Characterization of the Auxin Efflux Transporter PIN Proteins in Pear
Abstract
1. Introduction
2. Results
2.1. Dwarfing Pear Rootstock ‘OHF51’ Limits the Growth Vigor of the Scion
2.2. Genome-Wide Identification of PIN Genes in Pyrus Bretschneideri
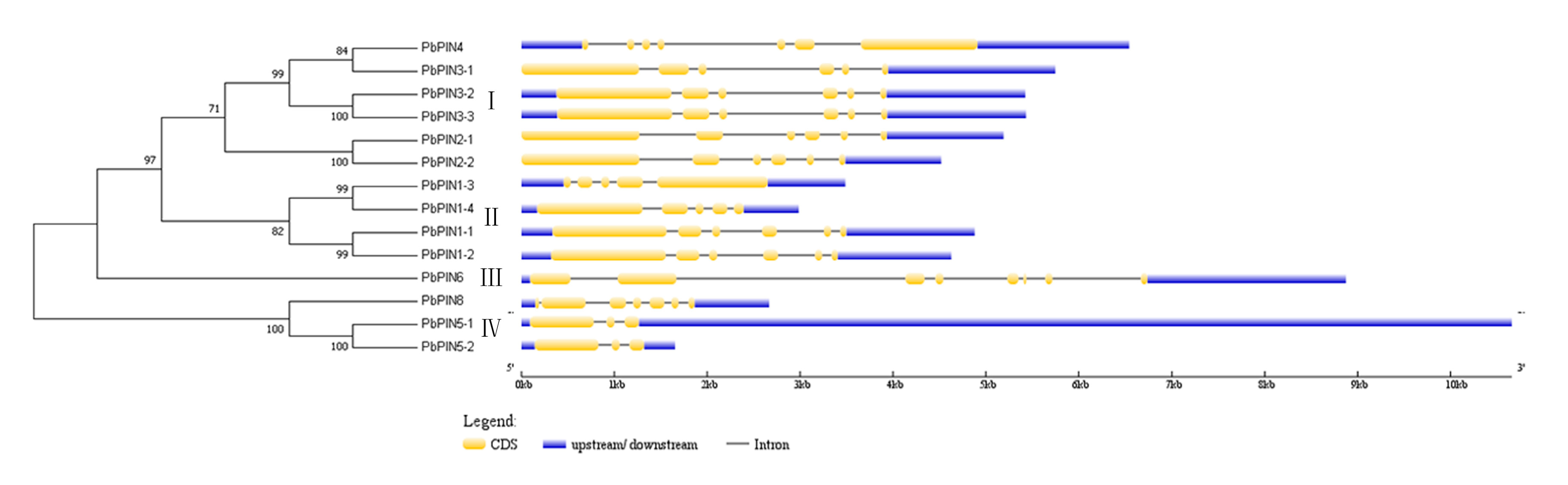
2.3. Phylogenetic Relationship Analysis of the PbPIN Family Genes
2.4. Exon-Intron Structure of PbPINs
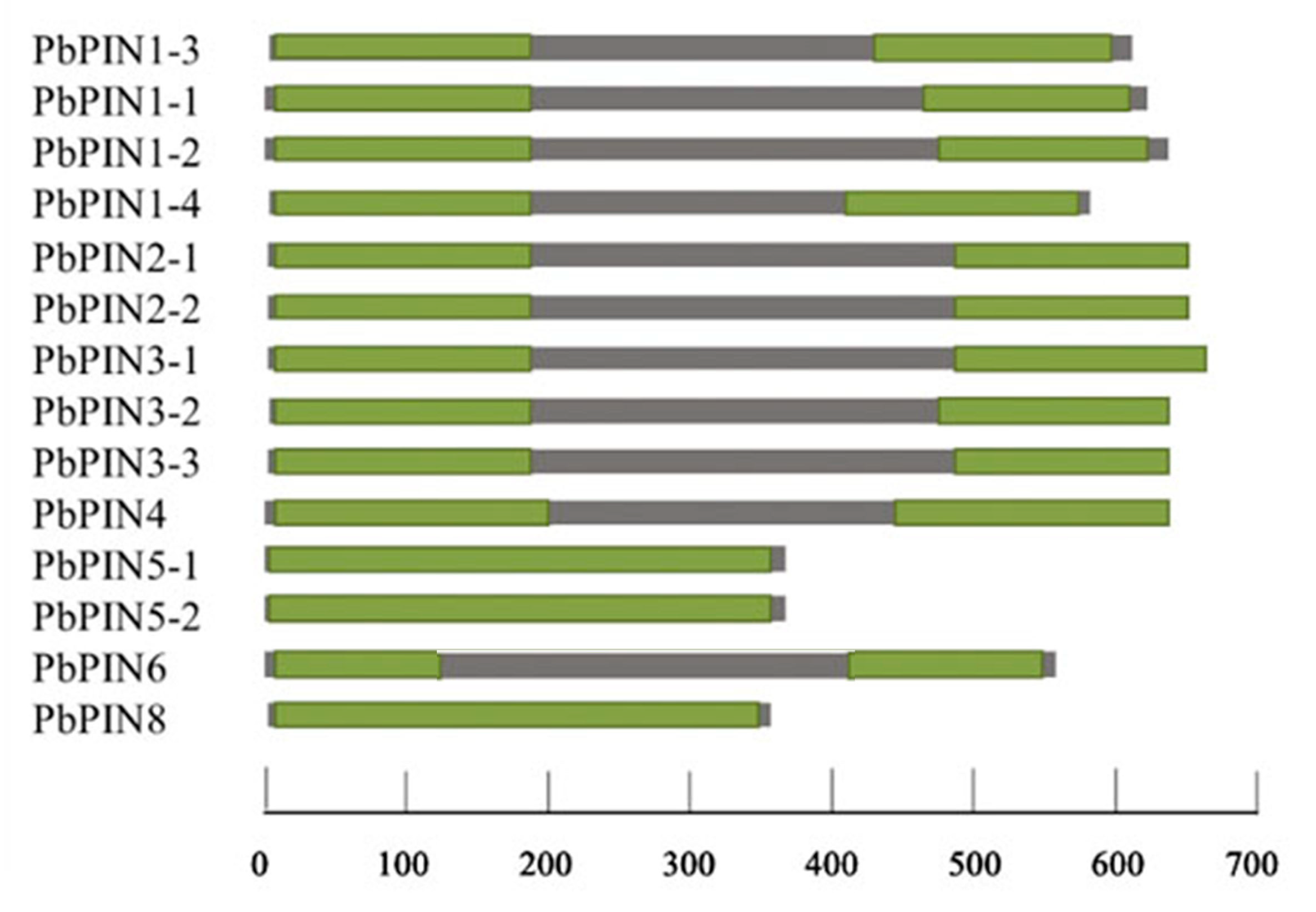
2.5. Analysis of Conservative Domain of PIN Family
2.6. Promoter Analysis of PbPINs
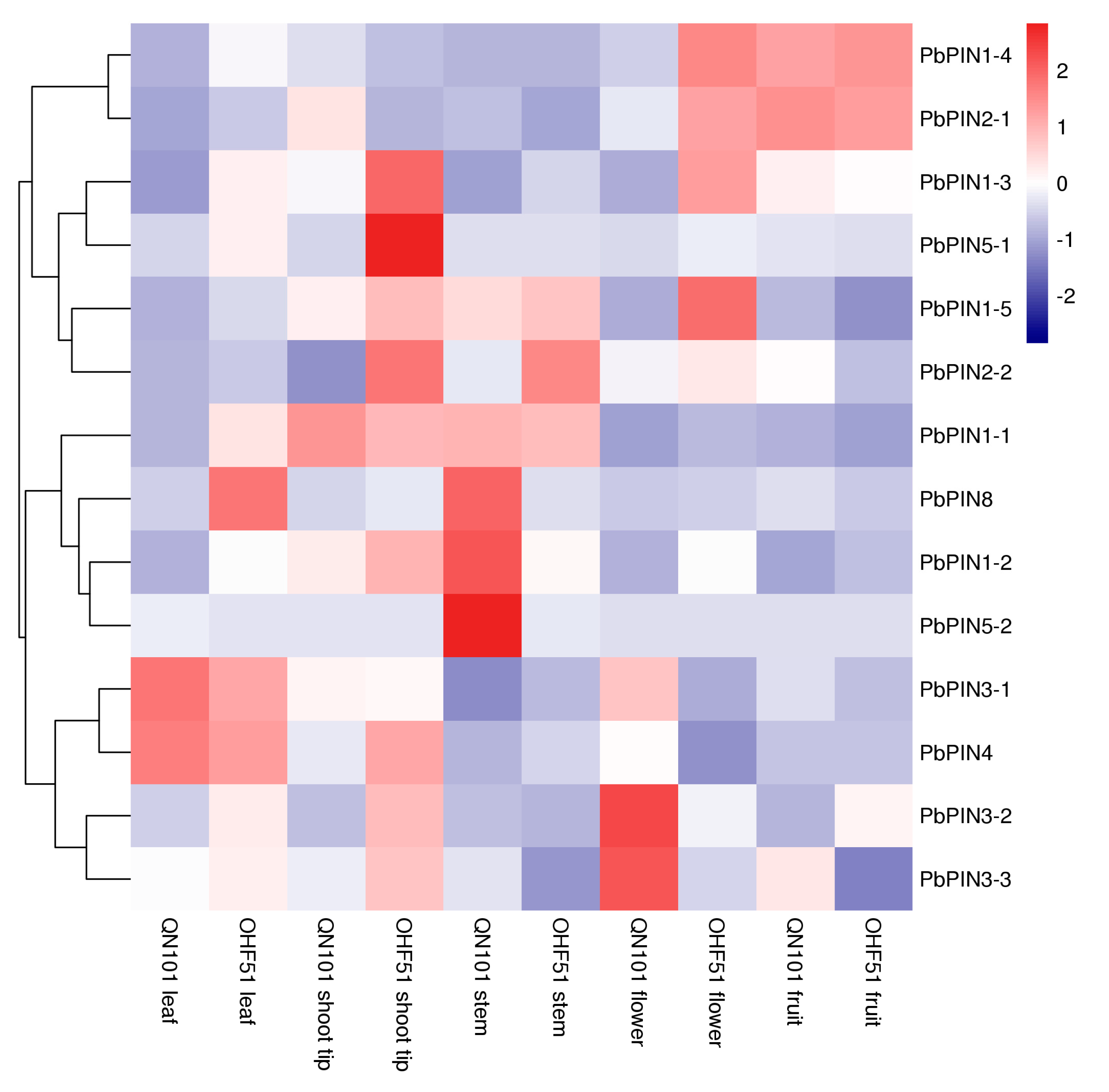
2.7. Expression of PbPINs in Different Organs
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Identification and Characteristics of PINs
4.3. Sequence Alignment and Phylogenetic Analysis
4.4. Sequence Conservation Analysis
4.5. Cis-Acting Regulatory Element Prediction in Promoters Regions of PbPINs
4.6. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Davis, T.D.; Curry, E.A.; Steffens, G.L. Chemical regulation of vegetative growth. Crit. Rev. Plant Sci. 1991, 10, 151–188. [Google Scholar] [CrossRef]
- Basak, A. Growth regulation of pome and stone fruit trees by use of prohexadione-Ca. Acta Hortic. 2000, 514, 41–50. [Google Scholar] [CrossRef]
- Bubán, T. The use of benzyladenine in orchard fruit growing: A mini review. Plant Growth Regul. 2000, 32, 381–390. [Google Scholar] [CrossRef]
- Medjdoub, R.; Val, J.; Blanco, A. Prohexadione-Ca inhibits vegetative growth of ‘Smoothee Golden Delicious’ apple trees. Sci. Hortic. 2004, 101, 243–253. [Google Scholar] [CrossRef]
- Reighard, G.L.; Ouellette, D.; Rauh, B.; Bridges, W., Jr. New rootstock cultivars for peach Influence growth, yield and fruit quality. Acta Hortic. 2014, 1058, 517–522. [Google Scholar] [CrossRef]
- Webster, A.D. A review of fruit tree rootstock research and development. Acta Hortic. 1997, 451, 53–73. [Google Scholar] [CrossRef]
- Webster, T. Dwarfing rootstocks:past, present and future. Compact Fruit Tree 2002, 35, 67–72. [Google Scholar]
- Atkinson, C.J.; Else, M.A.; Taylor, L. Root and stem hydraulic conductivity as determinants of growth potential in grafted trees of apple (Malus pumila Mill.). J. Exp. Bot. 2003, 54, 1221–1229. [Google Scholar] [CrossRef]
- Lockard, R.G.; Schneideri, G.W. Stock and scion growth relationships and the dwarfing mechanism in apple. Hort. Rev. 1981, 3, 315–375. [Google Scholar]
- Tustin, S.; Warrington, I.; Woolley, D.; van Hooijdonk, B.M. Rootstocks modify scion architecture, endogenous hormones, and root growth of newly grafted ‘royal gala’ apple trees. J. Am. Soc. Hortic. Sci. 2011, 136, 93–102. [Google Scholar]
- Richards, D.; Thompson, W.K.; Pharis, R.P. The influence of dwarfing interstocks on the distribution and metabolism of xylem-applied [3H]Gibberellin A4 in apple. Plant Physiol. 1986, 82, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Van Hooijdonk, B.M.; Woolley, D.J.; Warrington, I.J.; Tustin, D.S. Initial alteration of scion architecture by dwarfing apple rootstocks may involve shoot-root-shoot signalling by auxin, gibberellin, and cytokinin. J. Hortic. Sci. Biotechnol. 2010, 85, 59–65. [Google Scholar] [CrossRef]
- Kamboj, J.S.; Browning, G.; Blake, P.S.; Quinlan, J.D.; Baker, D.A. GC-MS-SIM analysis of abscisic acid and indole-3-acetic acid in shoot bark of apple rootstocks. Plant Growth Regul. 1999, 28, 21–27. [Google Scholar] [CrossRef]
- Tworkoski, T.; Fazio, G. Effects of size-controlling apple rootstocks on growth, abscisic acid, and hydraulic conductivity of scion of different vigor. Int. J. Fruit Sci. 2015, 15, 369–381. [Google Scholar] [CrossRef]
- Yu, C.; Dong, W.; Zhan, Y.; Huang, Z.A.; Li, Z.; Kim, I.S. Genome-wide identification and expression analysis of ClLAX, ClPIN and ClABCB genes families in Citrullus lanatus under various abiotic stresses and grafting. BMC Genet. 2017, 18, 33. [Google Scholar] [CrossRef]
- Li, K.; Kamiya, T.; Fujiwara, T. Differential roles of PIN1 and PIN2 in root meristem maintenance under low-B conditions in Arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 1205–1214. [Google Scholar] [CrossRef]
- Zhang, H.; An, H.S.; Wang, Y.; Zhang, X.Z.; Han, Z.H. Low expression of PIN gene family members is involved in triggering the dwarfing effect in M9 interstem but not in M9 rootstock apple trees. Acta Physiol. Plant. 2015, 37, 104. [Google Scholar] [CrossRef]
- Swarup, R.; Kargul, J.; Marchant, A.; Zadik, D.; Rahman, A.; Mills, R.; Yemm, A.; May, S.; Williams, L.; Millner, P.; et al. Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 2004, 16, 3069–3083. [Google Scholar] [CrossRef]
- Petrasek, J.; Mravec, J.; Bouchard, R.; Blakeslee, J.J.; Abas, M.; Seifertova, D.; Wisniewska, J.; Tadele, Z.; Kubes, M.; Covanova, M.; et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 2006, 312, 914–918. [Google Scholar] [CrossRef]
- Cho, M.; Lee, S.H.; Cho, H.T. P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells. Plant Cell 2007, 19, 3930–3943. [Google Scholar] [CrossRef]
- Li, H.L.; Zhang, H.; Yu, C. Possible roles of auxin and zeatin for initiating the dwarfing effect of M9 used as apple rootstock or interstock. Acta Physiol. Plant 2012, 34, 235–244. [Google Scholar] [CrossRef]
- Friml, J. Efux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 2003, 426, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Dhonukshe, P.; Brewer, P.B.; Friml, J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell. Mol. Life Sci. 2006, 63, 2738–2754. [Google Scholar] [CrossRef] [PubMed]
- Friml, J. Subcellular trafficking of PIN auxin efflux carriers in auxin transport. Eur. J. Cell Biol. 2010, 89, 231–235. [Google Scholar] [CrossRef]
- Viaene, T.; Delwiche, C.F.; Rensing, S.A.; Friml, J. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- Paponov, I.A.; Teale, W.D.; Trebar, M.; Blilou, I.; Palme, K. The PIN auxin efflux facilitators: Evolutionary and functional perspectives. Trends Plant Sci. 2005, 10, 170–177. [Google Scholar] [CrossRef]
- Ding, Z.; Wang, B.; Moreno, I.; Duplakova, N.; Simon, S.; Carraro, N.; Reemmer, J.; Pencik, A.; Chen, X.; Tejos, R.; et al. ER-localized auxin transporter PIN8 egulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 2012, 3, 941. [Google Scholar] [CrossRef]
- Mravec, J.; Skupa, P.; Bailly, A.; Hoyerova, K.; Krecek, P.; Bielach, A.; Petrasek, J.; Zhang, J.; Gaykova, V.; Stierhof, Y.D. Subcellular homeostasis of phytohormone auxin is mediated by the ER localized PIN5 transporter. Nature 2009, 459, 1136–1140. [Google Scholar] [CrossRef]
- Bennett, T.; Brockington, S.F.; Rothfels, C.; Graham, S.W.; Stevenson, D.; Kutchan, T.; Rolf, M.; Thomas, P.; Wong, G.K.; Leyser, O.; et al. Paralogous radiations of PIN proteins with multiple origins of noncanonical PIN structure. Mol. Biol. Evol. 2014, 31, 2042–2060. [Google Scholar] [CrossRef]
- Simon, S.; Skupa, P.; Viaene, T.; Zwiewka, M.; Tejos, R.; Klima, P.; Carna, M.; Rolcik, J.; De Rycke, R.; Moreno, I.; et al. PIN6 auxin transporter at endoplasmic reticulum and plasma membrane mediates auxin homeostasis and organogenesis in Arabidopsis. New Phytol. 2016, 211, 65–74. [Google Scholar] [CrossRef]
- Krecek, P.; Skupa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zazimalova, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.S.; Grones, P.; Stepanova, A.N.; Robles, L.M.; Lokerse, A.S.; Alonso, J.M.; Weijers, D.; Friml, J. Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr. Biol. 2013, 23, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Lee, S.H.; Cho, M.; Lee, O.R.; Yoo, H.; Cho, H.T. Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells. Plant Physiol. 2010, 153, 1046–1061. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Hu, H.; Wang, G.H.; Li, J.; Chen, J.Y.; Wu, P. Expression of PIN genes in rice (Oryza sativa L.): Tissue specificity and regulation by hormones. Mol. Plant. 2009, 2, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Yue, R.; Tie, S.; Sun, T.; Zhang, L.; Yang, Y.; Qi, J. Genome-wide identification and expression profiling analysis of ZmPIN, ZmPILS, ZmLAX and ZmABCB auxin transporter gene families in maize (Zea mays L.) under various abiotic stresses. PLoS ONE 2015, 10, e0118751. [Google Scholar] [CrossRef]
- Peng, H.; Peng, Z.; Wang, L.; Zhang, Y.; Wang, X.; Hui, X. The PIN gene family in cotton (gossypium hirsutum): Genome-wide identification and gene expression analyses during root development and abiotic stress responses. BMC Genom. 2017, 18, 507. [Google Scholar]
- Chen, Y.; Fan, X.; Song, W.; Zhang, Y.; Xu, G. Over-expression of OsPIN2 leads to increased tiller numbers, angle and shorter plant height through suppression of OsLAZY1. Plant Biotechnol. J. 2012, 10, 139–149. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Zhang, W.; Yan, S.; Wang, R.; Zhao, J.; Li, Y.; Qi, Z.; Sun, Z.; Zhu, Z. The putative auxin efflux carrier OsPIN3t is involved in the drought stress response and drought tolerance. Plant J. 2012, 72, 805–816. [Google Scholar] [CrossRef]
- Carraro, N.; Peer, W.A. Immunolocalization of PIN and ABCB transporters in plants. Methods Mol. Biol. 2016, 1398, 55–67. [Google Scholar]
- Forestan, C.; Meda, S.; Varotto, S. ZmPIN1-mediated auxin transport is related to cellular differentiation during maize embryogenesis and endosperm development. Plant Physiol. 2010, 152, 1373–1390. [Google Scholar] [CrossRef]
- Shen, C.; Bai, Y.; Wang, S.; Zhang, S.; Wu, Y.; Chen, M.; Jiang, D.; Qi, Y. Expression profile of PIN, AUX/LAX and PGP auxin transporter gene families in Sorghum bicolor under phytohormone and abiotic stress. FEBS J. 2010, 277, 2954–2969. [Google Scholar] [CrossRef] [PubMed]
- Pattison, R.J.; Catalá, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chai, C.; Valliyodan, B.; Maupin, C.; Annen, B.; Nguyen, H.T. Genome-wide analysis and expression profiling of the PIN auxin transporter gene family in soybean (glycine max). BMC Genom. 2015, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Qin, G.; Si, P.; Luo, Z.; Gao, J.; Chen, X. Analysis of Nicotiana tabacum PIN genes identifies NtPIN4 as a key regulator of axillary bud growth. Physiol. Plant. 2017, 160, 222–239. [Google Scholar] [CrossRef]
- Gao, L.W.; Lyu, S.W.; Tang, J.; Zhou, D.Y.; Bonnema, G.; Xiao, D.; Hou, X.L.; Zhang, C.W. Genome-wide analysis of auxin transport genes identifies the hormone responsive patterns associated with leafy head formation in Chinese cabbage. Sci. Rep. 2017, 7, 42229. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Wang, L. A survey of Populus PIN-FORMED family genes reveals their diversified expression patterns. J. Exp. Bot. 2014, 65, 2437–2448. [Google Scholar] [CrossRef]
- Fett-Neto, A.; Almeida, M.D.; Ruedell, C. Expression of auxin carrier genes during adventitious rooting in Eucalyptus globulus. BMC Proc. 2011, 5, 64. [Google Scholar] [CrossRef]
- Friml, J.; Wisniewska, J.; Benkova, E.; Mendgen, K.; Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 2002, 415, 806–809. [Google Scholar] [CrossRef]
- Cho, M.; Cho, H.T. The function of ABCB transporters in auxin transport. Plant Signal. Behav. 2013, 8, e22990. [Google Scholar] [CrossRef]
- Zhu, L.H. Adventitious shoot regeneration of two dwarfing pear rootstocks and the development of a transformation protocol. J. Hortic. Sci. Biotech. 2000, 75, 745–752. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Zhao, Y.; Li, Y.; Zhang, G.; Peng, Z.; Zhang, J. Enhancing auxin accumulation in maize root tips improves root growth and dwarfs plant height. Plant Biotechnol. J. 2018, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.B. The effect of intron location on intron-mediated enhancement of gene expression in Arabidopsis. Plant J. 2005, 40, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.B. Requirements for intron-mediated enhancement of gene expression in Arabidopsis. RNA 2002, 8, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhang, H.; Xiao, Y.; Wang, C.; Tian, Y. Deletion in the promoter of PcPIN-L affects the polar auxin transport in dwarf pear (Pyrus communis L.). Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Shi, Z.; Zhang, S.; Ming, R.; Zhu, S.; Khan, M.A.; Tao, S.; Korban, S.S.; Wang, H.; et al. The genome of the pear (Pyrus bretschneideri Rehd.). Genome Res. 2013, 23, 396–408. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Jeanmougin, F.; Thompson, J.D.; Gouy, M.; Higgins, D.G.; Gibson, T.J. Multiple sequence alignment with Clustal, X. Trends Biochem. Sci. 1998, 23, 403–405. [Google Scholar] [CrossRef]
- Schultz, J.; Milpetz, F.; Bork, P.; Ponting, C.P. Smart, a simple modular architecture research tool: Identification of signaling domains. PNAS 1998, 95, 5857–5864. [Google Scholar] [CrossRef]
- Bateman, A. The Pfam protein families database. Nucleic Acids Res. 2004, 32, 138–141. [Google Scholar] [CrossRef]
- Gasteiger, E.; Gattiker, A.; Hoogland, C.; Ivanyi, I.; Appel, R.D.; Bairoch, A. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003, 31, 3784–3788. [Google Scholar] [CrossRef]
- Guo, A.Y.; Zhu, Q.H.; Chen, X.; Luo, J.C. GSDS: A gene structure display server. Hereditas 2007, 29, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Kenichi, H.; Yoshihiro, U.; Masao, I.; Tomoko, K. Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar]


| Grafting Combinations | ‘Xueqing’/‘QN101’/‘Douli’ | ‘Xueqing’/‘OHF51’/‘Douli’ |
|---|---|---|
| Fruit weight (g) | 443.2 + 16.0 b | 537.0 ± 29.6 a |
| Total Soluble Solids (TSS) content | 13.73 ± 0.16 a | 13.56 ± 0.16 a |
| Firmness (Kg cm−2) | 6.53 ± 0.12 a | 6.42 ± 0.16 a |
| Tree height (m) | 2.93 ± 0.08 a | 2.62 ± 0.08 b |
| Scion diameter (mm) | 42.77 ± 2.62 a | 33.54 ± 0.98 b |
| Interstock diameter (mm) | 44.63 ± 2.12 a | 44.10 ± 1.41 a |
| Rootstock diameter (mm) | 50.78 ± 2.88 a | 43.46 ± 2.50 a |
| Diameter ratio of trunk amongScion/Interstock/Rootstock | 0.96/1/1.14 | 0.76/1/0.96 |
| Gene Name | Gene Locus ID | Genomic Position | CDS Length | Protein Length | Molecular Weight (Da) | Isoelectric Point (PI) | Instability Index | Aliphatic Index |
|---|---|---|---|---|---|---|---|---|
| PbPIN1-1 | LOC103946937 | 824,136..828,039 | 1863 | 620 | 67,663.97 | 9.11 | 34.52 | 88.55 |
| PbPIN1-2 | LOC103950573 | 343,331..347,039 | 1869 | 622 | 67,713.01 | 8.98 | 34.59 | 88.62 |
| PbPIN1-3 | LOC103933990 | 216,016..218,808 | 1854 | 617 | 66,514.59 | 8.89 | 37.38 | 94.05 |
| PbPIN1-4 | LOC103960490 | 472,382..474,775 | 1761 | 586 | 63,204.43 | 9.11 | 40.01 | 88.72 |
| PbPIN2-1 | LOC103941631 | 34496..38649 | 1944 | 647 | 69,852.83 | 9.32 | 42.75 | 89.68 |
| PbPIN2-2 | LOC103950477 | 728,493..732,110 | 1944 | 647 | 69,832.76 | 9.25 | 43.06 | 89.98 |
| PbPIN3-1 | LOC103947028 | 312,759..317,361 | 1980 | 659 | 71,419.31 | 8.47 | 36.62 | 90.17 |
| PbPIN3-2 | LOC103948593 | 3,901,582..3,905,923 | 1917 | 638 | 69,255.79 | 8.2 | 33.77 | 92.66 |
| PbPIN3-3 | LOC103948670 | 4,098,128..4,102,477 | 1917 | 638 | 69,255.79 | 8.2 | 33.77 | 92.66 |
| PbPIN4 | LOC103931858 | 51256..56492 | 1857 | 618 | 68,224.38 | 9.54 | 38.8 | 93.12 |
| PbPIN5-1 | LOC103930394 | 305,314..313,840 | 1080 | 359 | 39,296.44 | 7.65 | 35.98 | 116.71 |
| PbPIN5-2 | LOC103938552 | 149,838..151,159 | 1080 | 359 | 39,489.7 | 7.61 | 35.38 | 115.35 |
| PbPIN6 | LOC103951142 | 485,530..492,630 | 1662 | 553 | 60,371.48 | 8.93 | 39.93 | 98.43 |
| PbPIN8 | LOC103934837 | 52,931..55,068 | 1074 | 357 | 38,888.24 | 9.29 | 30.69 | 120.25 |
| Cis-Elements | Number of Gene | Functions of the Cis-Elements | Cis-Elements Types |
|---|---|---|---|
| AE-box | 8 | part of a module for light response | Light responsive |
| Box 4 | 12 | part of a conserved DNA module involved in light responsiveness | Light responsive |
| G-Box | 11 | cis-acting regulatory element involved in light responsiveness | Light responsive |
| G-box | 10 | cis-acting regulatory element involved in light responsiveness | Light responsive |
| GA-motif | 7 | part of a light responsive element | Light responsive |
| GT1-motif | 11 | light responsive element | Light responsive |
| TCT-motif | 8 | part of a light responsive element | Light responsive |
| ATCT-motif | 9 | part of a conserved DNA module involved in light responsiveness | Light responsive |
| GAG-motif | 13 | part of a light responsive element | Light responsive |
| Sp1 | 11 | light responsive element | Light responsive |
| Box I | 12 | light responsive element | Light responsive |
| CATT-motif | 8 | part of a light responsive element | Light responsive |
| I-box | 7 | part of a light responsive element | Light responsive |
| AAGAA-motif | 11 | Light responsive | |
| ABRE | 7 | cis-acting element involved in the abscisic acid responsiveness | Hormone responsive |
| CGTCA-motif | 11 | cis-acting regulatory element involved in the MeJA-responsiveness | Hormone responsive |
| TGACG-motif | 11 | cis-acting regulatory element involved in the MeJA-responsiveness | Hormone responsive |
| TCA-element | 11 | cis-acting element involved in salicylic acid responsiveness | Hormone responsive |
| GARE-motif | 7 | gibberellin-responsive element | Hormone responsive |
| P-box | 7 | gibberellin-responsive element | Hormone responsive |
| ERE | 7 | ethylene-responsive element | Hormone responsive |
| HSE | 11 | cis-acting element involved in heat stress responsiveness | Stress responsive |
| MBS | 9 | MYB binding site involved in drought-inducibility | Stress responsive |
| TC-rich repeats | 14 | cis-acting element involved in defense and stress responsiveness | Stress responsive |
| ARE | 8 | cis-acting regulatory element essential for the anaerobic induction | Stress responsive |
| Box-W1 | 8 | fungal elicitor responsive element | Stress responsive |
| W box | 8 | wounding and pathogen response | Stress responsive |
| circadian | 13 | cis-acting regulatory element involved in circadian control | development related elements |
| Skn-1_motif | 13 | cis-acting regulatory element required for endosperm expression | development related elements |
| O2-site | 7 | cis-acting regulatory element involved in zein metabolism regulation | development related elements |
| CAAT-box | 14 | common cis-acting element in promoter and enhancer regions | promoter related elements |
| TATA-box | 14 | core promoter element around −30 of transcription start | promoter related elements |
| Unnamed__1 | 8 | 60K protein binding site | site-binding related elements |
| Name | Sequence | Name | Sequence |
|---|---|---|---|
| Actin | U: CCCAGAAGTGCTCTTCCAAC | PbPIN3-2 | U: ATTTTACCCCTCCCCTCTTCT |
| D: TTGATCTTCATGCTGCTTGG | D: AATATCATCGCCACGTACAGC | ||
| PbPIN1-1 | U: ATTGTTCCCTTTGTCTTTGC | PbPIN3-3 | U: GCCATTCTTAGCACTGCGGTTAT |
| D: TTCTCATCATGTTTGCGTTT | D: GGCGGATTCTTCGTTGATCTTCAT | ||
| PbPIN1-2 | U: CCCAGAAACAGAGCAATAAGA | PbPIN4 | U: CATTCTTAGCACTGCGGTTATT |
| D: TGGAGATGAAGTGGAAGGAGA | D: TCGTTCTCCATCGTACTTAATCTA | ||
| PbPIN1-3 | U: GATTGTTCCGTTTGTCTT | PbPIN5-1 | U: TCTGGGTAGTCCTCAAGTCC |
| D: ATATTTCCTCCACGTCTC | D: GTCAGCCGCTATGAACCT | ||
| PbPIN1-4 | U: TTGATTTTCCTTCTGATTTCGG | PbPIN5-2 | U: ATTAATTACCAGTTGTGCTCCAA |
| D: GAAGATTTTCCACCATTTCACG | D: ATCAACATGTGCAGTGAATTCGA | ||
| PbPIN2-1 | U: GCCTTGCCTGTAACAATACT | PbPIN6 | U: GTTTCCTTACCAGTGACGCTCCT |
| D: ATCAAGGTACTCAGTTGTCACAT | D: CATGTACTAATATGACACCAGACCC | ||
| PbPIN2-2 | U: ACGCGGACATACTTAGCACTG | PbPIN8 | U: CATCCAGACATTTTGAGCAC |
| D: TGAACACTGAATCCAATGGCA | D: GTAAACATATCAGCCATATTACA | ||
| PbPIN3-1 | U: CTATTGTCCAGGCTGCTCTTC | ||
| D: TTCTTCGTTTTCCTAGCTTTGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, L.; Chen, L.; Wang, C.; Zhang, S.; Yang, Y.; Liu, J.; Li, D.; Song, J.; Wang, R. Characterization of the Auxin Efflux Transporter PIN Proteins in Pear. Plants 2020, 9, 349. https://doi.org/10.3390/plants9030349
Qi L, Chen L, Wang C, Zhang S, Yang Y, Liu J, Li D, Song J, Wang R. Characterization of the Auxin Efflux Transporter PIN Proteins in Pear. Plants. 2020; 9(3):349. https://doi.org/10.3390/plants9030349
Chicago/Turabian StyleQi, Liying, Ling Chen, Chuansen Wang, Shaoling Zhang, Yingjie Yang, Jianlong Liu, Dingli Li, Jiankun Song, and Ran Wang. 2020. "Characterization of the Auxin Efflux Transporter PIN Proteins in Pear" Plants 9, no. 3: 349. https://doi.org/10.3390/plants9030349
APA StyleQi, L., Chen, L., Wang, C., Zhang, S., Yang, Y., Liu, J., Li, D., Song, J., & Wang, R. (2020). Characterization of the Auxin Efflux Transporter PIN Proteins in Pear. Plants, 9(3), 349. https://doi.org/10.3390/plants9030349





