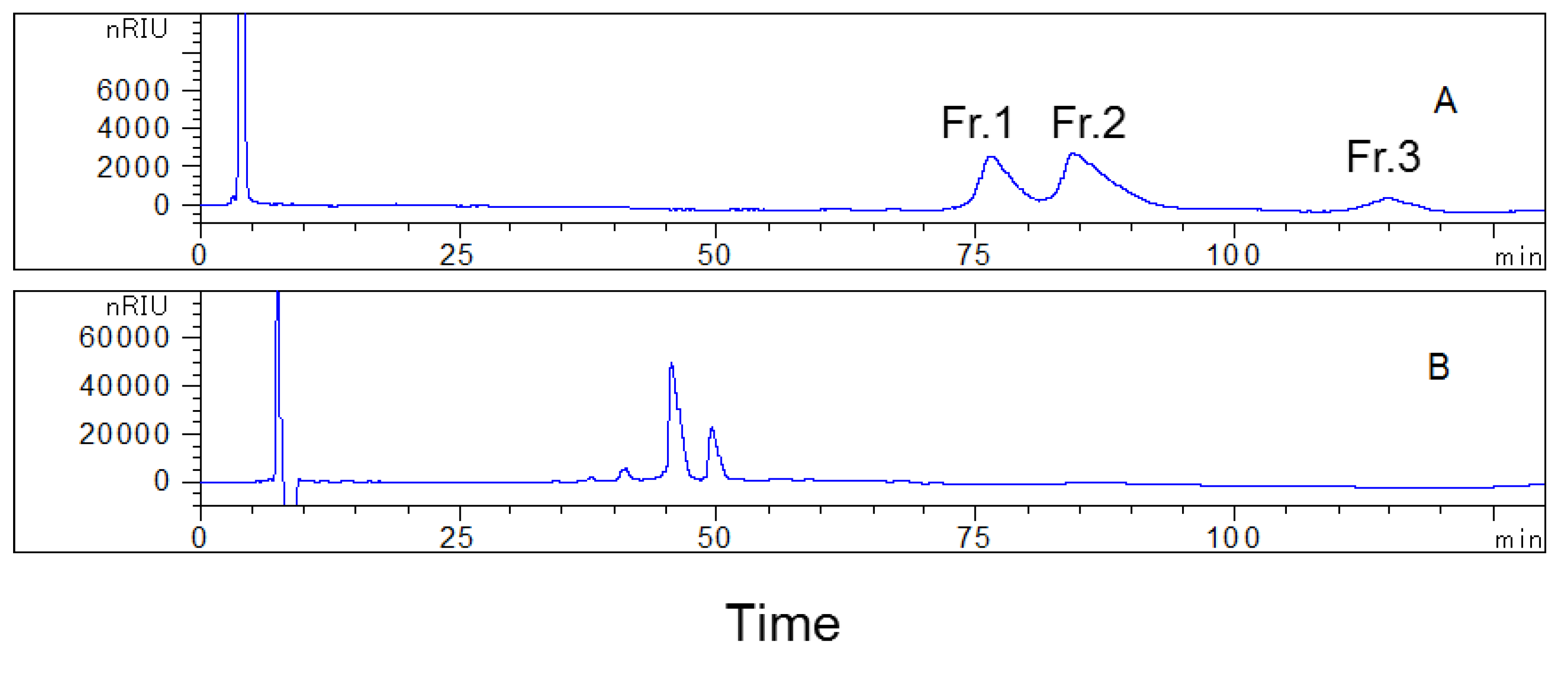
2.2. First HPLC Separation
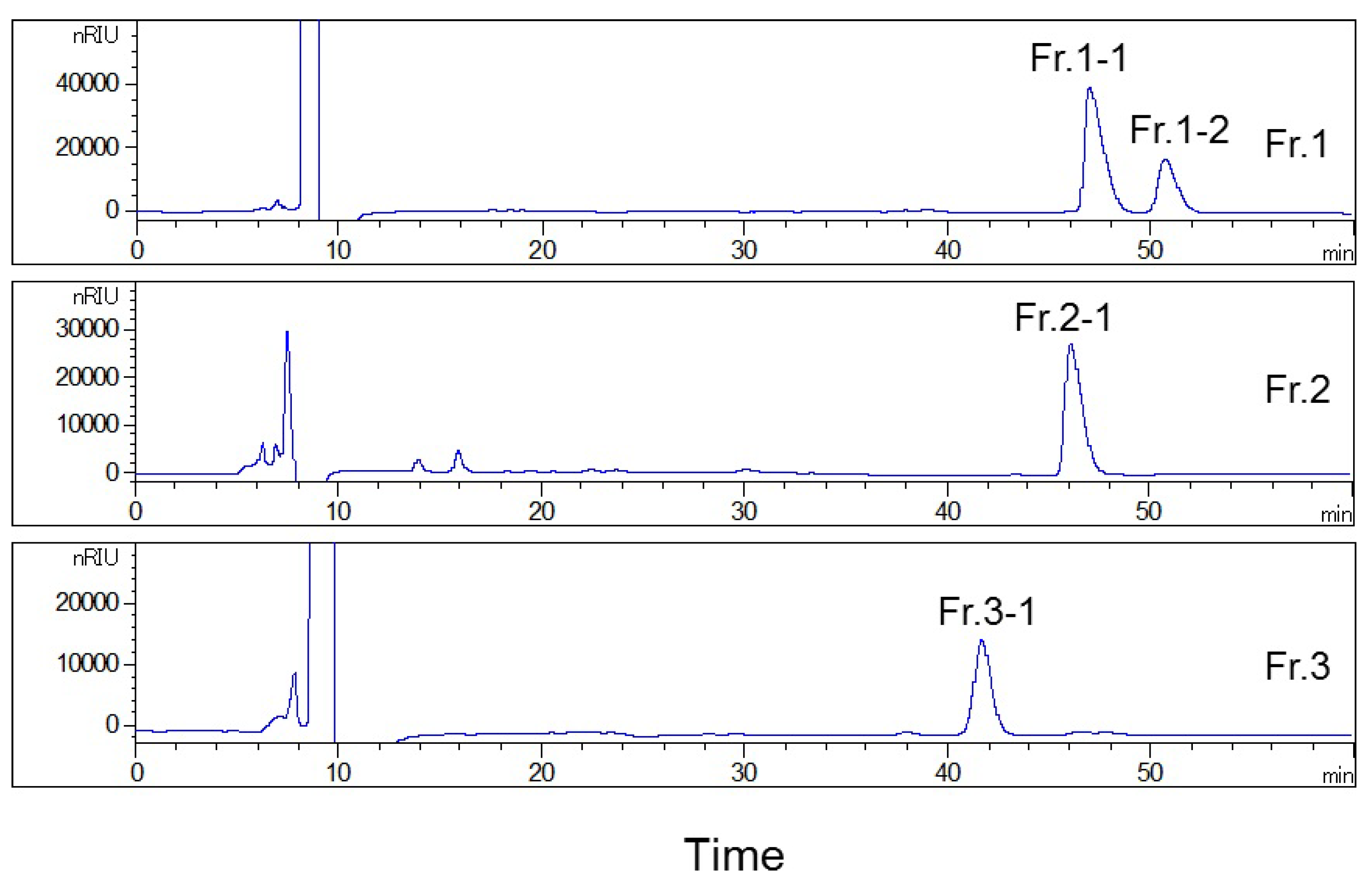
2.3. Analysis of the Collected Fractions on the YMC-Pack ODS-AQ Column
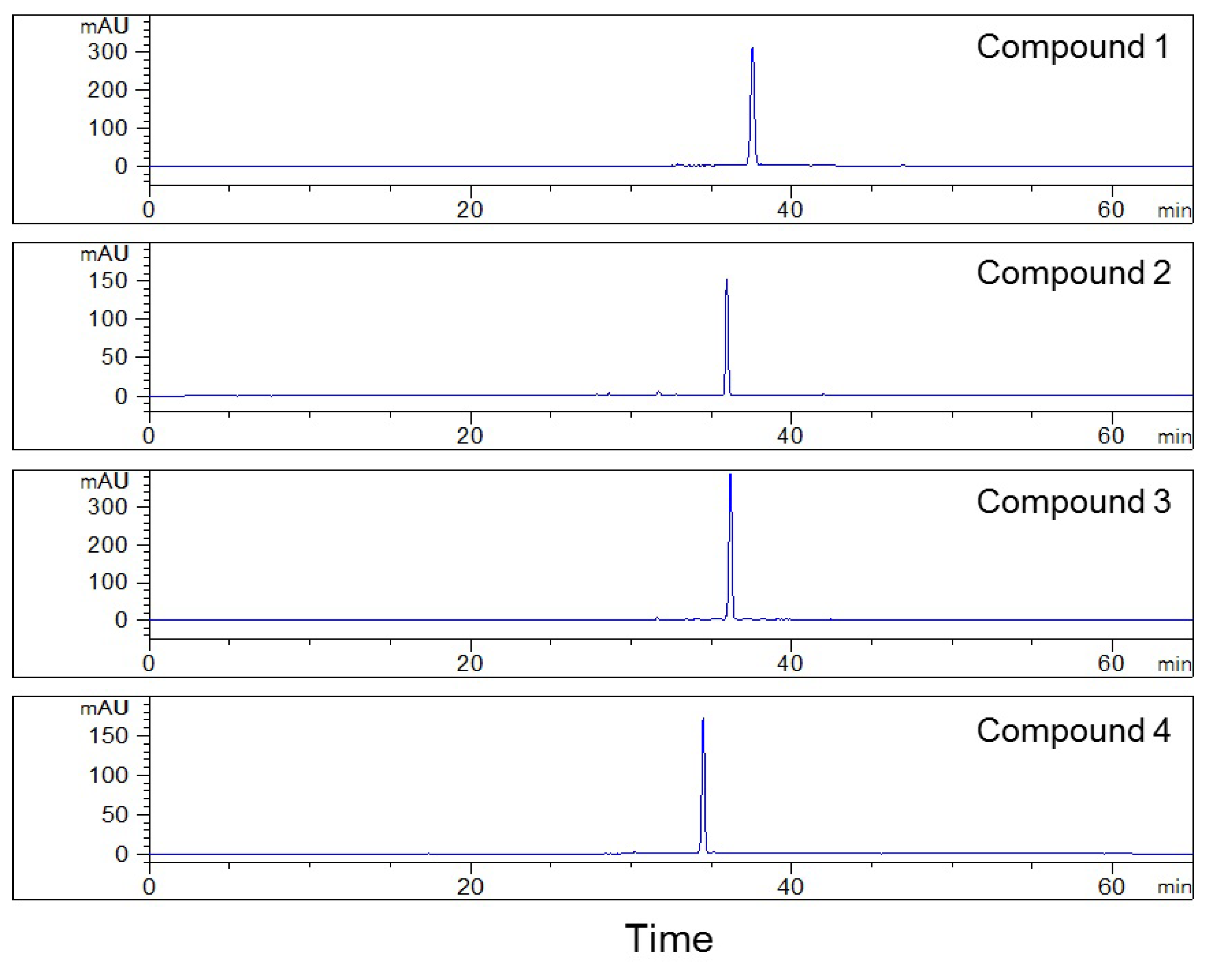
2.4. Second HPLC Preparation of High-Purity Compounds
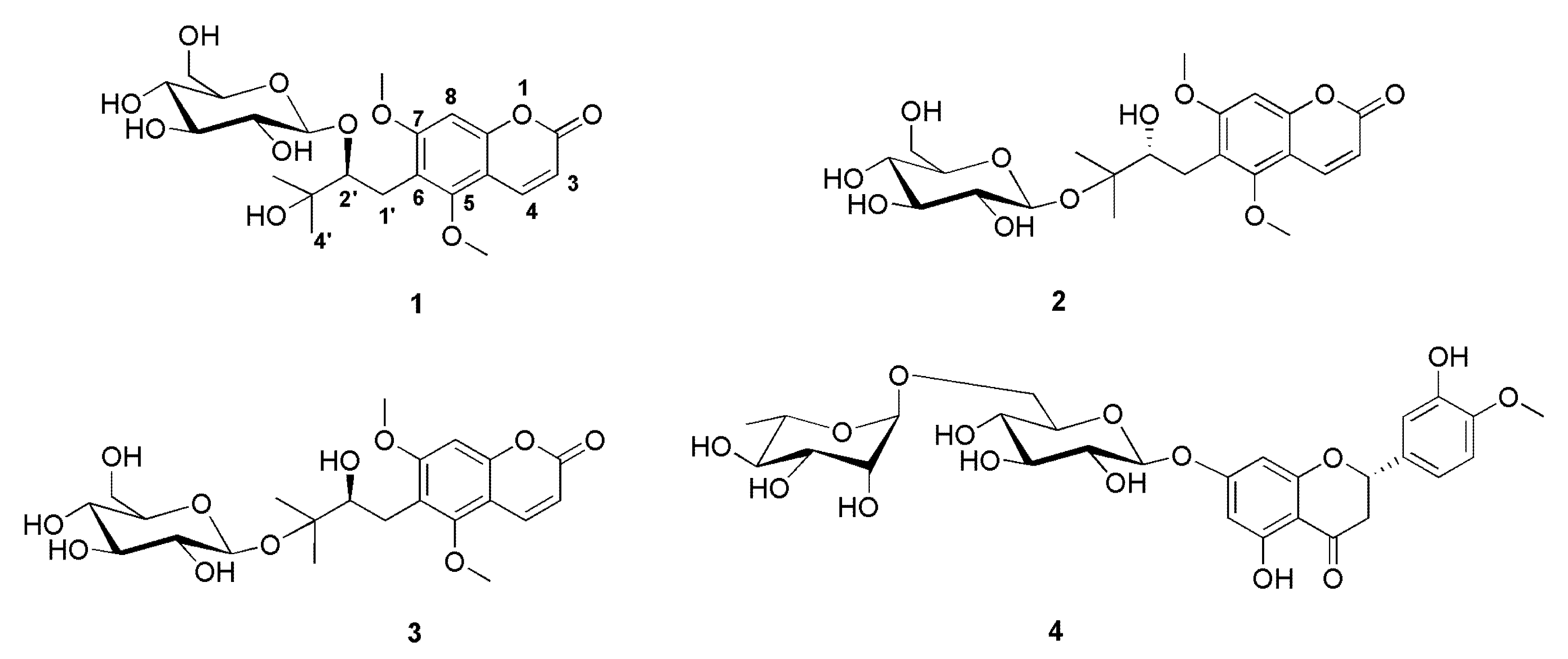
2.5. Structure Identification
3. Materials and Methods
3.1. Apparatus and Reagents
3.2. Sample Preparation
3.3. Chromatographic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Orwa, J.A.; Jondiko, I.J.O.; Minja, R.J.A.; Bekunda, M. The use of Toddalia asiatica (L.) Lam. (Rutaceae) in traditional medicine practice in East Africa. J. Ethnopharmacol. 2008, 115, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.S.; Huang, J.L.; Jiang, M.H.; Xu, Y.K.; Ahmed, A.; Yin, S.; Tang, G.H. New prenylated coumarins from the stems of Toddalia asiatica. RSC Adv. 2017, 7, 31061–31068. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Sun, W.B.; Yang, Z.; Liang, Y.; Zhou, W.; Tang, L. Hemostatic chemical constituents from natural medicine Toddalia asiatica root bark by LC-ESI Q-TOF MSE. Chem. Cent. J. 2017, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.J.; Wei, P.Q.; Peng, Q.; Qin, S.Y.; Zhou, Y.; Zhang, R.; Zhu, C.C.; Zhang, L. Simultaneous qualitative and quantitative evaluation of Toddalia asiatica root by using HPLC-DVD and UPLC-QTOF-MS/MS. Phytochem. Anal. 2019, 30, 164–181. [Google Scholar] [CrossRef]
- Fang, S.M.; Fang, G.; Liu, R.; Xu, Y.; Fan, G.W. Chemical constituents in Toddalia asiatica Lam.: Research advances. J. Int. Pharm. Res. 2016, 43, 239–248. [Google Scholar] [CrossRef]
- Hirunwong, C.; Sukieum, S.; Phatchana, R.; Yenjai, C. Cytotoxic and antimalarial constituents from the roots of Toddalia asiatica. Phytochem. Lett. 2016, 17, 242–246. [Google Scholar] [CrossRef]
- Tang, Z.H.; Liu, Y.B.; Ma, S.G.; Li, L.; Li, Y.; Jiang, J.D.; Qu, J.; Yu, S.S. Antiviral spirotriscoumarins A and B: Two pairs of oligomeric coumarin enantiomers with a spirodienone-sesquiterpene skeleton from Toddalia asiatica. Org. Lett. 2016, 18, 5146–5149. [Google Scholar] [CrossRef]
- Kumagai, M.; Watanabe, A.; Yoshida, I.; Mishima, T.; Nakamura, M.; Nishikawa, K.; Morimoto, Y. Evaluation of aculeatin and toddaculin isolated from Toddalia asiatica as anti-inflammatory agents in LPS-stimulated RAW264 macrophages. Biol. Pharma. Bull. 2018, 41, 132–137. [Google Scholar] [CrossRef]
- Raj, M.K.; Balachandran, C.; Duraipandiyan, V.; Agastian, P.; Ignacimuthu, S. Antimicrobial activity of ulopterol isolated from Toddalia asiatica (L.) Lam.: A traditional medicinal plant. J. Ethnopharmacol. 2012, 140, 161–165. [Google Scholar] [CrossRef]
- Tsai, I.L.; Wun, M.F.; Teng, C.M.; Ishikawa, T.; Chen, I.S. Anti-platelet aggregation constituents from formosan Toddalia asiatica. Phytochemistry 1998, 48, 1377–1382. [Google Scholar] [CrossRef]
- Nyahanga, T.; Jondiko, J.I.; Manguro, L.O.A.; Orwa, J.A. Antiplasmodial and larvicidal compounds of Toddalia asiatica root bark. J. Chem. Sci. 2013, 125, 1115–1121. [Google Scholar] [CrossRef]
- Lin, T.T.; Huang, Y.Y.; Tang, G.H.; Cheng, Z.B.; Liu, X.; Luo, H.B.; Yin, S. Prenylated coumarins: Natural phosphodiesterase-4 inhibitors from Toddalia asiatica. J. Nat. Prod. 2014, 77, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.; Kumagai, M.; Mishima, T.; Ito, J.; Otoki, Y.; Harada, T.; Kato, T.; Yoshida, M.; Suzuki, M.; Yoshida, I.; et al. Toddaculin, isolated from of Toddalia asiatica (L.) Lam., inhibited osteoclastogenesis in RAW 264 cells and enhanced osteoblastogenesis in MC3T3-E1 cells. PLoS ONE 2015, 10, e0127158. [Google Scholar] [CrossRef] [PubMed]
- Chorepsima, S.; Tentolouris, K.; Dimitroulis, D.; Tentolouris, N. Melilotus: Contribution to wound healing in the diabetic foot. J. Herb. Med. 2013, 3, 81–86. [Google Scholar] [CrossRef]
- Di Visconte, M.S.; Nicolì, F.; Giudice, R.D.; Mis, T.C. Effect of a mixture of diosmin, coumarin glycosides, and triterpenes on bleeding, thrombosis, and pain after stapled anopexy: A prospective, randomized, placebo-controlled clinical trial. Int. J. Colorectal. Dis. 2017, 32, 425–431. [Google Scholar] [CrossRef]
- Zhou, W.J.; Liu, Y.M.; Wang, J.X.; Guo, Z.M.; Shen, A.J.; Liu, Y.F.; Liang, X.M. Application of two-dimensional liquid chromatography in the separation of traditional Chinese medicine. J. Sep. Sci. 2020, 43, 87–104. [Google Scholar] [CrossRef]
- Bocian, S.; Buszewski, B. Phenyl-bonded stationary phases-the influence of polar functional groups on retention and selectivity in reversed-phase liquid chromatography. J. Sep. Sci. 2014, 37, 3435–3442. [Google Scholar] [CrossRef]
- Jiang, D.S.; Ke, Y.X.; Cai, J.F.; Zhang, H.H.; Fu, Q.; Jin, Y.; Liang, X.M. Evaluation of a series of phenyl-type stationary phases in supercritical fluid chromatography with the linear solvation energy relationship model and its application to the separation of phenolic compounds. J. Chromatogr. A 2020, 1614, 460700. [Google Scholar] [CrossRef]
- Janasa, P.; Bocianb, S.; Janderaa, P.; Kowalkowskib, T.; Buszewski, B. Separation of flavonoids on different phenyl-bonded stationary phases-the influence of polar groups in stationary phase structure. J. Chromatogr. A. 2016, 1429, 198–206. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sanders, J.K.M. The nature of π-π interactions. J. Am. Chem. Soc. 1990, 112, 5525–5534. [Google Scholar] [CrossRef]
- Liu, K.; Wang, W.; Guo, B.H.; Gao, H.; Liu, Y.; Liu, X.H.; Yao, H.L.; Cheng, K. Chemical evidence for potent xanthine oxidase inhibitory activity of ethyl acetate extract of Citrus aurantium L. dried immature fruits. Molecules 2016, 21, 302. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sun, S.-W.; Zhang, X.-Y.; Liu, Y.; Liu, X.-H.; Zhang, S.; Wang, W.; Wang, J.; Wang, W. Separation of New Coumarin Glycosides from Toddalia asiatica Using Offline Two-Dimensional High-Performance Liquid Chromatography. Plants 2020, 9, 428. https://doi.org/10.3390/plants9040428
Li Y, Sun S-W, Zhang X-Y, Liu Y, Liu X-H, Zhang S, Wang W, Wang J, Wang W. Separation of New Coumarin Glycosides from Toddalia asiatica Using Offline Two-Dimensional High-Performance Liquid Chromatography. Plants. 2020; 9(4):428. https://doi.org/10.3390/plants9040428
Chicago/Turabian StyleLi, Yan, Shi-Wei Sun, Xiao-Yi Zhang, Yang Liu, Xiao-Hong Liu, Shuang Zhang, Wei Wang, Jin Wang, and Wei Wang. 2020. "Separation of New Coumarin Glycosides from Toddalia asiatica Using Offline Two-Dimensional High-Performance Liquid Chromatography" Plants 9, no. 4: 428. https://doi.org/10.3390/plants9040428
APA StyleLi, Y., Sun, S.-W., Zhang, X.-Y., Liu, Y., Liu, X.-H., Zhang, S., Wang, W., Wang, J., & Wang, W. (2020). Separation of New Coumarin Glycosides from Toddalia asiatica Using Offline Two-Dimensional High-Performance Liquid Chromatography. Plants, 9(4), 428. https://doi.org/10.3390/plants9040428




