Vertical Strata and Stem Carbon Dioxide Efflux in Cycas Trees
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Cycad Stems
3.2. Stem Respiration Literature Bias
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, J.; He, Y.; Aubrey, D.P.; Zhuang, Q.; Teskey, O. Global patterns and predictors of stem CO2 efflux in forest ecosystems. Glob. Chang. Biol. 2016, 22, 1433–1444. [Google Scholar] [CrossRef] [PubMed]
- Vargas, R.; Barba, J. Greenhouse gas fluxes from tree stems. Trends Plant Sci. 2019, 24, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Bloemen, J.; McGuire, M.A.; Aubrey, D.P.; Teskey, R.O.; Steppe, K. Transport of root-respired CO2 via the transpiration stream affects aboveground carbon assimilation and CO2 efflux in trees. New Phytol. 2013, 197, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Kunert, N. A case study on the vertical and diurnal variation of stem CO2 effluxes in an Amazonian forest tree. Trees 2018, 32, 913–917. [Google Scholar] [CrossRef]
- Tarvainen, L.; Wallin, G.; Lim, H.; Linder, S.; Oren, R.; Ottosson Löfvenius, M.; Räntfors, M.; Tor-ngern, P.; Marshall, J. Photosynthetic refixation varies along the stem and reduces CO2 efflux in mature boreal Pinus sylvestris trees. Tree Physiol. 2018, 38, 558–569. [Google Scholar] [CrossRef]
- Martin, T.A.; Teskey, R.O.; Dougherty, P.M. Movement of respiratory CO2 in stems of loblolly pine (Pinus taeda L.) seedlings. Tree Physiol. 1994, 14, 481–495. [Google Scholar] [CrossRef]
- Bowman, W.P.; Barbour, M.M.; Turnbull, M.H.; Tissue, D.T.; Whitehead, D.; Griffin, K.L. Sap flow rates and sapwood density are critical factors in within- and between-tree variation in CO2 efflux from stems of mature Dacrydium cupressinum trees. New Phytol. 2005, 167, 815–828. [Google Scholar] [CrossRef]
- McGuire, M.A.; Cerasoli, S.; Teskey, R.O. CO2 fluxes and respiration of branch segments of sycamore (Platanus occidentalis L.) examined at different sap velocities, branch diameters, and temperatures. J. Exp. Bot. 2007, 58, 2159–2168. [Google Scholar] [CrossRef]
- Saveyn, A.; Steppe, K.; Lemeur, R. Daytime depression in tree stem CO2 efflux rates: Is it caused by low stem turgor pressure? Ann. Bot. 2007, 99, 477–485. [Google Scholar] [CrossRef]
- Hilman, B.; Muhr, J.; Trumbore, S.E.; Kunert, N.; Carbone, M.S.; Yuval, P.; Wright, S.J.; Moreno, G.; Pérez-Priego, O.; Migliavacca, M.; et al. Comparison of CO2 and O2 fluxes demonstrate retention of respired CO2 in tree stems from a range of tree species. Biogeosciences 2019, 16, 177–191. [Google Scholar] [CrossRef]
- Sprugel, D.G. Components of woody-tissue respiration in young Abies amabilis (Dougl.) Forbes trees. Trees 1990, 4, 88–98. [Google Scholar] [CrossRef]
- Damesin, C.; Ceschia, E.; Le Goff, N.; Ottorini, J.-M.; Dufrêne, E. Stem and branch respiration of beech: From tree measurements to estimations at the stand level. New Phytol. 2002, 153, 159–172. [Google Scholar] [CrossRef]
- Ryan, M.G.; Hubbard, R.M.; Pongratic, S.; Raison, R.J.; McMurtrie, R.E. Foliage, fine-root, woody-tissue and stand respiration in Pinus radiata in relation to nitrogen status. Tree Physiol. 1996, 16, 333–343. [Google Scholar] [CrossRef]
- Cavaleri, M.A.; Oberbauer, S.F.; Ryan, M.G. Wood CO2 efflux in a primary tropical rain forest. Glob. Chang. Biol. 2006, 12, 2442–2458. [Google Scholar] [CrossRef]
- Katayama, A.; Kume, T.; Komatsu, H.; Ohashi, M.; Matsumoto, K.; Ichihashi, R.; Kumagai, T.O.; Otsuki, K. Vertical variations in wood CO2 efflux for live emergent trees in a Bornean tropical rainforest. Tree Physiol. 2014, 34, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, L.; Räntfors, M.; Wallin, G. Vertical gradients and seasonal variation in stem CO2 efflux within a Norway spruce stand. Tree Physiol. 2014, 34, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E. Stem CO2 efflux of Cycas micronesica is reduced by chronic non-native insect herbivory. Plant Signal. Behav. 2020, 15, e1716160. [Google Scholar] [CrossRef]
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithaca, NY, USA, 1997. [Google Scholar]
- Niklas, K.J.; Cobb, E.D.; Marler, T. A comparison between the record height-to-stem diameter allometries of pachycaulis and leptocaulis species. Ann. Bot. 2006, 97, 79–83. [Google Scholar] [CrossRef]
- Fosberg, A.S. Structure and growth of the shoot apex of Cycas revoluta. Am. J. Bot. 1939, 26, 372–385. [Google Scholar]
- Fosberg, A.S. Further studies on zonal structure and growth of the shoot apex of Cycas revoluta Thunb. Am. J. Bot. 1940, 27, 487–500. [Google Scholar]
- Stevenson, D.W. Radial growth in the Cycadales. Am. J. Bot. 1980, 67, 465–475. [Google Scholar] [CrossRef]
- Fisher, J.B.; Lindström, A.; Marler, T. Tissue responses and solution movement after stem wounding in six Cycas species. HortScience 2009, 44, 848–851. [Google Scholar] [CrossRef]
- Terrazas, T. Origin and activity of successive cambia in Cycas (Cycadales). Am. J. Bot. 1991, 78, 1335–1344. [Google Scholar] [CrossRef]
- Marler, T.E.; Lindström, A.; Fisher, J.B. Stem tissue dimensions correlate with ease of horticultural management for six Cycas species. HortScience 2010, 45, 1293–1296. [Google Scholar] [CrossRef]
- Steppe, K.; Saveyn, A.; McGuire, M.A.; Lemeur, R.; Teskey, R.O. Resistance to radial CO2 diffusion contributes to between-tree variation in CO2 efflux of Populus deltoides stems. Funct. Plant Biol. 2007, 34, 785–792. [Google Scholar] [CrossRef]
- Ryan, M.G.; Cavaleri, M.A.; Almeida, A.C.; Penchel, R.; Senock, R.S.; Stape, J.L. Wood CO2 efflux and foliar respiration for Eucalyptus in Hawaii and Brazil. Tree Physiol. 2009, 29, 1213–1222. [Google Scholar] [CrossRef]
- Langdon, L.M. Stem anatomy of Dioon spinulosum. Bot. Gaz. 1920, 70, 110–120. [Google Scholar] [CrossRef]
- Chamberlain, C.J. Gymnosperms: Structure and Evolution; University Chicago Press: Chicago, IL, USA, 1935. [Google Scholar]
- Salomón, R.L.; Steppe, K.; Crous, K.Y.; Noh, N.J.; Ellsworth, D.S. Elevated CO2 does not affect stem CO2 efflux nor stem respiration in a dry Eucalyptus woodland, but it shifts the vertical gradient in xylem [CO2]. Plant Cell Environ. 2019, 42, 2151–2164. [Google Scholar]
- Marler, T.E.; Krishnapillai, M.V. Cycas micronesica trees alter local soil traits. Forests 2018, 9, 565. [Google Scholar] [CrossRef]
- Marler, T.E.; Krishnapillai, M.V. Does plant size influence leaf elements in an arborescent cycad? Biology 2018, 7, 51. [Google Scholar] [CrossRef]
- Marler, T.E.; Krishnapillai, M.V. Distribution of elements along the rachis of Cycas micronesica leaves: A cautionary note for sampling design. Horticulturae 2019, 5, 33. [Google Scholar] [CrossRef]
- Marler, T.E.; Krishnapillai, M.V. Incident light and leaf age influence leaflet element concentrations of Cycas micronesica trees. Horticulturae 2019, 5, 58. [Google Scholar] [CrossRef]
- Marler, T.E.; Ferreras, U.F. Disruption of leaf nutrient remobilization in coastal Cycas trees by tropical cyclone damage. J. Geogr. Nat. Disasters 2015, 5, 2. [Google Scholar] [CrossRef]
- Marler, T.E.; Ferreras, U.F. Current status, threats, and conservation needs of the endemic Cycas wadei Merrill. J. Biodivers Endanger. Species 2017, 5, 193. [Google Scholar] [CrossRef]
- Xu, M.; DeBiase, T.A.; Qi, Y. A simple technique to measure stem respiration using a horizontally oriented soil chamber. Can. J. For. Res. 2000, 30, 1555–1560. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, G.; Zhu, W.; Zhao, J.; Wang, X.; Wang, Y.; Jia, M. Stem CO2 efflux of Abies fabri in subalpine forests in the Gongga Mountains, Eastern Tibetan Plateau. J. Plant Ecol. 2017, 10, 1001–1011. [Google Scholar]
- Ávila-Lovera, E.; Tezara, W. Water-use efficiency is higher in green stems than in leaves of tropical tree species. Trees 2018, 32, 1547–1558. [Google Scholar] [CrossRef]

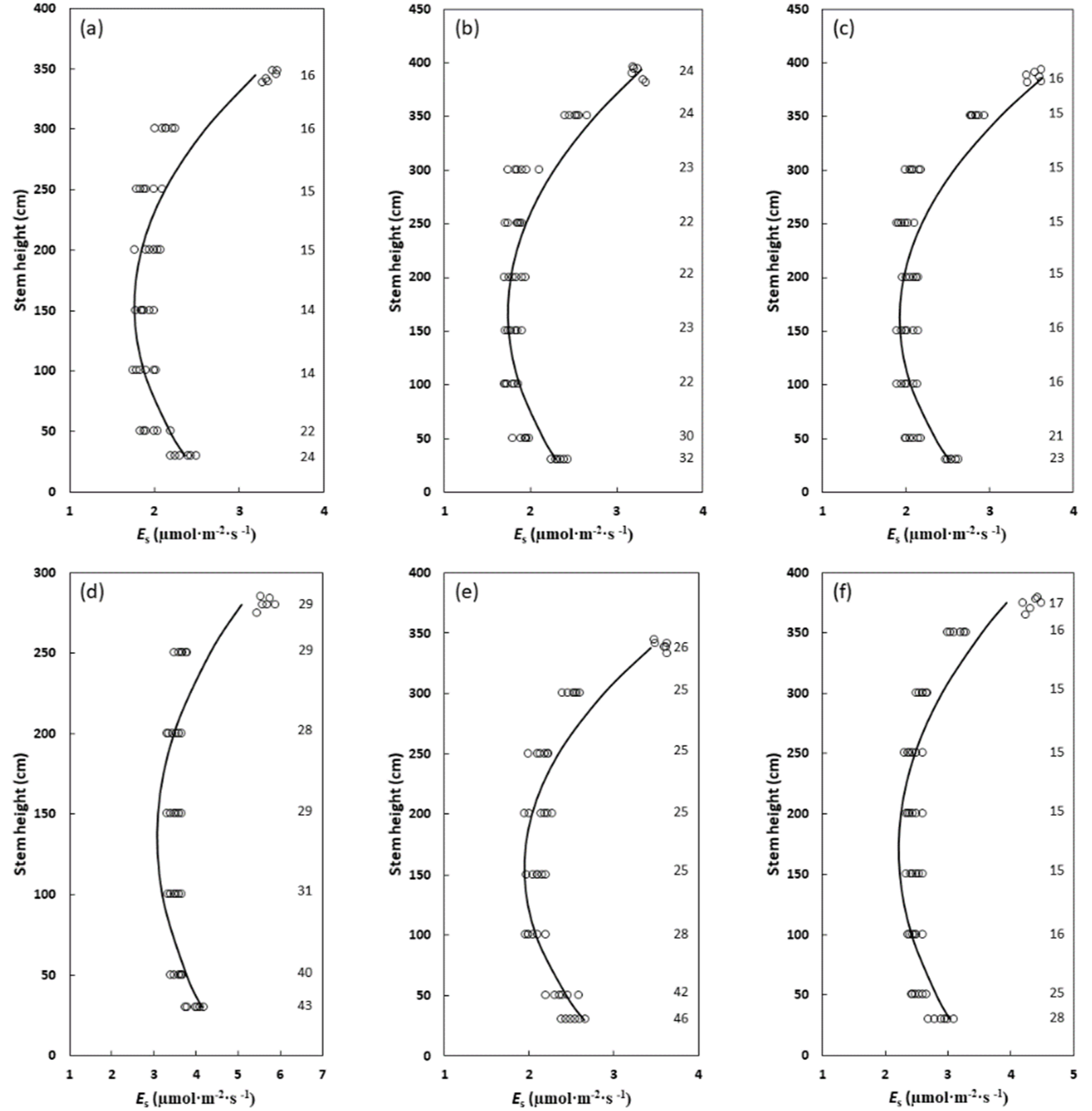
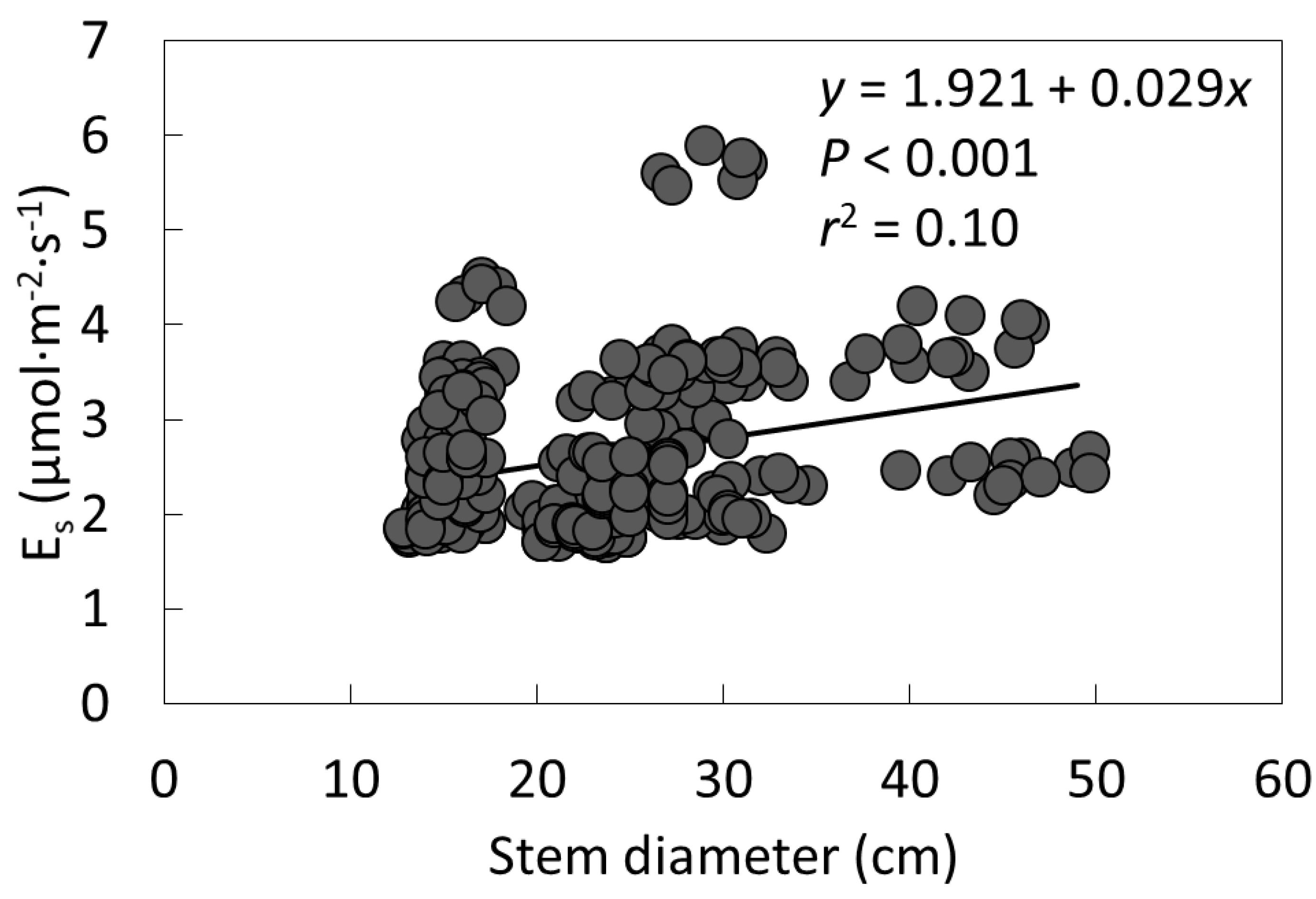
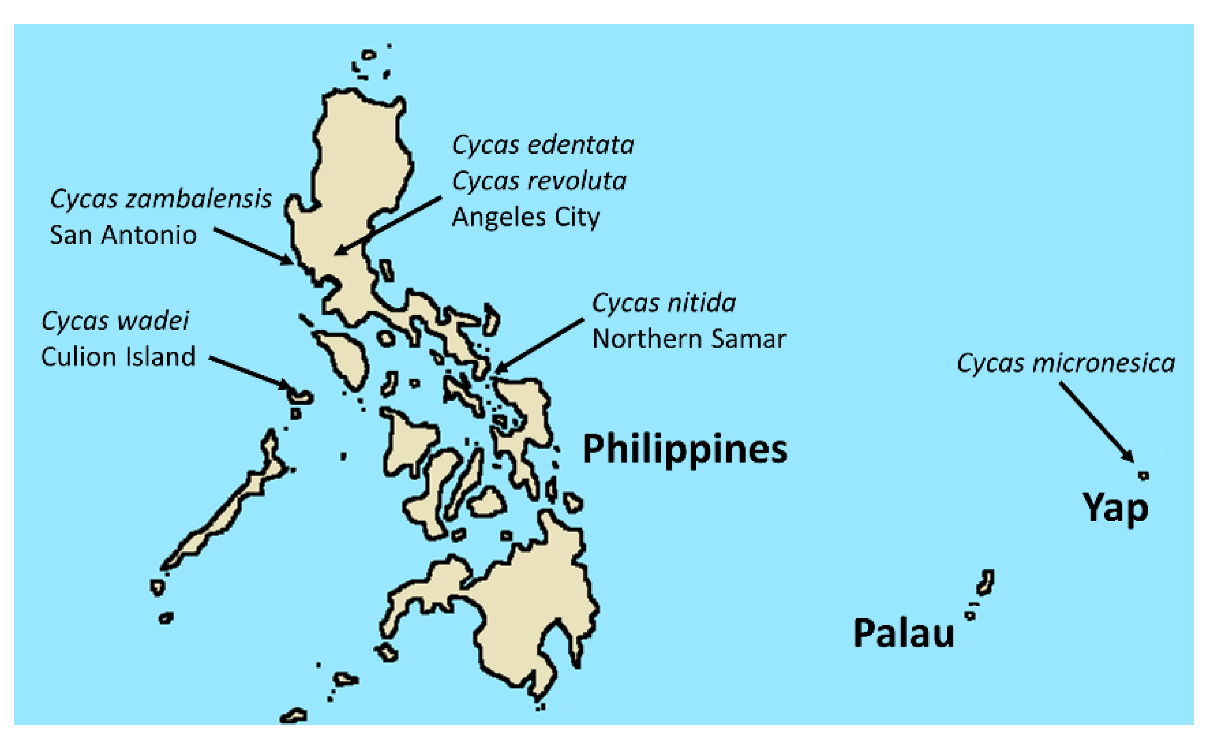

| Species | Air Temperature (°C) | Stem Temperature (°C) | Relative Humidity (%) | Quadratic Model P Value | Quadratic Model R2 |
|---|---|---|---|---|---|
| Cycas edentata | 27–32 | 26–32 | 59–65 | 0.0079–0.0018 | 0.78–0.82 |
| Cycas micronesica | 28–31 | 26–31 | 60–69 | 0.0021–0.0011 | 0.88–0.90 |
| Cycas nitida | 26–30 | 25–30 | 63–71 | 0.0014–0.0018 | 0.87–0.90 |
| Cycas revoluta | 27–33 | 27–31 | 59–66 | 0.0087–0.0015 | 0.71–0.77 |
| Cycas wadei | 29–34 | 27–33 | 60–68 | 0.0500–0.0501 | 0.85–0.88 |
| Cycas zambalensis | 26–30 | 25–29 | 58–64 | 0.0139–0.0110 | 0.79–0.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E.; Krishnapillai, M.V. Vertical Strata and Stem Carbon Dioxide Efflux in Cycas Trees. Plants 2020, 9, 230. https://doi.org/10.3390/plants9020230
Marler TE, Krishnapillai MV. Vertical Strata and Stem Carbon Dioxide Efflux in Cycas Trees. Plants. 2020; 9(2):230. https://doi.org/10.3390/plants9020230
Chicago/Turabian StyleMarler, Thomas E., and Murukesan V. Krishnapillai. 2020. "Vertical Strata and Stem Carbon Dioxide Efflux in Cycas Trees" Plants 9, no. 2: 230. https://doi.org/10.3390/plants9020230
APA StyleMarler, T. E., & Krishnapillai, M. V. (2020). Vertical Strata and Stem Carbon Dioxide Efflux in Cycas Trees. Plants, 9(2), 230. https://doi.org/10.3390/plants9020230





