An Efficient Method for In Vitro Shoot-Tip Culture and Sporophyte Production Using Selaginella martensii Spring Sporophyte
Abstract
:1. Introduction
2. Results
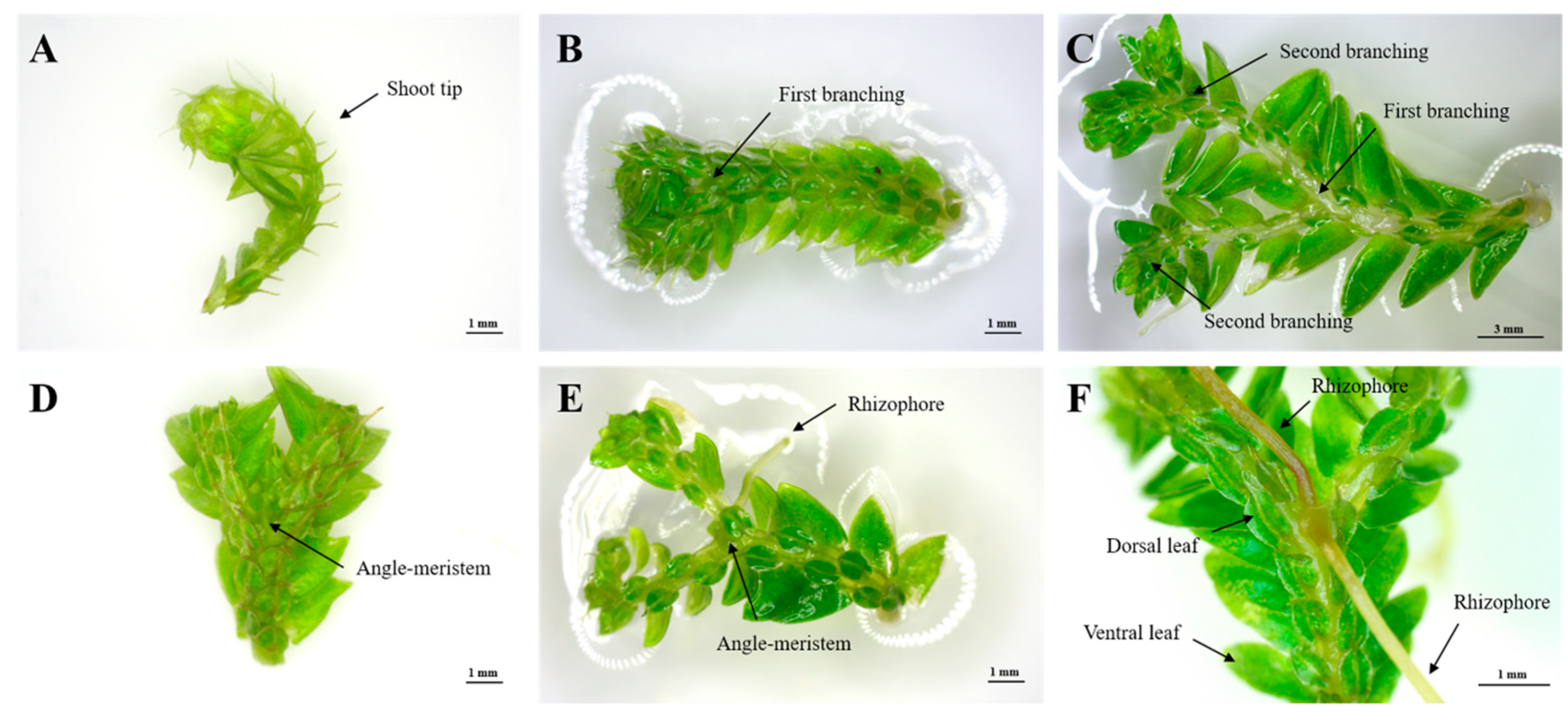
2.1. Selection of Explant Type
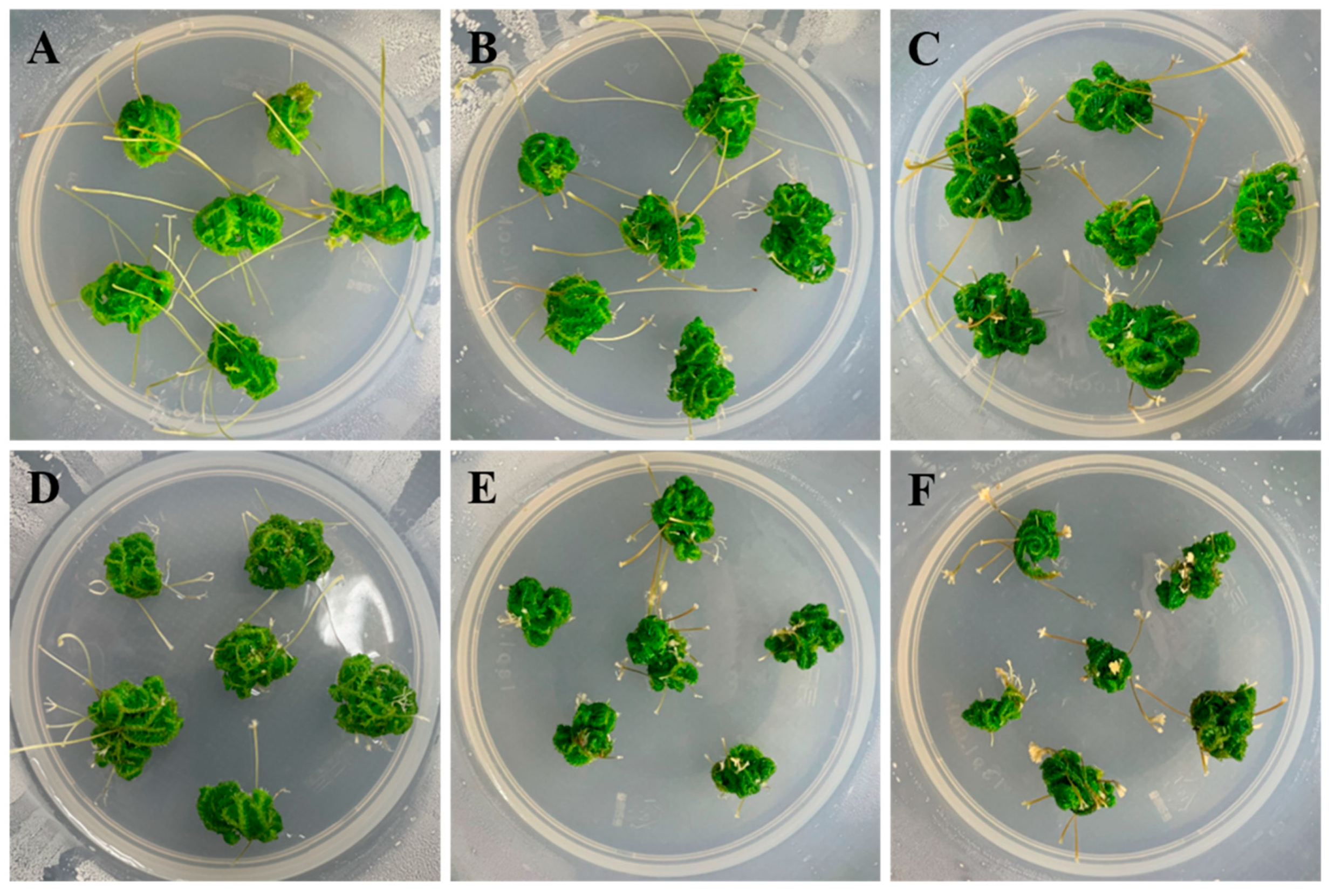
2.2. Effect of Medium Components on Shoot-Tip Proliferation
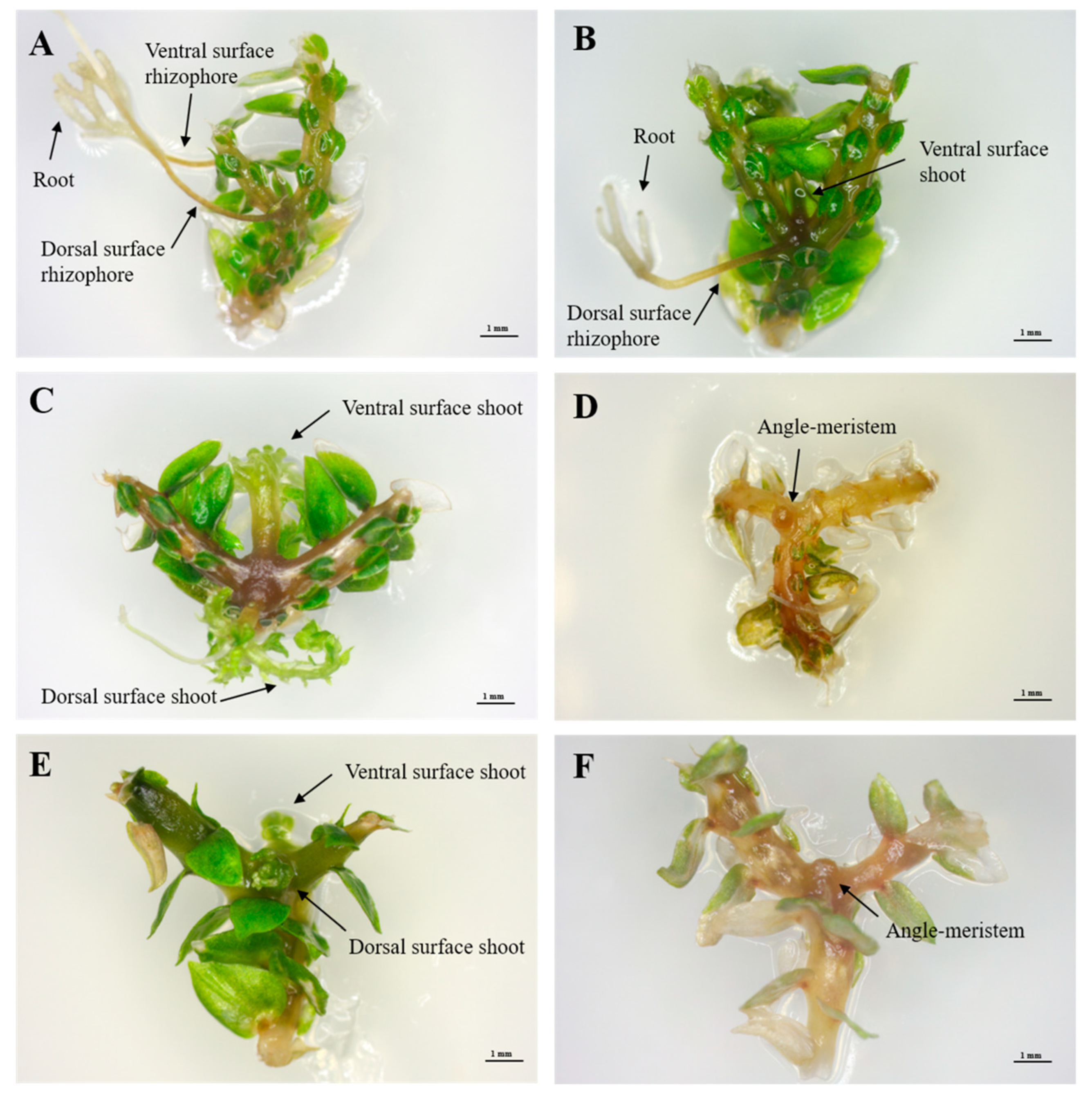
2.3. Effects of Plant-Growth Regulators (PGRs) on the Induction of Branching Segments
2.4. Ex Vitro Production of Sporophyte Seedlings
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Medium Optimization for Shoot-Tip Proliferation
4.3. Effects of PGRs on the Induction of Branching Segments
4.4. Evaluation of Sporophyte Seedling Growth in ex Vitro Conditions
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Webster, T.R.; Steeves, T.A. Developmental morphology of the root of Selaginella martensii Spring. Can. J. Bot. 1967, 45, 395–404. [Google Scholar] [CrossRef]
- Williams, S. An analysis of the vegetative organs of Selaginella grandis Moore, together with some observations on abnormalities and experimental results. Trans. Roy. Soc. Edinb. 1931, 57, 1–24. [Google Scholar] [CrossRef]
- Jernstedt, J.A.; Cutter, E.G.; Gifford, E.M.; Lu, P. Angle meristem origin and development in Selaginella martensii. Ann. Bot. 1992, 69, 351–363. [Google Scholar] [CrossRef]
- Banks, J.A. Selaginella and 400 million years of separation. Annu. Rev. Plant Biol. 2009, 60, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Baniaga, A.E.; Jorgensen, S.A.; Barker, M.S. A successful in vitro propagation technique for resurrection plants of the Selaginellaceae. Am. Fern J. 2017, 107, 96–104. [Google Scholar] [CrossRef]
- Fernández, H.; Bertrand, A.M.; Sánchez-Tamés, R. In vitro regeneration of Asplenium nidus L. from gametophytic and sporophytic tissue. Sci. Hort. 1993, 56, 71–77. [Google Scholar] [CrossRef]
- Barnicoat, H.; Cripps, R.; Kendon, J.; Sarasan, V. Conservation in vitro of rare and threatened ferns—case studies of biodiversity hotspot and island species. In Vitro Cell. Dev. Biol. Plant. 2011, 47, 37–45. [Google Scholar] [CrossRef]
- Kromer, K.; Raj, A.; Ż.ołnierz, L.; Poturała, D. Propagation in Vitro and Ex Situ Cultivation of Woodsia Alpina (Bolton) S.F. Gray. Available online: https://docplayer.pl/115577272-Propagation-in-vitro-and-ex-situ-cultivation-of-woodsia-alpina-bolton-s-f-gray.html (accessed on 11 February 2020).
- Jang, B.K.; Cho, J.S.; Park, K.T.; Lee, C.H. A methodology for large-scale Athyrium sheareri gametophyte proliferation and sporophyte production using tissue culture. In Vitro Cell. Dev. Biol. Plant. 2019, 55, 519–526. [Google Scholar] [CrossRef]
- Jang, B.K.; Cho, JS.; Park, K.T.; Lee, C.H. Practical methodology for gametophyte proliferation and sporophyte production of green penny fern (Lemmaphyllum microphyllum C. Presl) using mechanical fragmentation. In Vitro Cell. Dev. Biol. Plant. 2020. [Google Scholar] [CrossRef]
- Amaki, W.; Higuch, H. A possible propagation system of Nephrolepis, Asplenium, Pteris, Adiantum and Rhumora through tissue culture. Acta Hortic. 1991, 300, 237–243. [Google Scholar]
- Bristow, J.M. The controlled in vitro differentiation of callus derived from a fern, Pteris cretica L., into gametophytic or sporophytic tissues. Dev. Biol. 1962, 4, 361–375. [Google Scholar] [CrossRef]
- Fernández, H.; Revilla, M.A. In vitro culture of ornamental ferns. Plant Cell Tissue Organ Cult. 2003, 73, 1–13. [Google Scholar] [CrossRef]
- Hegde, S.; Kumar Menon, V.; Noronha, R.; D’Souza, L. Callus culture and an unconventional pattern of sporophyte regeneration in Drynaria quercifolia a medicinal fern. In Vitro Cell. Dev. Biol. Plant. 2006, 42, 508–513. [Google Scholar] [CrossRef]
- Menéndez, V.; Revilla, M.A.; Bernard, P.; Gotor, V.; Fernández, H. Gibberellins and antheridiogen on sex in Blechnum spican L. Plant Cell Rep. 2006, 25, 1104–1110. [Google Scholar] [CrossRef] [PubMed]
- Avila-Pérez, M.C.R.; White-Olascoaga, L.; Arzate-Fernández, A.M. In vitro regeneration of leatherleaf fern [Rumohra adiantiformis (G. Forst.) Ching]. Am. Fern J. 2011, 101, 25–35. [Google Scholar] [CrossRef]
- Mikuła, A.; Pożoga, M.; Tomiczak, K.; Rybczyński, J.J. Somatic embryogenesis in ferns: A new experimental system. Plant Cell Rep. 2015, 34, 783–794. [Google Scholar] [CrossRef] [Green Version]
- Thakur, R.C.; Hosoi, Y.; Ishii, K. Rapid in vitro propagation of Matteuccia struthiopteris (L.) Todaro – an edible fern. Plant Cell Rep. 1998, 18, 203–208. [Google Scholar] [CrossRef]
- Jung, J.A.; Lee, C.H. Mass propagation of Dryopteris varia (L.) O. Kuntze by sporophyte culture. Korean Soc. Floricult. Sci. 2006, 14, 25–34. [Google Scholar]
- Somer, A.; Arbesú, R.; Menéndez, V.; Revilla, M.A.; Fernández, H. Sporophyte induction studies in ferns in vitro. Euphytica 2010, 171, 203–210. [Google Scholar] [CrossRef]
- Park, K.T.; Jang, B.K.; Lee, C.H. Culture condition for gametophyte and sporophyte mass propagation of bamboo fern (Coniogramme japonica) using tissue culture. J. Plant Biotechnol. 2019, 46, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Webster, T.R. An investigation of angle-meristem development in excised stem segments of Selaginella martensii. Can. J. Bot. 1969, 47, 717–722. [Google Scholar] [CrossRef]
- Hegde, S.; D’Souza, L. Recent advances in biotechnology of ferns. In Plant Biotechnology Recent Advances; Trivedi, P.C., Ed.; Panima Publishing corporation: Bangalore, India, 2000. [Google Scholar]
- Shin, S.L.; Lee, C.H. In vitro medium composition and culture method affecting mass propagation of Osmunda japonica Thunb. prothalli. Korean J. Hortic. Sci. 2009, 27, 299–304. [Google Scholar]
- Cho, J.S.; Han, J.H.; Lee, C.H. Effects of medium components and composition on mass propagation of Arachniodes aristata (G. Forst.) Tindale. Hortic. Sci. Technol. 2017, 35, 131–141. [Google Scholar]
- Cho, J.S.; Lee, C.H. Several factors affecting mass production of Microlepia strigosa (Thunb.) C. Presl sporophytes. Korean J. Hortic. Sci. 2017, 35, 46–58. [Google Scholar]
- Fernández, H.; Bertrand, A.M.; Feito, I.; Sánchez-Tamés, R. Gametophyte culture in vitro and antheridiogen activity in Blechnum spicant. Plant. Cell Tissue Org. Cult. 1997, 50, 71–74. [Google Scholar] [CrossRef]
- Hirsch, A.M. The effect of sucrose on the differentiation of excised fern leaf tissue into either gametophytes or sporophytes. Plant. Physiol. 1975, 56, 390–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittier, D.P. Effects of nitrogen source on spore germination and gametophyte growth in Psilotum. Bot. Gaz. 1990, 151, 50–53. [Google Scholar] [CrossRef]
- Melan, M.A.; Whittier, D.P. Effects of inorganic nitrogen sources on spore germination and gametophyte growth in Botrychium dissectum. Plant Cell Environ. 1990, 13, 477–482. [Google Scholar] [CrossRef]
- Kuriyama, A.; Kobayashi, T.; Hayashi, S.; Maeda, M. Medium composition for the production of sporophytes of the fern Adiantum capillus-veneris. J. Jpn. Soc. Hortic. Sci. 2004, 73, 580–582. [Google Scholar] [CrossRef]
- Jang, B.K.; Cho, J.S.; Lee, K.C.; Lee, C.H. Culture conditions affecting spore germination, prothallus propagation and sporophyte formation of Dryopteris nipponensis Koidz. Hortic Sci Technol 2017, 35, 480–489. [Google Scholar]
- Haynes, R.; Goh, K.M. Ammonium and nitrate nutrition of plants. Biol. Rev. 1978, 53, 465–510. [Google Scholar] [CrossRef]
- Menéndez, V.; Arbesú, R.; Somer, M.; Revilla, A.; Fernández, H. From spore to sporophyte: How to proceed in vitro. In Working with Ferns: Issues and Applications, 1st ed.; Fernández, H., Kumar, A., Revilla, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Menéndez, V.; Abul, Y.; Bohanec, B.; Lafont, F.; Fernández, H. The effect of exogenous and endogenous phytohormones on the in vitro development of gametophyte and sporophyte in Asplenium nidus L. Acta Physiol. Plant. 2011, 33, 2493–2500. [Google Scholar] [CrossRef]
- Marszał-Jagacka, J.; Kromer, K. In vitro propagation of rare and endangered serpentine. In Working with Ferns: Issues and Applications, 1st ed.; Fernández, H., Kumar, A., Revilla, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Hazarika, B.N. Acclimatization of tissue-cultured plants. Curr. Sci. 2003, 85, 1704–1712. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]



| Medium Strength | Fresh Weight/Explant (g) | Maximum No. of Nodes/Explants | Total No. of Branches/Explant | No. of Rhizophores/Explant |
|---|---|---|---|---|
| 1/4 MS | 0.22 ± 0.02 c | 5.63 ± 0.08 b | 31.92 ± 1.70 c | 29.08 ± 1.06 b |
| 1/2 MS | 0.40 ± 0.02 b | 5.92 ± 0.21 b | 43.29 ± 5.26 b | 32.63 ± 2.70 b |
| 1 MS | 0.68 ± 0.06 a | 6.77 ± 0.35 a | 56.93 ± 3.00 a | 52.49 ± 4.77 a |
| 2 MS | 0.15 ± 0.04 c | 2.78 ± 0.22 c | 7.22 ± 1.13 d | 2.22 ± 0.84 c |
| Sucrose (%) | Fresh Weight/Explant (g) | Maximum No. of Nodes/Explant | Total No. of Branches/Explant | No. of Rhizophores/Explants |
|---|---|---|---|---|
| 0 | 0.21 ± 0.01 c | 4.96 ± 0.14 b | 13.79 ± 0.92 c | 11.17 ± 0.91 c |
| 1 | 0.35 ± 0.06 b | 5.31 ± 0.32 b | 26.24 ± 3.39 b | 22.60 ± 1.91 ab |
| 2 | 0.54 ± 0.05 a | 6.24 ± 0.39 a | 43.01 ± 7.17 a | 28.60 ± 4.01 a |
| 3 | 0.36 ± 0.05 b | 5.52 ± 0.29 ab | 31.29 ± 3.23 b | 22.03 ± 1.67 ab |
| 4 | 0.29 ± 0.01 bc | 5.15 ± 0.15 b | 24.13 ± 1.57 bc | 20.02 ± 1.45 b |
| 5 | 0.27 ± 0.01 bc | 5.14 ± 0.21 b | 24.16 ± 1.69 bc | 21.12 ± 1.21 b |
| Total Nitrogen Conc. (mM) | Fresh Weight/Explant (g) | Maximum No. of Nodes/Explant | Total no. of Branches/Explant | No. of Rhizophores/Explants |
|---|---|---|---|---|
| 30 | 0.04 ± 0.01 b z | 1.72 ± 0.24 b | 1.83 ± 0.29 b | 2.00 ± 0.17 b |
| 60 | 0.17 ± 0.05 a | 5.00 ± 0.20 a | 20.63 ± 0.98 a | 16.13 ± 1.09 a |
| 120 | 0.01 ± 0.00 c | 0.00 c | 0.00 c | 0.00 c |
| NH4+:NO3– | Fresh Weight/Explant (g) | Maximum No. of Nodes/Explant | Total No. of Branches/Explant | No. of Rhizophores/Explants |
|---|---|---|---|---|
| 3:0 | 0.01 ± 0.00 c | 0.00 c | 0.00 c | 0.00 d |
| 2:1 | 0.33 ± 0.02 a | 5.39 ± 0.37 a | 21.99 ± 1.65 b | 6.96 ± 0.70 c |
| 1:1 | 0.36 ± 0.02 a | 5.85 ± 0.32 a | 28.81 ± 1.10 a | 13.95 ± 2.85 b |
| 1:2 | 0.37 ± 0.04 a | 5.51 ± 0.34 a | 31.56 ± 3.67 a | 22.21 ± 3.28 a |
| 0:3 | 0.14 ± 0.05 b | 2.58 ± 0.25 b | 5.25 ± 0.08 c | 5.57 ± 0.23 cd |
| PGRs | Concentration (μM) | Survival Rate (%) | Organs Formed in Angle Meristem (%) | ||
|---|---|---|---|---|---|
| S/S | S/R | R/R | |||
| 0.00 | 100.0 | 0.0 | 0.0 | 100.0 | |
| IAA | 2.85 | 91.7 | 72.7 | 18.2 | 9.1 |
| 5.71 | 100.0 | 0.0 | 16.7 | 83.3 | |
| 11.42 | 100.0 | 8.3 | 8.3 | 83.3 | |
| IBA | 2.46 | 91.7 | 18.2 | 54.5 | 27.3 |
| 4.92 | 100.0 | 16.7 | 8.3 | 75.0 | |
| 9.84 | 100.0 | 0.0 | 0.0 | 100.0 | |
| Kinetin | 2.32 | 100.0 | 100.0 | 0.0 | 0.0 |
| 4.65 | 100.0 | 100.0 | 0.0 | 0.0 | |
| 9.30 | 0.0 | 0.0 | 0.0 | 0.0 | |
| BA | 2.22 | 100.0 | 91.7 | 8.3 | 0.0 |
| 4.44 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 8.88 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Substrate | Length (cm) | Plant Height (cm) | Root Length (cm) | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|---|---|
| Hs1 | 1 | 5.50 ± 0.38 a–d | 2.78 ± 0.22 c–e | 0.28 ± 0.10 d | 0.03 ± 0.01 ef |
| 2 | 5.82 ± 0.38 a-c | 4.30 ± 0.32 ab | 0.40 ± 0.06 d | 0.06 ± 0.01 dc | |
| 4 | 6.14 ± 0.56 a | 4.54 ± 0.29 a | 1.21 ± 0.18 ab | 0.13 ± 0.02 ab | |
| Hs2-D1 | 1 | 4.54 ± 0.26 de | 3.64 ± 0.25 bc | 0.29 ± 0.06 d | 0.04 ± 0.01 d-f |
| 2 | 4.80 ± 0.31 c-e | 3.50 ± 0.33 bc | 0.31 ± 0.04 d | 0.04 ± 0.01 d-f | |
| 4 | 5.30 ± 0.31 a-d | 4.74 ± 0.50 a | 0.97 ± 0.13 b | 0.12 ± 0.01 b | |
| Hs3-D1 | 1 | 5.66 ± 0.45 a-d | 3.60 ± 0.22 bc | 0.19 ± 0.06 d | 0.05 ± 0.01 de |
| 2 | 4.94 ± 0.18 b-e | 4.32 ± 0.18 ab | 0.26 ± 0.03 d | 0.04 ± 0.00 d-f | |
| 4 | 6.00 ± 0.37 ab | 4.40 ± 0.34 ab | 1.19 ± 0.08 ab | 0.16 ± 0.00 a | |
| Hs2-Pr1 | 1 | 5.20 ± 0.19 a-d | 2.14 ± 0.19 e | 0.21 ± 0.02 d | 0.02 ± 0.00 ef |
| 2 | 4.54 ± 0.24 de | 2.50 ± 0.09 de | 0.23 ± 0.04 d | 0.03 ± 0.00 ef | |
| 4 | 4.96 ± 0.27 b-e | 3.10 ± 0.28 cd | 0.68 ± 0.07 c | 0.08 ± 0.01 c | |
| Hs3-Pr1 | 1 | 5.08 ± 0.29 a-e | 2.12 ± 0.26 e | 0.18 ± 0.05 d | 0.02 ± 0.00 f |
| 2 | 4.06 ± 0.36 e | 2.32 ± 0.36 de | 0.25 ± 0.05 d | 0.03 ± 0.01 ef | |
| 4 | 5.52 ± 0.42 a-e | 3.58 ± 0.07 bc | 1.35 ± 0.10 a | 0.13 ± 0.01 b | |
| Significance | |||||
| A (substrate) | ** | *** | ** | *** | |
| B (length) | ** | *** | *** | *** | |
| A × B | NS | NS | * | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.; Jang, B.K.; Lee, H.M.; Cho, J.S.; Lee, C.H. An Efficient Method for In Vitro Shoot-Tip Culture and Sporophyte Production Using Selaginella martensii Spring Sporophyte. Plants 2020, 9, 235. https://doi.org/10.3390/plants9020235
Park K, Jang BK, Lee HM, Cho JS, Lee CH. An Efficient Method for In Vitro Shoot-Tip Culture and Sporophyte Production Using Selaginella martensii Spring Sporophyte. Plants. 2020; 9(2):235. https://doi.org/10.3390/plants9020235
Chicago/Turabian StylePark, Kyungtae, Bo Kook Jang, Ha Min Lee, Ju Sung Cho, and Cheol Hee Lee. 2020. "An Efficient Method for In Vitro Shoot-Tip Culture and Sporophyte Production Using Selaginella martensii Spring Sporophyte" Plants 9, no. 2: 235. https://doi.org/10.3390/plants9020235
APA StylePark, K., Jang, B. K., Lee, H. M., Cho, J. S., & Lee, C. H. (2020). An Efficient Method for In Vitro Shoot-Tip Culture and Sporophyte Production Using Selaginella martensii Spring Sporophyte. Plants, 9(2), 235. https://doi.org/10.3390/plants9020235





