Tetraploid Induction by Colchicine Treatment and Crossing with a Diploid Reveals Less-Seeded Fruit Production in Pointed Gourd (Trichosanthes dioica Roxb.)
Abstract
1. Introduction
2. Results
2.1. Effect of Colchicine Treatments on the Germination Rate and Surviving Seedling Rate
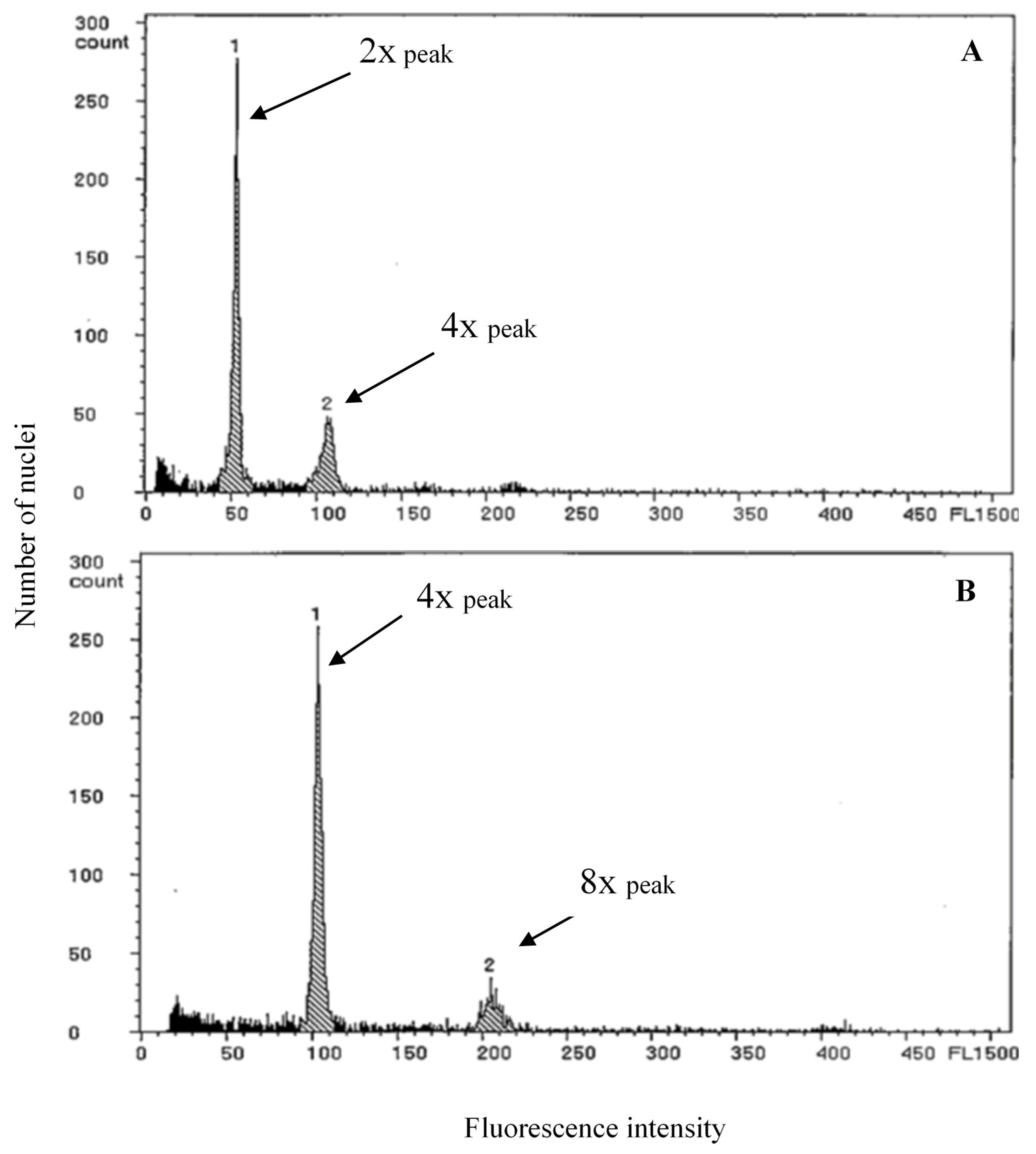
2.2. Confirmation of Ploidy Level
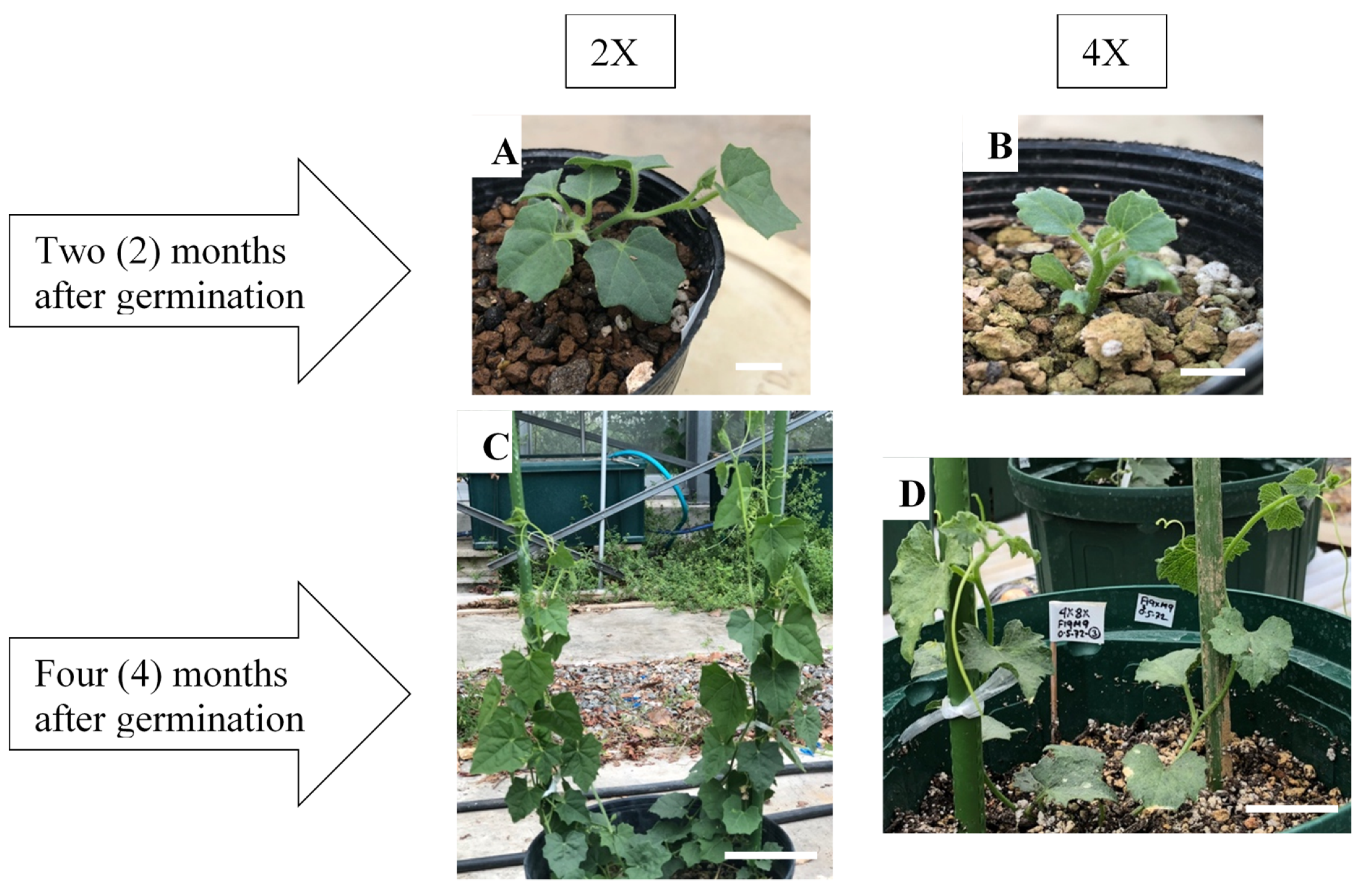
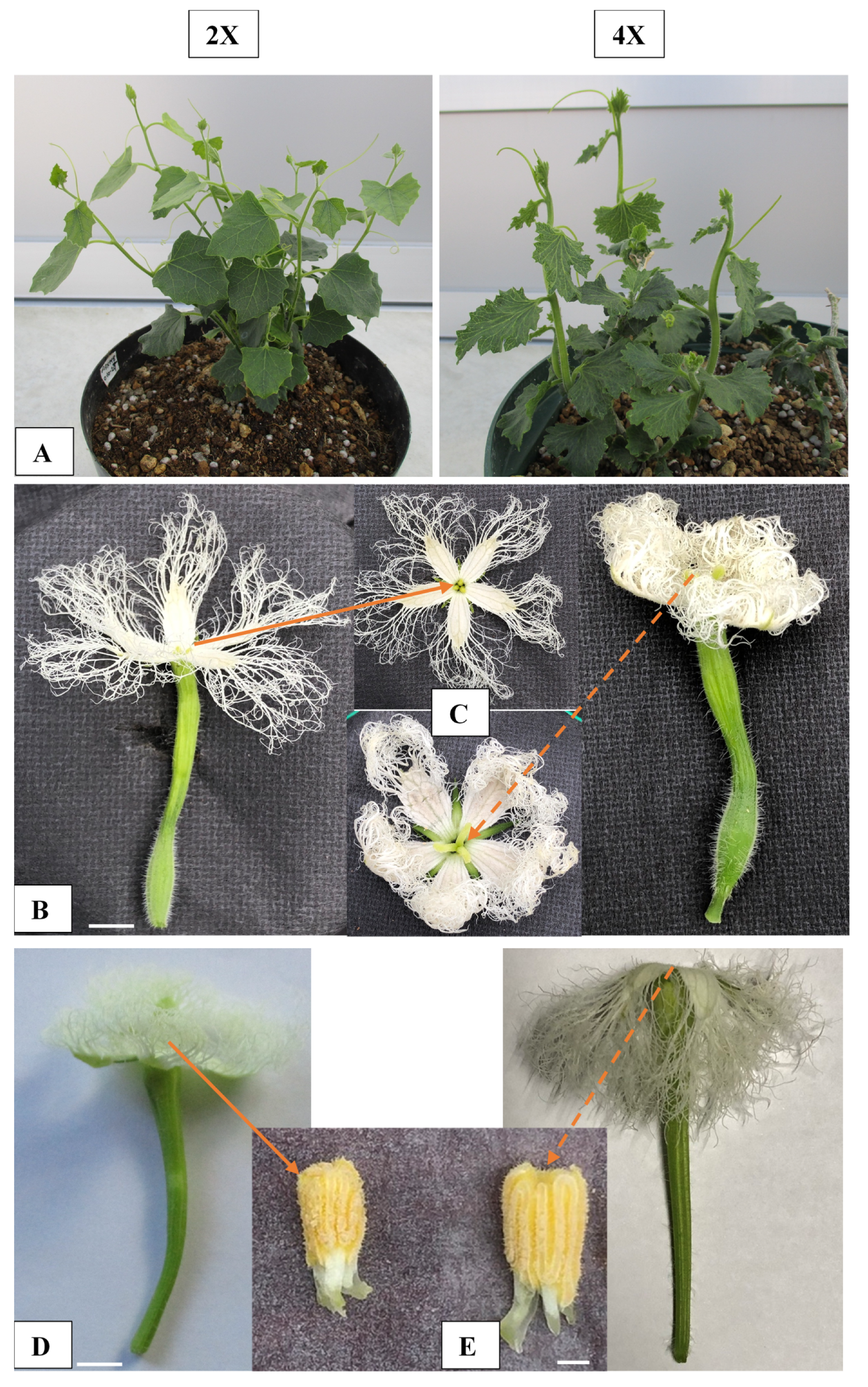
2.3. Morphological Characteristics of Diploid and Colchicine-Induced Tetraploid Plants
3. Discussion
4. Materials and Methods
4.1. Colchicine Treatment
4.2. Confirmation of Ploidy Level
4.3. Vegetative and Reproductive Traits
4.4. Crosspollination of Diploids with Colchicine-Induced Tetraploids
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sinha, S.; Guha, A.; Sinha, R.K. Karyotype and sex expression in Trichosanthes dioica. Cytologia 2003, 68, 357–361. [Google Scholar] [CrossRef]
- Hassan, J.; Miyajima, I. Induction of Parthenocarpy in Pointed Gourd (Trichosanthes dioica Roxb.) by Application of Plant Growth Regulators. J. Hortic. Plant Res. 2019, 8, 12–21. [Google Scholar] [CrossRef]
- Dubey, K.C.; Nair, P.K.R. Induced parthenocarpic fruit-set in pointed gourd (Trichosanthes dioica Roxb.). Indian J. Agric. Sci. 1972, 42, 765–768. [Google Scholar]
- Kim, I.S.; Okubo, H.; Fujieda, K. Endogenous levels of IAA in relation to parthenocarpy in cucumber (Cucumis sativus L.). Sci. Hortic. 1992, 52, 1–8. [Google Scholar] [CrossRef]
- Rasul, M.G.; Mian, M.A.K.; Cho, Y.; Ozaki, Y.; Okubo, H. Application of plant growth regulators on the parthenocarpic fruit development in teasle gourd (kakrol, Momordica dioica Roxb.). J. Fac. Agric. Kyushu Univ. 2008, 53, 39–42. [Google Scholar]
- Chen, L.P.; Wang, Y.J.; Zhao, M. In vitro induction and characterization of tetraploid Lychnis senno Siebold et Zucc. HortScience 2006, 41, 759–761. [Google Scholar] [CrossRef]
- Liu, Z.; Min, Z.; Sun, X.; Cheng, J.; Hu, Y. Study on induction and characterization of tetraploid plants in pumpkin. J. North China Agric. Univ. 2015, 30, 125–129. [Google Scholar]
- Maynard, D.N. An introduction to the watermelon. In Watermelons: Characteristics, Production and Marketing; ASHS Press: Alexandria, Egypt, 2001; pp. 9–20. [Google Scholar]
- Kazi, N.A. Polyploidy in vegetables. J. Glob. Biosci. 2015, 4, 1774–1779. [Google Scholar]
- Hazra, P. Induced polyploidy as a breeding approach in pointed gourd. J. Breed. Genet. 2001, 33, 47–48. [Google Scholar]
- Allum, J.F.; Bringloe, D.H.; Roberts, A.V. Chromosome doubling in a Rosa rugosa Thunb. Hybrid by exposure of in vitro nodes to oryzalin: The effects of node length, oryzalin concentration and exposure time. Plant Cell Rep. 2007, 26, 1977–1984. [Google Scholar] [CrossRef]
- Kihara, H. Tetraploid watermelons. Proc. Am. Soc. Hort. Sci. 1951, 58, 217–230. [Google Scholar]
- Zlesak, D.C.; Thill, C.A.; Anderson, N.O. Trifluralin-mediated polyploidization of Rosa chinensis minima (Sims) Voss seedlings. Euphytica 2005, 141, 281–290. [Google Scholar] [CrossRef]
- Dermen, H.; Emsweller, S.L. The use of colchicine in plant breeding. In Crops Research; Agricultural Research Service, U.S. Department of Agriculture, FEDLINK, USDA-arsseries: Washington, DC, USA, 1961; pp. 1–10. [Google Scholar]
- Stebbins, G.L. Chromosomal Evolution in Higher Plants; Addison-Wesley: London, UK, 1971. [Google Scholar]
- Jaskani, M.J.; Kwon, S.W.; Kim, D.H. Comparative study on vegetative, reproductive and qualitative traits of seven diploid and tetraploid watermelon lines. Euphytica 2005, 145, 259–268. [Google Scholar] [CrossRef]
- Jaskani, M.J.; Khan, I.A.; Husnain, S. Morphological description of citrus colchiploids. Proc. Int. Citrus Congr. 1996, 1, 130–132. [Google Scholar]
- Thao, N.T.P.; Ureshino, K.; Miyajima, I.; Ozaki, Y.; Okubo, H. Induction of tetraploids in ornamental Alocasia through colchicine and oryzaling treatments. Plant Cell Tissue Organ Cult. 2003, 72, 19–25. [Google Scholar] [CrossRef]
- Rose, J.B.; Kubba, J.; Tobutt, K.R. Chromosome doubling in sterile Syringa vulgaris × S. pinnatifolia hybrids by in vitro culture of nodal explants. Plant Cell Tissue Organ Cult. 2000, 63, 127–132. [Google Scholar] [CrossRef]
- Wehner, T.C.; Shetty, N.V.; Elmstrom, G.W. Breeding and seed production. In Watermelons: Characteristics, Production and Marketing; Maynard, D.N., Ed.; ASHS Press: Alexandria, Egypt, 2001; pp. 27–73. [Google Scholar]
- Tiwari, S.; Spielman, M.; Schulz, R.; Oakey, R.J.; Kelsey, G.; Salazar, A.; Zhang, K.; Pennell, R.; Scott, R.J. Transcriptional profiles underlying parent-of-origin effects in seeds of Arabidopsis thaliana. BMC Plant Biol. 2010, 10, 70. [Google Scholar] [CrossRef]
- Stebbins, G.L., Jr.; Polyploidy, I. Occurrences and nature of polyploid types. In Variation and Evolution in Plants; Dunn, L.C., Ed.; Columbia Univ. Press: New York, NY, USA, 1950; pp. 298–339. [Google Scholar]
- Burton, T.L.; Husband, B.C. Fitness differences among diploids, tetraploids, and their triploid progeny in Chamerion angustifolium: Mechanism of inviability and implications for polyploid evolution. Evolution 2000, 54, 1182–1191. [Google Scholar] [CrossRef]
- Walters, S.A.; Wehner, T.C. Incompatibility in diploid and tetraploid crosses of Cucumis sativus and Cucumis metuliferus. Euphytica 2002, 128, 371–374. [Google Scholar] [CrossRef]
- Wall, J.R. Use of marker genes in producing triploid watermelon. Proc. Am. Soc. Hort. Sci. 1960, 76, 577–581. [Google Scholar]
- Earhart, D.R.; Dainello, F.J.; Baker, M.L. Seedless (Triploid) watermelon evaluations for East Texas. Prog. Rep. 1994, 9, 5148. [Google Scholar]
- Gray, D.T.; Elmstrom, G.W. Process for Accelerated Production of Triploid Seed, for Seedless Watermelon Cultivar. U.S. Patent US5007198, 16 April 1991. [Google Scholar]
- Hassan, J.; Miyajima, I. Morphological and Ecological Characteristics of Pointed Gourd (Trichosanthes dioica Roxb.). J. Fac. Agric. Kyushu Univ. 2019, 64, 183–189. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]



| Colchicine Treatments | PGF02 × PGM03 | PGF17 × PGM08 | PGF18 × PGM08 | PGF19 × PGM09 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Conc. (%) | Duration (h) | Germinated Seeds (%) | Surviving Seedling (%) z | Germinated Seeds (%) | Surviving Seedling (%) | Germinated Seeds (%) | Surviving Seedling (%) | Germinated Seeds (%) | Surviving Seedling (%) |
| 0.05 | 24 | 1 (4.0) | 1 (4.0) | 3 (12.0) | 2 (8.0) | 2 (8.0) | 2 (8.0) | 1 (4.0) | 1 (4.0) |
| 48 | 5 (20.0) | 5 (20.0) | 1 (4.0) | 1 (4.0) | 3 (12.0) | 1 (4.0) | 2 (8.0) | 2 (8.0) | |
| 72 | 12 (48.0) | 9 (36.0) | 4 (16.0) | 4 (16.0) | 0 (0.0) | 0 (0.0) | 9 (36.0) | 9 (36.0) | |
| 0.1 | 24 | 1 (4.0) | 1 (4.0) | 2 (8.0) | 1 (4.0) | 5 (20.0) | 5 (20.0) | 7 (28.0) | 7 (28.0) |
| 48 | 5 (20.0) | 5 (20.0) | 0 (0.0) | 0 (0.0) | 4 (16.0) | 4 (16.0) | 7 (28.0) | 6 (24.0) | |
| 72 | 8 (32.0) | 6 (24.0) | 3 (12.0) | 3 (12.0) | 2 (8.0) | 2 (8.0) | 5 (20.0) | 4 (16.0) | |
| 0.5 | 24 | 1 (4.0) | 1 (4.0) | 5 (20.0) | 5 (20.0) | 2 (8.0) | 2 (8.0) | 3 (12.0) | 2 (8.0) |
| 48 | 4 (16.0) | 3 (12.0) | 1 (4.0) | 1 (4.0) | 5 (20.0) | 2 (8.0) | 5 (20.0) | 5 (20.0) | |
| 72 | 5 (20.0) | 4 (16.0) | 2 (8.0) | 2 (8.0) | 2 (8.0) | 1 (4.0) | 6 (24.0) | 5 (20.0) | |
| Total | 42 | 35 | 21 | 19 | 25 | 19 | 45 | 41 | |
| Colchicine Treatments | PGF02 × PGM03 | PGF17 × PGM08 | PGF18 × PGM08 | PGF19 × PGM09 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conc. (%) | Duration (h) | Ploidy Levels z | Ploidy Levels | Ploidy Levels | Ploidy Levels | ||||||||
| 2x | 4x | 2x + 4x | 2x | 4x | 2x + 4x | 2x | 4x | 2x + 4x | 2x | 4x | 2x + 4x | ||
| 0.05 | 24 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 1 | 0 | 0 |
| 48 | 4 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | |
| 72 | 8 | 0 | 1 | 4 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 | |
| 0.1 | 24 | 1 | 0 | 0 | 1 | 0 | 0 | 5 | 0 | 0 | 7 | 0 | 0 |
| 48 | 4 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 1 | 6 | 0 | 0 | |
| 72 | 3 | 0 | 3 | 2 | 0 | 1 | 1 | 0 | 1 | 4 | 0 | 0 | |
| 0.5 | 24 | 1 | 0 | 0 | 5 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 |
| 48 | 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 3 | 1 | 1 | |
| 72 | 3 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 2 | 3 | |
| Total | 28 | 0 | 7 | 16 | 0 | 3 | 14 | 0 | 5 | 34 | 3 | 4 | |
| Ploidy Level | No. of Evaluated Plants | Leaf Length (cm) | Leaf Diameter (cm) | Internode Length (cm) | Internode Diameter (mm) |
|---|---|---|---|---|---|
| Diploid (2x) | 3 | 5.6 ± 0.2 b z | 6.5 ± 0.1 b | 9.9 ± 1.0 ns | 2.6 ± 0.1 ns |
| Tetraploid (4x) | 3 | 8.7 ± 0.1 a | 9.7 ± 0.1 a | 8.6 ± 0.5 | 3.6 ± 0.5 |
| Ploidy Levels z | No. of Days to First Flowering | Node No. of First Flowering | Flowers No./ Plant y | Ovary Length (mm) | Ovary Diameter (mm) | Stalk Length (mm) | Petal Length (mm) | Petal Diameter (mm) |
|---|---|---|---|---|---|---|---|---|
| 2x | 74.3 ± 1.5 b x | 12.7 ± 1.1 b | 51.0 ± 2.0 a | 15.5 ± 1.1 b | 5.4 ± 0.6 b | 28.5 ± 0.9 b | 19.5 ± 0.8 b | 6.3 ± 0.6 b |
| 4x | 129.7 ± 3.2 a | 30.3 ± 2.3 a | 31.7 ± 2.5 b | 29.0 ± 1.2 a | 10.4 ± 1.0 a | 31.1 ± 0.8 a | 33.0 ± 1.2 a | 17.6 ± 1.0 a |
| Ploidy Levels z | Stalk Length (mm) | Stalk Diameter (mm) | Anther Length (mm) | Anther Diameter (mm) | No. of Anthers | Petal Length (mm) | Petal Diameter (mm) |
|---|---|---|---|---|---|---|---|
| 2x | 17.5 ± 0.9 b y | 3.6 ± 0.6 b | 3.1 ± 0.1 b | 5.1 ± 0.2 b | 3.0 ± 0.0 ns | 16.1 ± 0.6 b | 5.6 ± 0.1 b |
| 4x | 45.8 ± 1.2 a | 6.0 ± 0.9 a | 4.5 ± 0.2 a | 9.4 ± 0.3 a | 3.0 ± 0.0 | 24.5 ± 0.5 a | 14.1 ± 0.3 a |
| Cross Combination w (Seed Parent × Pollen Parent) | No. of Flowers Pollinated/Repetition z | No. of Flowers that Set Fruits | Fruit Set Rate (%) | Fruit Length (cm) | Fruit Diameter (cm) | Fruit Weight (g) |
|---|---|---|---|---|---|---|
| 2x × 2x | 5 | 5.0 ± 0.0 a y | 100.0 ± 00.0 a | 11.0 ± 0.9 a | 3.3 ± 0.0 a | 45.7 ± 2.3 a |
| 2x × 4x | 5 | – x | – | – | – | – |
| 4x × 4x | 5 | 0.7 ± 0.6 b | 13.3 ± 11.5 b | 2.9 ± 0.1 c | 0.9 ± 0.8 b | 4.3 ± 3.7 c |
| 4x × 2x | 5 | 4.7 ± 0.5 a | 93.3 ± 11.5 a | 8.8 ± 0.5 b | 3.3 ± 0.2 a | 33.4 ± 0.9 b |
| Cross Combination z (Seed Parent × Pollen Parent) | No. of Evaluated Fruits y | No. of Developed Seeds | No. of Aborted Seeds | Seed Diameter (mm) | 100 Seeds Weight (g) | Seed Germination (%) |
|---|---|---|---|---|---|---|
| 2x × 2x | 3 | 26.4 ± 0.9 a x | 1.3 ± 0.5 b | 5.5 ± 0.1 b | 6.4 ± 0.3 b | 100.0 |
| 2x × 4x | 3 | – w | – | – | – | – |
| 4x × 4x | 3 | 0.0 ± 0.0 b | 1.0 ± 0.9 b | – | – | – |
| 4x × 2x | 3 | 1.8 ± 0.0 b | 2.7 ± 0.3 a | 7.8 ± 0.2 a | 12.4 ± 0.6 a | – |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, J.; Miyajima, I.; Ozaki, Y.; Mizunoe, Y.; Sakai, K.; Zaland, W. Tetraploid Induction by Colchicine Treatment and Crossing with a Diploid Reveals Less-Seeded Fruit Production in Pointed Gourd (Trichosanthes dioica Roxb.). Plants 2020, 9, 370. https://doi.org/10.3390/plants9030370
Hassan J, Miyajima I, Ozaki Y, Mizunoe Y, Sakai K, Zaland W. Tetraploid Induction by Colchicine Treatment and Crossing with a Diploid Reveals Less-Seeded Fruit Production in Pointed Gourd (Trichosanthes dioica Roxb.). Plants. 2020; 9(3):370. https://doi.org/10.3390/plants9030370
Chicago/Turabian StyleHassan, Jahidul, Ikuo Miyajima, Yukio Ozaki, Yuki Mizunoe, Kaori Sakai, and Wasimullah Zaland. 2020. "Tetraploid Induction by Colchicine Treatment and Crossing with a Diploid Reveals Less-Seeded Fruit Production in Pointed Gourd (Trichosanthes dioica Roxb.)" Plants 9, no. 3: 370. https://doi.org/10.3390/plants9030370
APA StyleHassan, J., Miyajima, I., Ozaki, Y., Mizunoe, Y., Sakai, K., & Zaland, W. (2020). Tetraploid Induction by Colchicine Treatment and Crossing with a Diploid Reveals Less-Seeded Fruit Production in Pointed Gourd (Trichosanthes dioica Roxb.). Plants, 9(3), 370. https://doi.org/10.3390/plants9030370




