Effect of Plant Growth Regulators on Coloured Callus Formation and Accumulation of Azadirachtin, an Essential Biopesticide in Azadirachta indica
Abstract
1. Introduction
2. Materials and Methods
2.1. Explant Source and Surface Sterilization
2.2. Callus Cultures Establishment
2.3. Isolation and Extraction of Azadirachtin
2.4. HPLC Quantification of Azadirachtin
2.5. Toxicity Analysis
2.5.1. Sample Preparations and Zebrafish Maintenance and Breeding
2.5.2. Zebrafish Embryo Toxicity (ZFET) Assay
2.6. Statistical Analysis
3. Results
3.1. Development of Callus in vitro
3.2. Extraction and Quantification of Azadirachtin (AZA) from Colored Callus
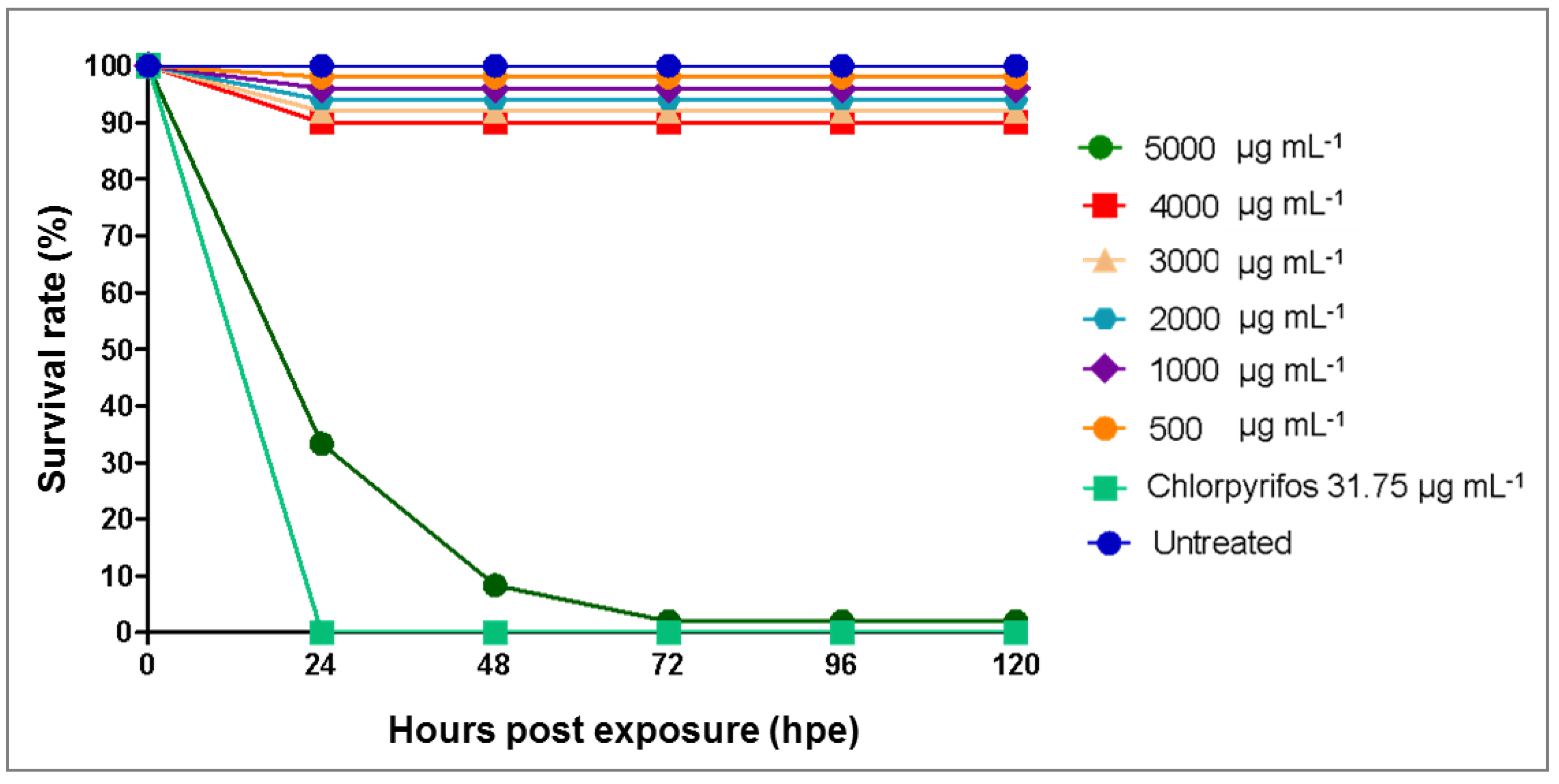
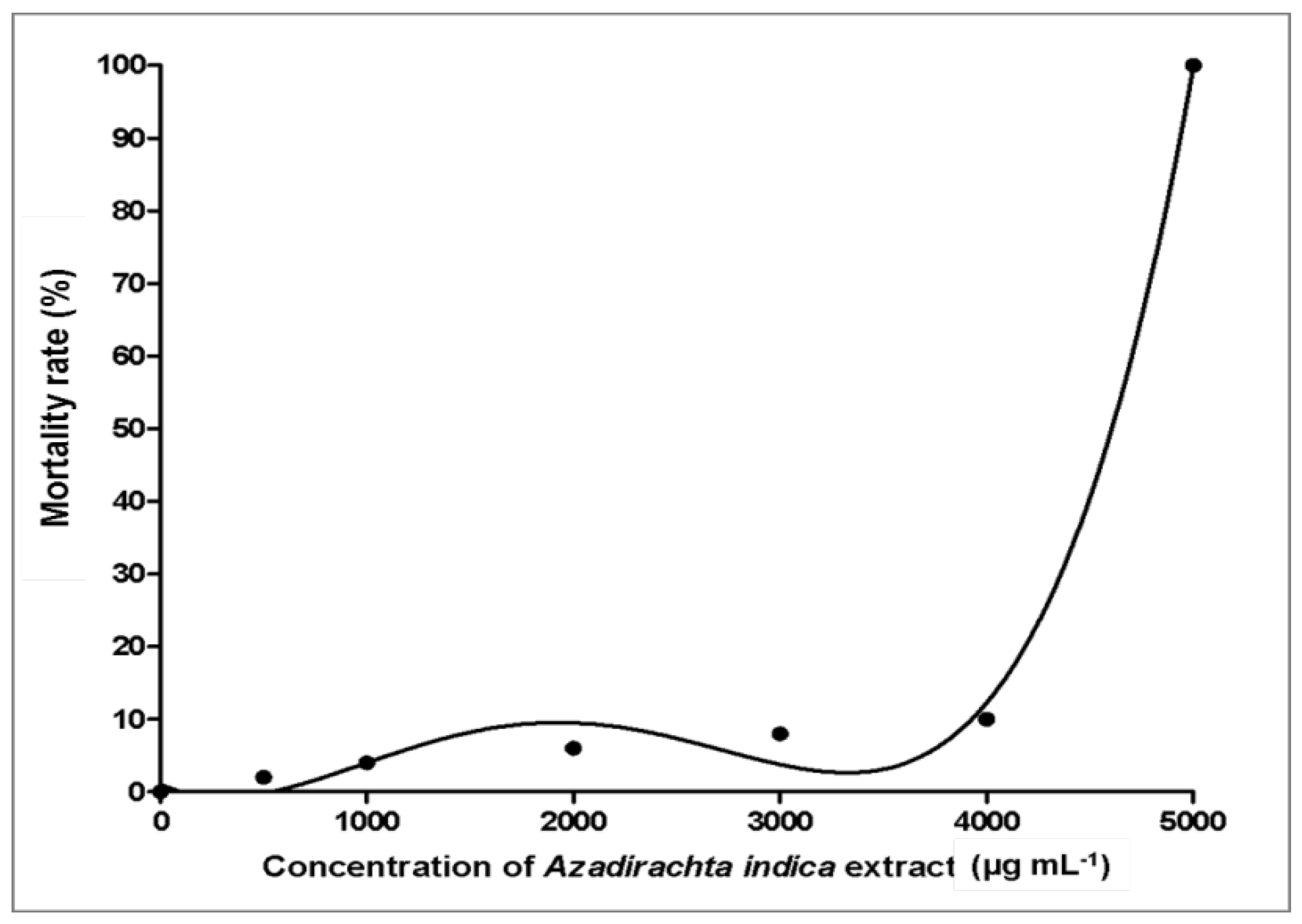
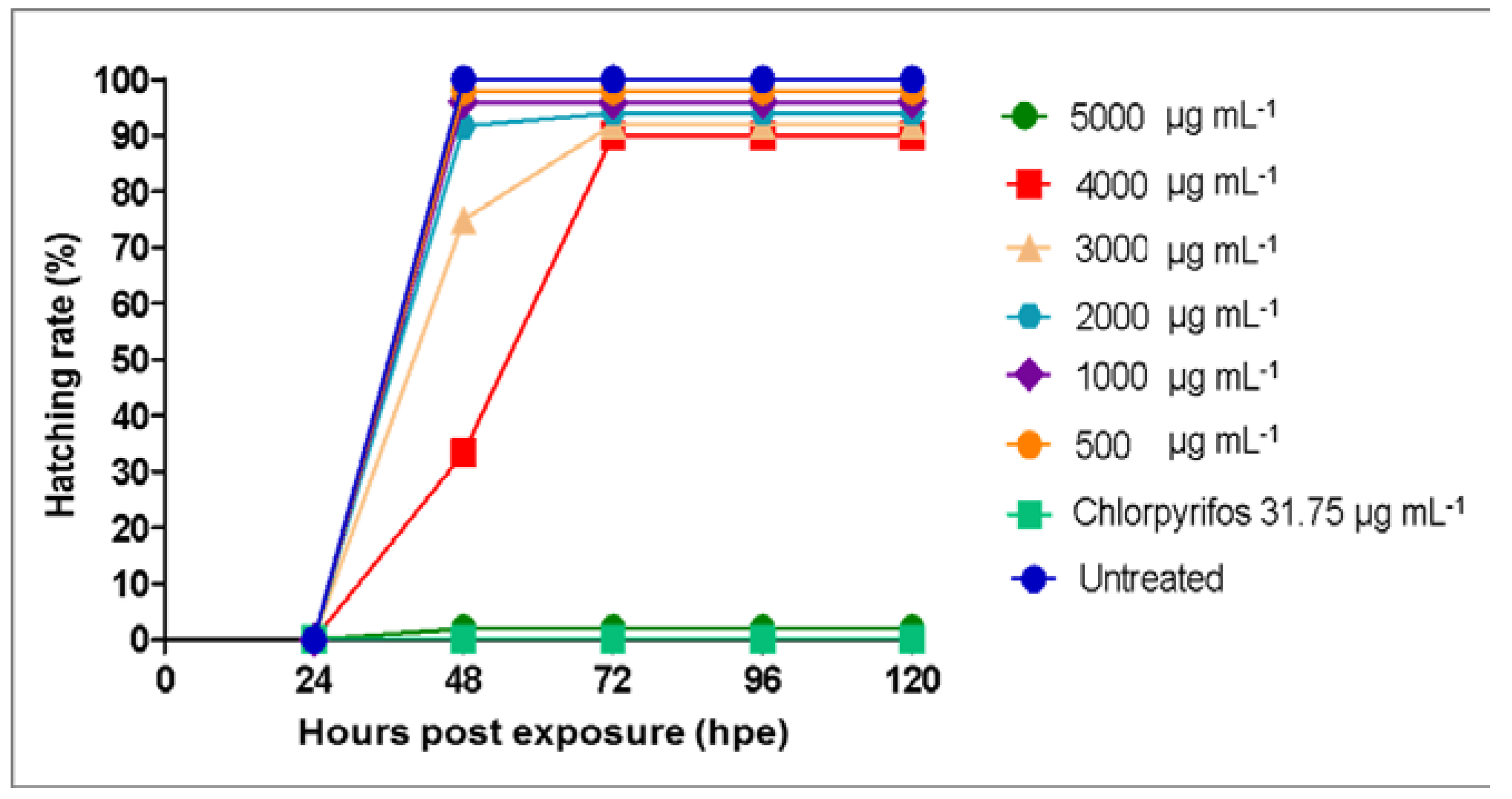
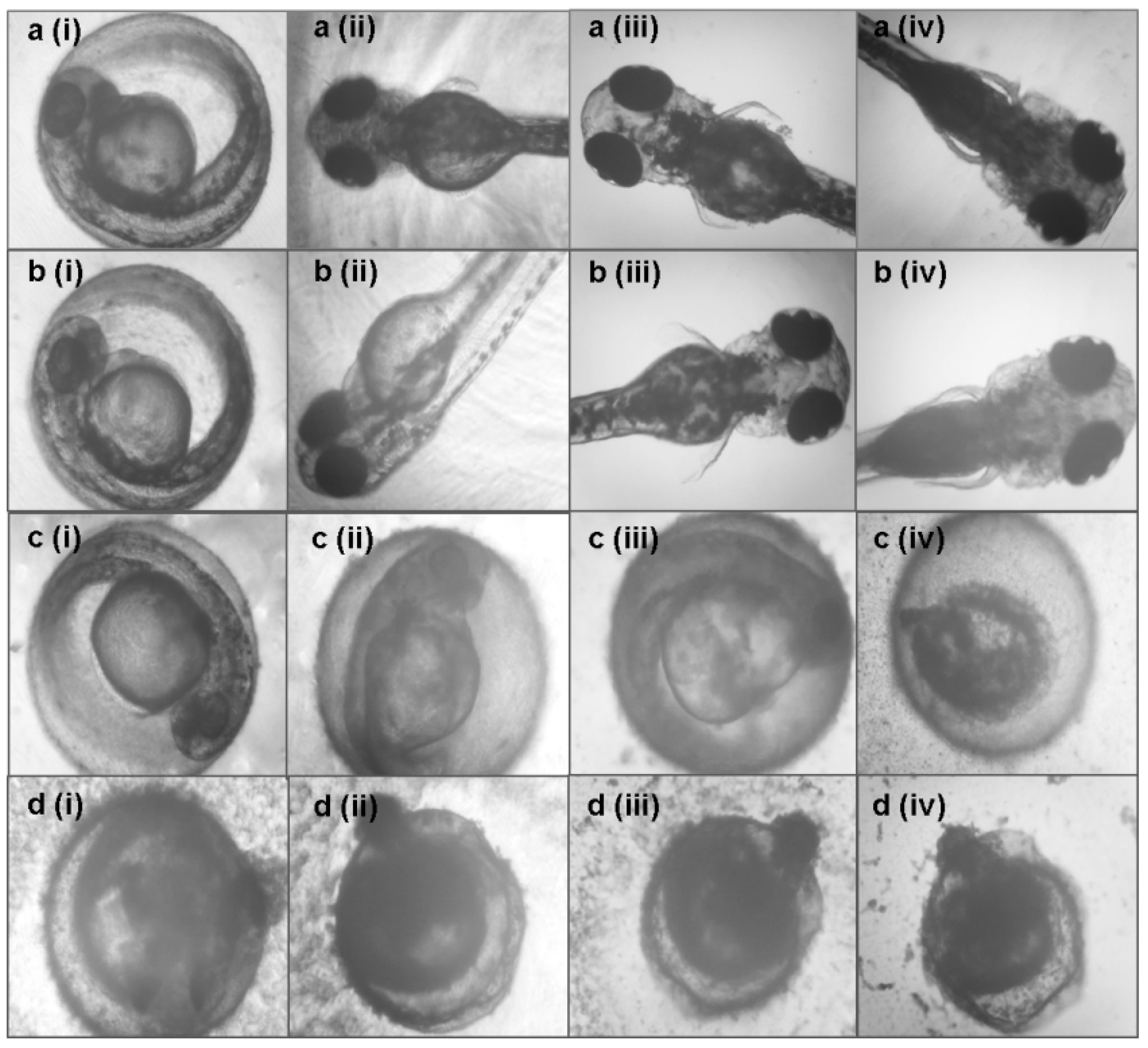
3.3. Toxicity Effects of Azadirachta indica Extracts on Zebrafish Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parsaeimehr, A.; Martinez-Chapa, S.O.; Parra-Saldívar, R. Chapter 13—Medicinal plants versus skin disorders: A survey from ancient to modern herbalism. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Kon, K., Rai, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 2, pp. 205–221. [Google Scholar]
- Okoye, T.C.; Uzor, P.F.; Onyeto, C.A.; Okereke, E.K. 18—Safe African medicinal plants for clinical studies. In Toxicological Survey of African Medicinal Plants; Kuete, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 535–555. [Google Scholar]
- Veeresham, C. Natural Products Derived from Plants as a Source of Drugs; Wolters Kluwer-Medknow Publications: Mumbai, India, 2012. [Google Scholar]
- Gupta, S.C.; Prasad, S.; Tyagi, A.K.; Kunnumakkara, A.B.; Aggarwal, B.B. Neem (Azadirachta indica): An indian traditional panacea with modern molecular basis. Phytomedicine 2017, 34, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Sujarwo, W.; Keim, A.P.; Caneva, G.; Toniolo, C.; Nicoletti, M. Ethnobotanical uses of neem (Azadirachta indica a. Juss.; meliaceae) leaves in Bali (Indonesia) and the indian subcontinent in relation with historical background and phytochemical properties. J. Ethnopharmacol. 2016, 189, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Venkata, K.C.N.; Bhattacharyya, P.; Sethi, G.; Bishayee, A. Seminars in cancer biology. In Potential of Neem (Azadirachta indica L.) for Prevention and Treatment of Oncologic Diseases; Elsevier: Amsterdam, The Netherlands, 2016; pp. 100–115. [Google Scholar]
- Biswas, K.; Chattopadhyay, I.; Banerjee, R.K.; Bandyopadhyay, U. Biological activities and medicinal properties of neem (Azadirachta indica). Curr. Sci. Bangalore 2002, 82, 1336–1345. [Google Scholar]
- Al-Hashemi, Z.S.S.; Hossain, M.A. Biological activities of different neem leaf crude extracts used locally in Ayurvedic medicine. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 128–131. [Google Scholar] [CrossRef]
- Sidhu, O.P.; Kumar, V.; Behl, H.M. Variability in triterpenoids (nimbin and salanin) composition of neem among different provenances of India. Ind. Crop. Prod. 2004, 19, 69–75. [Google Scholar] [CrossRef]
- Gehlot, A.; Arya, I.D.; Arya, S. Regeneration of complete plantlets from callus culture of Azadirachta indica a. Juss using immature flower buds. Adv. For. Sci. 2017, 4, 71–76. [Google Scholar]
- Wang, Y.; Chen, X.; Wang, J.; Xun, H.; Sun, J.; Tang, F. Comparative analysis of the terpenoid biosynthesis pathway in azadirachta indica and melia azedarach by RNA-seq. SpringerPlus 2016, 5, 819. [Google Scholar] [CrossRef]
- Quraishi, A.; Koche, V.; Sharma, P.; Mishra, S. In vitro clonal propagation of neem (Azadirachta indica). Plant Cell Tissue Organ Cult. 2004, 78, 281–284. [Google Scholar] [CrossRef]
- Chaturvedi, R.; Razdan, M.K.; Bhojwani, S.S. An efficient protocol for the production of triploid plants from endosperm callus of neem, Azadirachta indica a. Juss. J. Plant Physiol. 2003, 160, 557–564. [Google Scholar] [CrossRef]
- Ashrafuzzaman, M.; Haque, M.S.; Luna, L.N. In vitro clonal propagation of the neem tree (Azadirachta indica a. Juss.). Afr. J. Biotechnol. 2008, 7, 386–391. [Google Scholar]
- Sanyal, M.; Das, A.; Banerjee, M.; Datta, P. In vitro hormone induced chemical & histological differentation in stem callus of neem Azadirachta indica a. Juss. Indian J. Exp. Biol. 1981, 19, 1067–1068. [Google Scholar]
- Srinidhi, H.; Gill, R.; Sidhu, D. Micropropagation of adult and juvenile neem (Azadirachta indica a. Juss). J. Crop Improv. 2008, 21, 221–232. [Google Scholar] [CrossRef]
- Arora, K.; Sharma, M.; Srivastava, J.; Ranade, S.; Sharma, A. Rapid in vitro cloning of a 40-year-old tree of Azadirachta indica a. Juss.(neem) employing nodal stem segments. Agrofor. Syst. 2010, 78, 53–63. [Google Scholar] [CrossRef]
- Gautam, V.K.; Nanda, K.; Gupta, S.C. Development of shoots and roots in anther-derived callus of Azadirachta indica a. Juss—A medicinal tree. Plant Cell Tissue Organ Cult. 1993, 34, 13–18. [Google Scholar] [CrossRef]
- Ramesh, K.; Padhya, M. In vitro propagation of neem, Azadirachta indica (a. Juss.), from leaf discs. Indian J. Exp. Biol 1990, 28, 932–935. [Google Scholar]
- Gonçalves, S.; Romano, A. Production of plant secondary metabolites by using biotechnological tools. Second. Metab. Sources Appl. 2018, 1, 81–99. [Google Scholar]
- Cardoso, J.C.; Oliveira, M.E.B.d.; Cardoso, F.d.C. Advances and challenges on the in vitro production of secondary metabolites from medicinal plants. Hortic. Bras. 2019, 37, 124–132. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Zhong, J.-J.; Paek, K.-Y. Strategies for enhanced production of plant secondary metabolites from cell and organ cultures. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 471–508. [Google Scholar]
- Ruffoni, B.; Pistelli, L.; Bertoli, A.; Pistelli, L. Plant cell cultures: Bioreactors for industrial production. In Bio-Farms for Nutraceuticals: Functional Food and Safety Control by Biosensors; Giardi, M.T., Rea, G., Eds.; Springer US: Boston, MA, USA, 2010; pp. 203–221. [Google Scholar]
- Nathan, S.S.; Kalaivani, K.; Murugan, K. Effects of neem limonoids on the malaria vector anopheles Stephensi liston (diptera: Culicidae). Acta Trop. 2005, 96, 47–55. [Google Scholar] [CrossRef]
- Hossain, M.A.; Al-Toubi, W.A.; Weli, A.M.; Al-Riyami, Q.A.; Al-Sabahi, J.N. Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. J. Taibah Univ. Sci. 2013, 7, 181–188. [Google Scholar] [CrossRef]
- Priyadarsini, R.V.; Manikandan, P.; Kumar, G.H.; Nagini, S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Radic. Res. 2009, 43, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Baligar, N.; Aladakatti, R.; Ahmed, M.; Hiremath, M. Hepatoprotective activity of the neem-based constituent azadirachtin-a in carbon tetrachloride intoxicated wistar rats. Can. J. Physiol. Pharmacol. 2014, 92, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Chaturvedi, R. Increased production of azadirachtin from an improved method of androgenic cultures of a medicinal tree azadirachta indica a. Juss. Plant Signal. Behav. 2011, 6, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Akula, C.; Akula, A.; Drew, R. Somatic embryogenesis in clonal neem, Azadirachta indica a. Juss. And analysis for in vitro azadirachtin production. Vitr. Cell. Dev. Biol. Plant 2003, 39, 304–310. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A. Azadirachtin production by hairy root cultivation of Azadirachta indica in a modified stirred tank reactor. Bioprocess Biosyst. Eng. 2012, 35, 1549–1553. [Google Scholar] [CrossRef] [PubMed]
- Sujanya, S.; Devi, B.P.; Sai, I. In vitro production of azadirachtin from cell suspension cultures of Azadirachta indica. J. Biosci. 2008, 33, 113–120. [Google Scholar] [CrossRef]
- Daud, N.H.; Jayaraman, S.; Mohamed, R. Methods paper: An improved surface sterilization technique for introducing leaf, nodal and seed explants of Aquilaria malaccensis from field sources into tissue culture. Asia Pac. J. Mol. Biol. Biotech 2012, 20, 55–58. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Singh, M.; Chaturvedi, R. Sustainable production of azadirachtin from differentiated in vitro cell lines of neem (Azadirachta indica). Aob Plants 2013, 5, plt034. [Google Scholar] [CrossRef]
- Organisation de coopération et de développement économiques. Test No. 236: Fish Embryo Acute Toxicity (Fet) Test; OECD Publishing: Paris, France, 2013. [Google Scholar]
- Ohikhena, F.U.; Wintola, O.A.; Afolayan, A.J. Toxicity assessment of different solvent extracts of the medicinal plant, phragmanthera capitata (sprengel) balle on brine shrimp (Artemia salina). Int. J. Pharmacol. 2016, 12, 701–710. [Google Scholar]
- Ashokhan, S.; Ramasamy, S.; Karsani, S.A.; Othman, R.; Yaacob, J.S. Analysis of bioactive pigments in coloured callus of Azadirachta indica for possible use as functional natural colourants. Pigment Resin Technol. 2019, 48, 9–19. [Google Scholar] [CrossRef]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Warren, K.S.; Yellen, G.; Fishman, M.C. Defective “pacemaker” current (ih) in a zebrafish mutant with a slow heart rate. Proc. Natl. Acad. Sci. USA 1997, 94, 4554–4559. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chang, C.; Chang, W. Direct somatic embryogenesis on leaf explants of oncidium gower ramsey and subsequent plant regeneration. Plant Cell Rep. 1999, 19, 143–149. [Google Scholar] [CrossRef]
- Horsch, R.; Fry, J.; Hoffman, N.; Eichholtz, D.; Rogers, S.a.; Fraley, R. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1232. [Google Scholar]
- Davies, P.J. The Plant Hormones: Their Nature, Occurrence, and Functions; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Gaspar, T.; Kevers, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. Vitr. Cell. Dev. Biol. Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Cabrini, D.A.; Moresco, H.H.; Imazu, P.; Silva, C.D.d.; Pietrovski, E.F.; Mendes, D.A.G.B.; Prudente, A.d.S.; Pizzolatti, M.G.; Brighente, I.M.C.; Otuki, M.F. Analysis of the potential topical anti-inflammatory activity of Averrhoa carambola L. In mice. Evid. Based Complementary Altern. Med. 2011, 2011, 7. [Google Scholar] [CrossRef]
- Kreis, W.; Haug, B.; Yücesan, B. Somaclonal variation of cardenolide content in heywood’s foxglove, a source for the antiviral cardenolide glucoevatromonoside, regenerated from permanent shoot culture and callus. Vitr. Cell. Dev. Biol. Plant 2015, 51, 35–41. [Google Scholar] [CrossRef]
- Faisal, M.; Anis, M. Rapid mass propagation of tylophora indica merrill via leaf callus culture. Plant Cell Tissue Organ Cult. 2003, 75, 125–129. [Google Scholar] [CrossRef]
- Singh, S.K.; Joshi, T.; Kanojiya, S.; Tripathi, V.; Mishra, D.K. Callus culture and in vitro biosynthesis of echitamine from Alstonia scholaris (l.) r. Br. Plant Cell Tissue Organ Cult. 2015, 120, 367–372. [Google Scholar] [CrossRef]
- Byamukama, R.; Namukobe, J.; Kiremire, B. Anthocyanins from leaf stalks of cassava (Manihot esculenta crantz). Afr. J. Pure Appl. Chem. 2009, 3, 20–25. [Google Scholar]
- Ling, A.P.K.; Tan, K.P.; Hussein, S. Comparative effects of plant growth regulators on leaf and stem explants of Labisia pumila var. Alata. J. Zhejiang Univ. Sci. B 2013, 14, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, O.; Carra, A.; Oddo, E.; Carimi, F.; Sajeva, M. Is in vitro micrografting a possible valid alternative to traditional micropropagation in cactaceae? Pelecyphora aselliformis as a case study. SpringerPlus 2016, 5, 201. [Google Scholar] [CrossRef]
- Giusti, P.; Vitti, D.; Fiocchetti, F.; Colla, G.; Saccardo, F.; Tucci, M. In vitro propagation of three endangered cactus species. Sci. Hortic. 2002, 95, 319–332. [Google Scholar] [CrossRef]
- Srivastava, P.; Singh, M.; Mathur, P.; Chaturvedi, R. In vitro organogenesis and plant regeneration from unpollinated ovary cultures of Azadirachta indica. Biol. Plant. 2009, 53, 360–364. [Google Scholar] [CrossRef]
- Kou, Y.; Ma, G.; da Silva, J.A.T.; Liu, N. Callus induction and shoot organogenesis from anther cultures of Curcuma attenuata wall. Plant Cell Tissue Organ Cult. 2013, 112, 1–7. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, N.; Sheng, A.; Ma, G.; Wu, G. In vitro plant regeneration from organogenic callus of Curcuma kwangsiensis lindl.(zingiberaceae). Plant Growth Regul. 2011, 64, 141–145. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rekha, K.; Yang, C.-H.; Jayabalan, N.; Chung, I.-M. High-frequency shoot regeneration from leaf explants through organogenesis in bitter melon (Momordica charantia L.). Plant Biotechnol. Rep. 2010, 4, 321–328. [Google Scholar] [CrossRef]
- Al-Mayahi, A.M.W. Thidiazuron-induced in vitro bud organogenesis of the date palm (Phoenix dactylifera L.) cv. Hillawi. Afr. J. Biotechnol. 2014, 13, 3581–3590. [Google Scholar]
- Gill, R.; Ozias-Akins, P. Thidiazuron-induced highly morphogenic callus and high frequency regeneration of fertile peanut (Arachis hypogaea L.) plants. Vitr. Cell. Dev. Biol. Plant 1999, 35, 445–450. [Google Scholar] [CrossRef]
- Garcia, R.; Pacheco, G.; Falcão, E.; Borges, G.; Mansur, E. Influence of type of explant, plant growth regulators, salt composition of basal medium, and light on callogenesis and regeneration in Passiflora suberosa L. (passifloraceae). Plant Cell Tissue Organ Cult. 2011, 106, 47–54. [Google Scholar] [CrossRef]
- Misra, P.; Gupta, N.; Toppo, D.D.; Pandey, V.; Mishra, M.K.; Tuli, R. Establishment of long-term proliferating shoot cultures of elite Jatropha curcas L. By controlling endophytic bacterial contamination. Plant Cell Tissue Organ Cult. 2010, 100, 189–197. [Google Scholar] [CrossRef]
- Pandreka, A.; Dandekar, D.S.; Haldar, S.; Uttara, V.; Vijayshree, S.G.; Mulani, F.A.; Aarthy, T.; Thulasiram, H.V. Triterpenoid profiling and functional characterization of the initial genes involved in isoprenoid biosynthesis in neem (Azadirachta indica). BMC Plant Biol. 2015, 15, 214. [Google Scholar] [CrossRef]
- Phukan, H.; Kumar, R.; Mitra, P.K. Plant regeneration by somatic embryogenesis in Azadirachta indica a. Juss (neem). Int. Res. J. Eng. Technol. 2017, 4, 3212–3217. [Google Scholar]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. The influence of cytokinins on proliferation and polyphenol accumulation in shoot cultures of Scutellaria altissima L. Phytochem. Lett. 2017, 20, 449–455. [Google Scholar] [CrossRef]
- Ozeki, Y.; Komamine, A. Effects of growth regulators on the induction of anthocyanin synthesis in carrot suspension cultures. Plant Cell Physiol. 1986, 27, 1361–1368. [Google Scholar] [CrossRef]
- Nitnaware, K.; Naik, D.; Nikam, T. Thidiazuron-induced shoot organogenesis and production of hepatoprotective lignan phyllanthin and hypophyllanthin in Phyllanthus amarus. Plant Cell Tissue Organ Cult. 2011, 104, 101–110. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Kayani, W.K.; Khan, T.; Khan, R.S. Thidiazuron regulated growth, secondary metabolism and essential oil profiles in shoot cultures of Ajuga bracteosa. Ind. Crop. Prod. 2018, 121, 418–427. [Google Scholar] [CrossRef]
- Caballero, M.V.; Candiracci, M. Zebrafish as screening model for detecting toxicity and drugs efficacy. J. Unexplored Med. Data 2018, 3, 1–14. [Google Scholar] [CrossRef]
- Bambino, K.; Chu, J. Zebrafish in toxicology and environmental health. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2017; Volume 124, pp. 331–367. [Google Scholar]
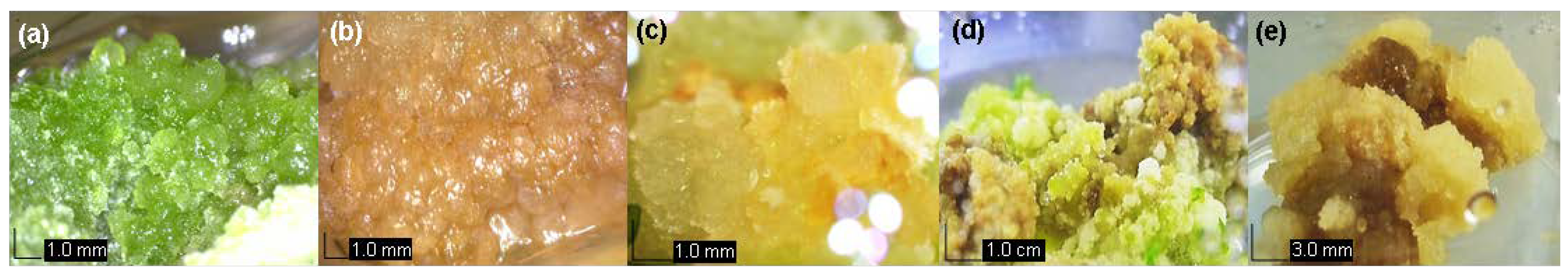


| MS + Hormone (mg L−1) | Fresh Weight (g) of Callus | Percentage (%) of Explants That Produced A Green Callus | Percentage (%) of Explants That Produced A Brown Callus | Percentage (%) of Explants That Produced A Cream Callus | Number of Days for Explants to Respond | Callus Texture |
|---|---|---|---|---|---|---|
| Control | 0.22 ± 0.01 a | NR | 100.00 ± 0.00 a | NR | 41 ± 0.66 d | watery |
| 0.2 TDZ | 2.08 ± 0.05 d | 100.00 ± 0.00 a | NR | NR | 20 ± 0.57 b | compact |
| 0.4 TDZ | 2.41 ± 0.09 e | 100.00 ± 0.00 a | NR | NR | 15 ± 0.88 a | compact |
| 0.6 TDZ | 3.38 ± 0.08 f | 100.00 ± 0.00 a | NR | NR | 14 ± 0.33 a | compact |
| 0.2 2,4-D | 0.96 ± 0.06 c | NR | 100.00 ± 0.00 a | 60.00 ± 2.08 | 25 ± 0.33 c | watery |
| 0.4 2,4-D | 0.82 ± 0.07 b,c | NR | 100.00 ± 0.00 a | 3.33 ± 1.52 | 16 ± 0.88 a | watery |
| 0.6 2,4-D | 0.76 ± 0.03 b | NR | 100.00 ± 0.00 a | 16.67 ± 1.15 | 14 ± 0.33 a | watery |
| MS + Hormone (mg L−1) | Fresh Weight (g) of Callus | Percentage of (%) Explants That Produced A Green Callus | Percentage of (%) Explants That Produced A Brown Callus | Percentage of (%) Explants That Produced A Cream Callus | Number of Days for Explants to Respond | Callus Texture |
|---|---|---|---|---|---|---|
| Control | 0.22 ± 0.01 a,b | NR | 100.00 ± 0.00 b | NR | 41 ± 0.66 e | watery |
| 0.2 T + 0.2 D | 0.43 ± 0.04 d | 100.00 ± 0.00 a | NR | NR | 32 ± 1.15 d | compact |
| 0.2 T + 0.4 D | 0.31 ± 0.03 c | 100.00 ± 0.00 a | NR | NR | 29 ± 0.58 c,d | compact |
| 0.2 T + 0.6 D | 0.56 ± 0.03 e | 100.00 ± 0.00 a | NR | NR | 29 ± 0.66 c,d | compact |
| 0.4 T + 0.2 D | 0.34 ± 0.04 c | 100.00 ± 0.00 a | NR | NR | 31 ± 0.57 d | compact |
| 0.4 T + 0.4 D | 0.34 ± 0.03 c | 100.00 ± 0.00 a | 100.00 ± 0.00 b | NR | 31 ± 0.57 d | watery + compact |
| 0.4 T + 0.6 D | 0.29 ± 0.03 b,c | 100.00 ± 0.00 a | 100.00 ± 0.00 b | NR | 31 ± 0.57 d | watery + compact |
| 0.6 T + 0.2 D | 0.19 ± 0.03 a | 100.00 ± 0.00 a | 100.00 ± 0.00 b | NR | 18 ± 1.52 a | watery + compact |
| 0.6 T + 0.4 D | 0.18 ± 0.02 a | 100.00 ± 0.00 a | 100.00 ± 0.00 b | NR | 23 ± 1.73 b | watery + compact |
| 0.6 T + 0.6 D | 0.16 ± 0.02 a | 100.00 ± 0.00 a | 100.00 ± 0.00 b | NR | 27 ± 1.00 c | watery + compact |
| MS + Hormone (mg L−1) | Fresh Weight (g) of Callus | Percentage of (%) Explants That Produced A Green Callus | Percentage of (%) Explants That Produced A Brown Callus | Percentage of (%) Explants That Produced A Cream Callus | Number of Days for Explants to Respond | Callus Texture |
|---|---|---|---|---|---|---|
| Control | NR | NR | NR | NR | NR | NR |
| 0.2 TDZ | 0.56 ± 0.17 d | 100 ± 0.00 a | NR | NR | 5 ± 1.52 a | compact |
| 0.4 TDZ | 0.30 ± 0.08 a,b | 100 ± 0.00 a | NR | NR | 5 ± 0.67 a | compact |
| 0.6 TDZ | 0.35 ± 0.12 b | 100 ± 0.00 a | NR | NR | 9 ± 1.54 c | compact |
| 0.2 2,4-D | 0.57 ± 0.18 d | 100 ± 0.00 a | 100 ± 0.00 a | NR | 8 ± 0.53 b | watery |
| 0.4 2,4-D | 0.34 ± 0.15 a | 100 ± 0.00 a | 100 ± 0.00 a | NR | 11 ± 1.66 e | watery |
| 0.6 2,4-D | 0.42 ± 0.17 c | 100 ± 0.00 a | 100 ± 0.00 a | NR | 10 ± 0.75 d | watery |
| MS + Hormone (mg L−1) | Fresh Weight (g) of Callus | Percentage of (%) Explants That Produced A Green Callus | Percentage of (%) Explants That Produced A Brown Callus | Percentage of (%) Explants That Produced A Cream Callus | Number of Days for Explants to Respond | Callus Texture |
|---|---|---|---|---|---|---|
| Control | NR | NR | NR | NR | NR | NR |
| 0.2 T + 0.2 D | 0.20 ± 0.02 e | 100 ± 0.00 a | 100 ± 0.00 a | NR | 32 ± 1.15 b | watery |
| 0.2 T + 0.4 D | 0.08 ± 0.00 d | 100 ± 0.00 a | NR | NR | 29 ± 1.15 b | compact |
| 0.2 T + 0.6 D | 0.05 ± 0.00 c | 100 ± 0.00 a | NR | NR | 30 ± 0.58 b | compact |
| 0.4 T + 0.2 D | 0.08 ± 0.01 d | 100 ± 0.00 a | NR | NR | 31 ± 0.58 b | compact |
| 0.4 T + 0.4 D | 0.05 ± 0.00 c | 100 ± 0.00 a | 100 ± 0.00 a | NR | 31 ± 2.10 5 | watery + compact |
| 0.4 T + 0.6 D | 0.04 ± 0.00 b,c | 100 ± 0.00 a | 100 ± 0.00 a | NR | 31 ± 1.90 d | watery + compact |
| 0.6 T + 0.2 D | 0.05 ± 0.00 c | 100 ± 0.00 a | 100 ± 0.00 b | NR | 18 ± 3.10 e | watery + compact |
| 0.6 T + 0.4 D | 0.05 ± 0.00 c | 100 ± 0.00 a | 100 ± 0.00 b | NR | 23 ± 1.73 f | watery + compact |
| 0.6 T + 0.6 D | 0.02 ± 0.02 b | 100 ± 0.00 a | 100 ± 0.00 b | NR | 27 ± 1.16 f | watery + compact |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashokhan, S.; Othman, R.; Abd Rahim, M.H.; Karsani, S.A.; Yaacob, J.S. Effect of Plant Growth Regulators on Coloured Callus Formation and Accumulation of Azadirachtin, an Essential Biopesticide in Azadirachta indica. Plants 2020, 9, 352. https://doi.org/10.3390/plants9030352
Ashokhan S, Othman R, Abd Rahim MH, Karsani SA, Yaacob JS. Effect of Plant Growth Regulators on Coloured Callus Formation and Accumulation of Azadirachtin, an Essential Biopesticide in Azadirachta indica. Plants. 2020; 9(3):352. https://doi.org/10.3390/plants9030352
Chicago/Turabian StyleAshokhan, Sharmilla, Rashidi Othman, Muhamad Hafiz Abd Rahim, Saiful Anuar Karsani, and Jamilah Syafawati Yaacob. 2020. "Effect of Plant Growth Regulators on Coloured Callus Formation and Accumulation of Azadirachtin, an Essential Biopesticide in Azadirachta indica" Plants 9, no. 3: 352. https://doi.org/10.3390/plants9030352
APA StyleAshokhan, S., Othman, R., Abd Rahim, M. H., Karsani, S. A., & Yaacob, J. S. (2020). Effect of Plant Growth Regulators on Coloured Callus Formation and Accumulation of Azadirachtin, an Essential Biopesticide in Azadirachta indica. Plants, 9(3), 352. https://doi.org/10.3390/plants9030352





