The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures
Abstract
1. Introduction
2. Results
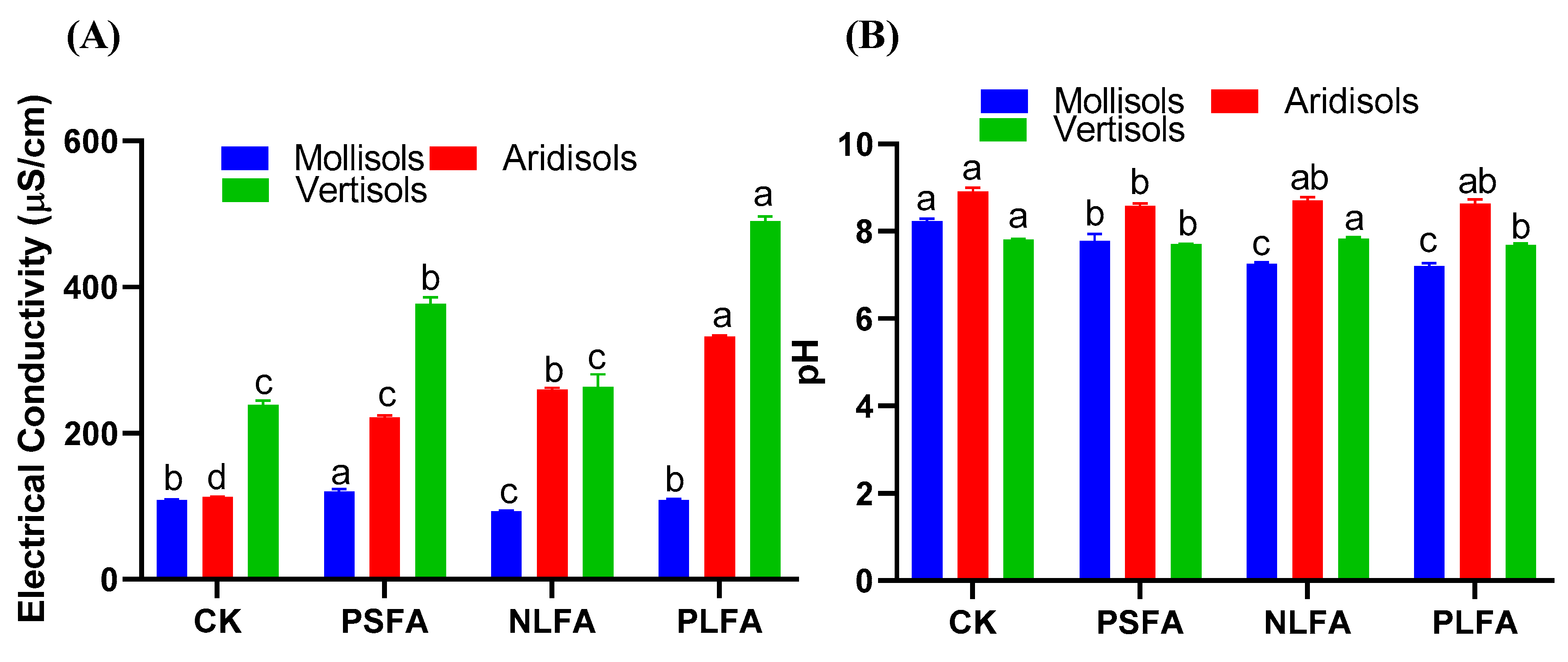
2.1. Effect of FA on Electrical Conductivity and Soil pH
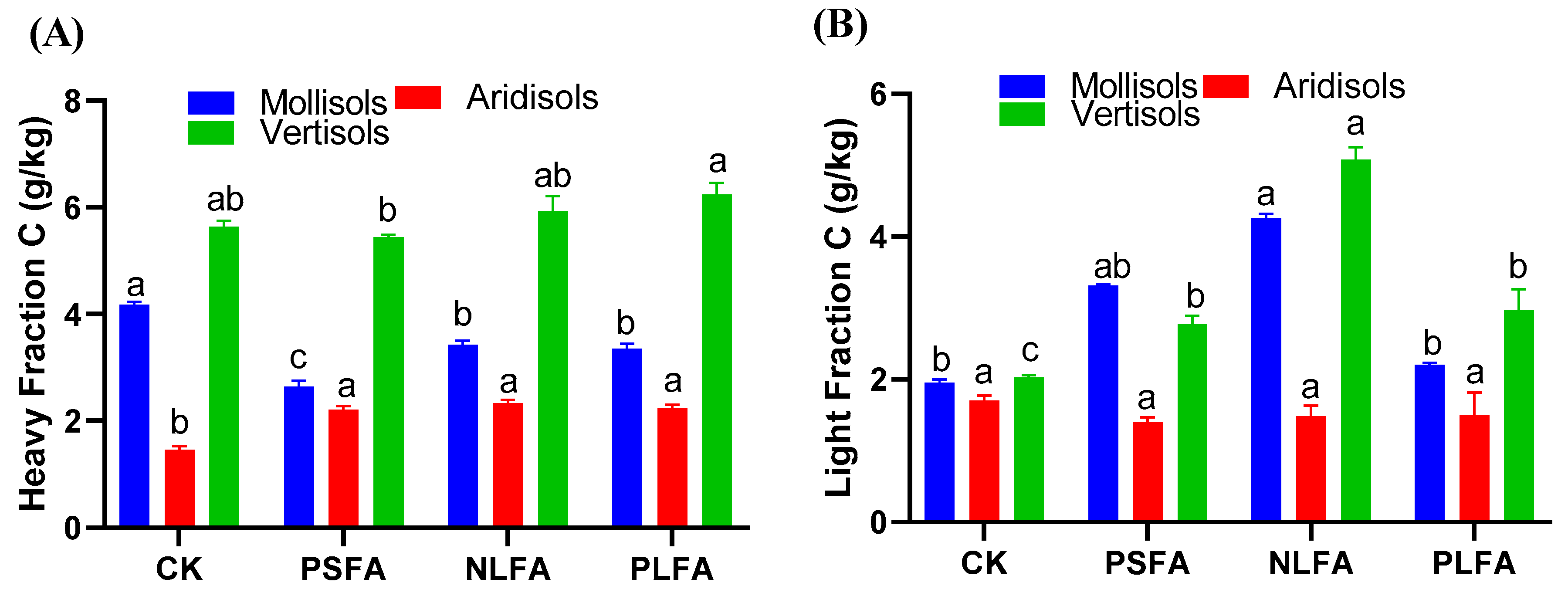
2.2. Effect of FAs on Heavy and Light Fractions of Organic Carbon
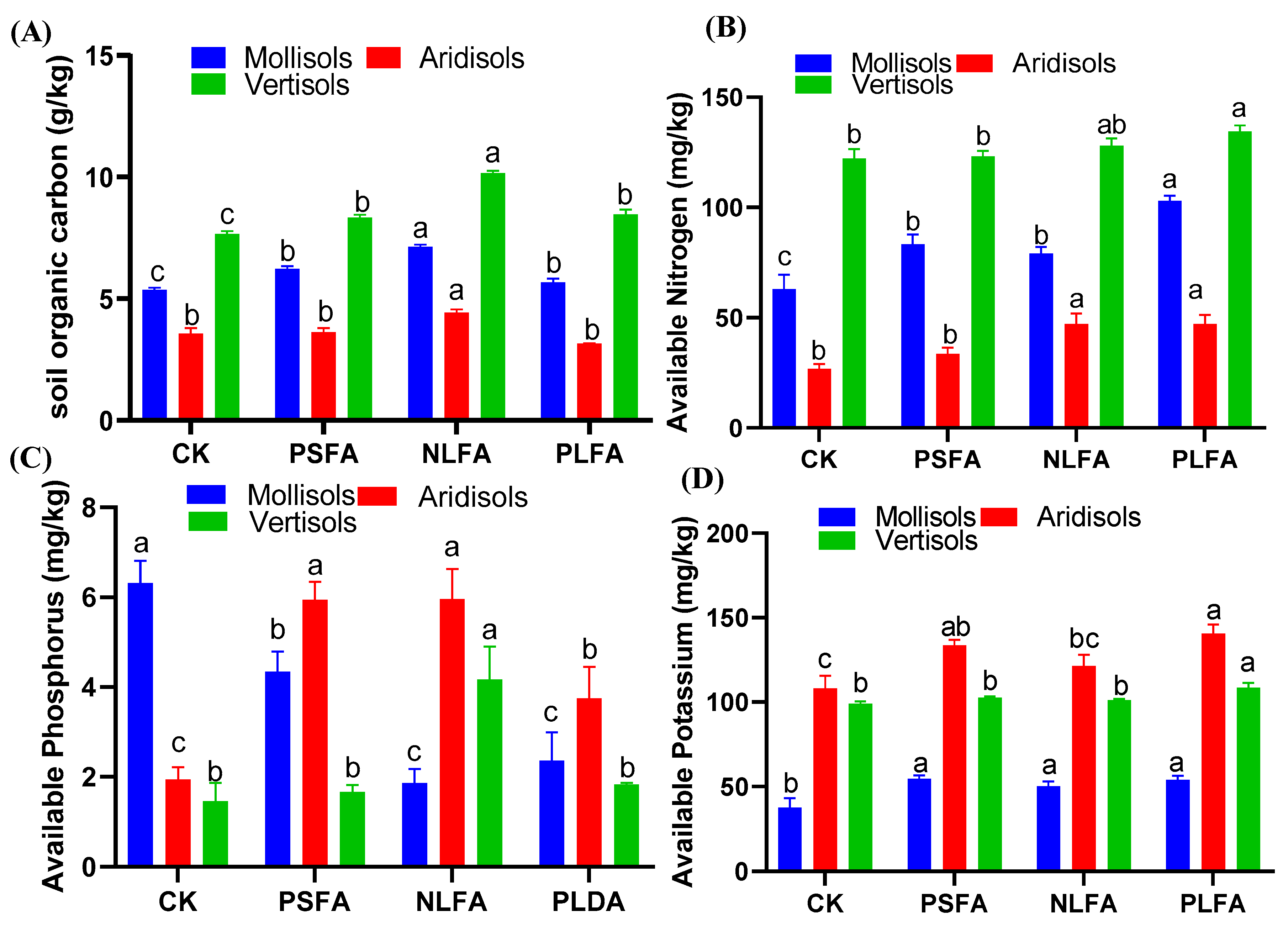
2.3. Effect of FAs on Soil Chemical Properties
2.4. Effect of FAs on the Organic–Inorganic Compounds and Their Complexes
2.5. Effect of FA on Wheat Grain and Spike Grain weight (g pot−1)
2.6. Effect of FAs on Plant Growth and Biomass Accumulation
2.7. Effect of FAs on the Nutrient Content of Wheat
3. Discussion
3.1. Influences of FAs on the Physiochemical Properties of Mollisol, Ardisol, and Vertisol Soils
3.2. The Influence of FAs on Heavy and Light Fraction C and Organic Complexes
3.3. Influence of FAs on the Plant Growth Characteristics of Wheat
4. Materials and Methods
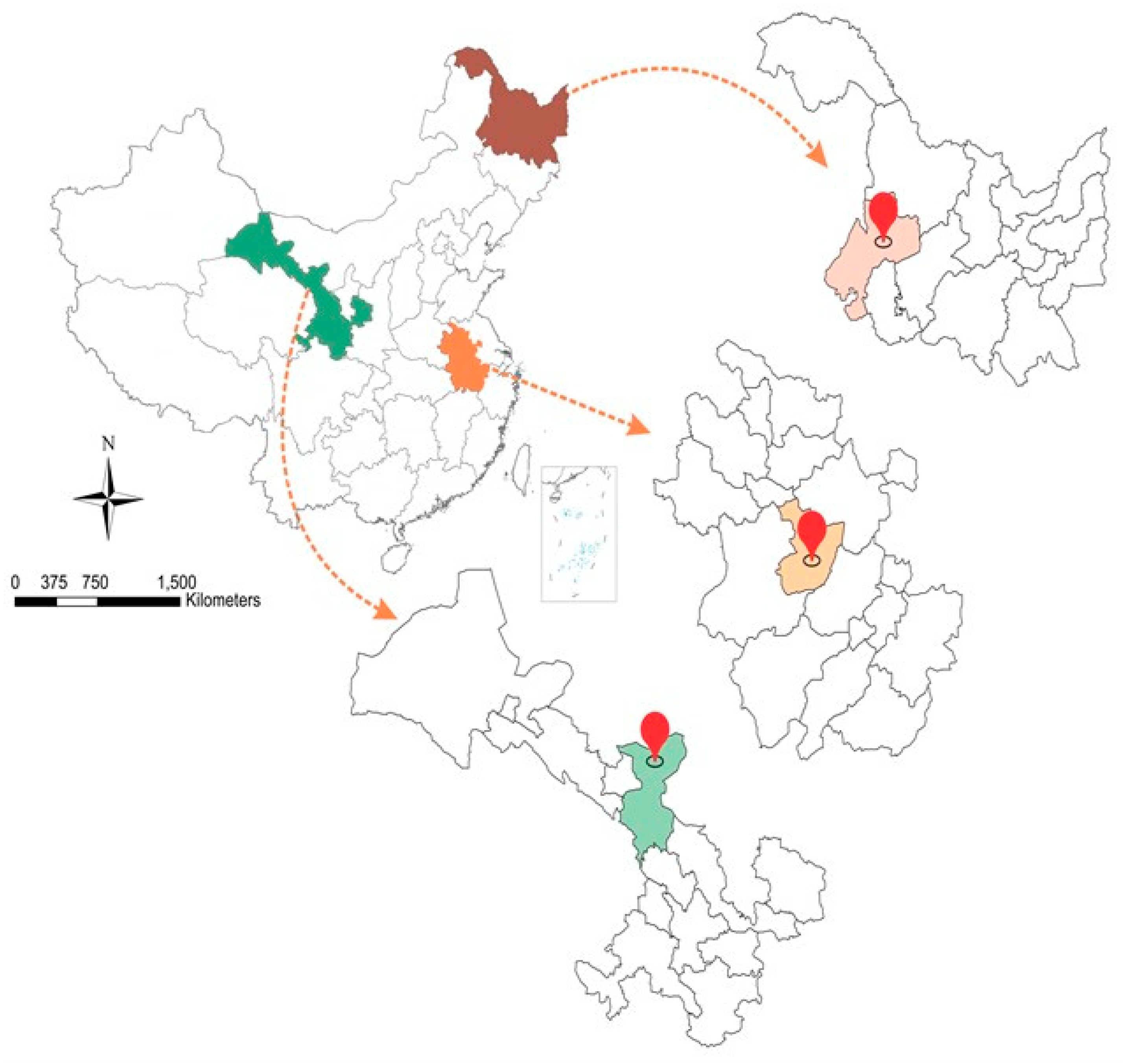
4.1. Collection and Preparation of Soil and Plant Samples
4.2. Organic–Inorganic Compound and Organic–Inorganic Composite Analysis
4.3. Experimental Design and Crop Maintenance
4.4. Collection of Plant- and Mineral-Derived FA
4.5. Elemental Analysis of FAs
4.6. Calculation and Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Lavkulich, L.M.; Arocena, J.M. Luvisolic soils of Canada: Genesis, distribution, and classification. Can. J. Soil Sci. 2011, 91, 781–806. [Google Scholar] [CrossRef]
- Dinka, T.M.; Morgan, C.L.S.; McInnes, K.J.; Kishné, A.S.; Daren Harmel, R. Shrink-swell behavior of soil across a Vertisol catena. J. Hydrol. 2013, 476, 352–359. [Google Scholar] [CrossRef]
- Millán, H.; Tarquís, A.M.; Pérez, L.D.; Mato, J.; González-Posada, M. Spatial variability patterns of some Vertisol properties at a field scale using standardized data. Soil Tillage Res. 2012, 120, 76–84. [Google Scholar] [CrossRef]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Kazmi, M.H. Lignite-derived humic acid effect on growth of wheat plants in different soils. Pedosphere 2011, 21, 124–131. [Google Scholar] [CrossRef]
- Xing, B.; Liu, X.; Liu, J.; Han, X. Physical and chemical characteristics of a typical Mollisol in China. Commun. Soil Sci. Plant Anal. 2004, 35, 1829–1838. [Google Scholar] [CrossRef]
- Kravchenko, Y.S.; Zhang, X.; Liu, X.; Song, C.; Cruse, R.M. Mollisols properties and changes in Ukraine and China. Chin. Geogr. Sci. 2011, 21, 257–266. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, W.; Liang, G.; Wang, X.; Sun, J.; He, P.; Li, L. Effects of different organic manures on the biochemical and microbial characteristics of albic paddy soil in a short-term experiment. PLoS ONE 2015, 10, e0124096. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.; Zhao, B.; Ma, M.; Guan, D.; Li, J.; Chen, S.; Cao, F.; Shen, D.; et al. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Han, X.Z.; Tang, C.; Song, C.Y.; Wang, S.Y.; Qiao, Y.F. Phosphorus characteristics correlate with soil fertility of albic luvisols. Plant Soil 2005, 270, 47–56. [Google Scholar] [CrossRef]
- Ling, N.; Sun, Y.; Ma, J.; Guo, J.; Zhu, P.; Peng, C.; Yu, G.; Ran, W.; Guo, S.; Shen, Q. Response of the bacterial diversity and soil enzyme activity in particle-size fractions of Mollisol after different fertilization in a long-term experiment. Biol. Fertil. Soils 2014, 50, 901–911. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Zhu, P.; Peng, C.; Wang, J.; He, H.; Zhang, X. Long-term manure amendments enhance neutral sugar accumulation in bulk soil and particulate organic matter in a mollisol. Soil Biol. Biochem. 2014, 78, 45–53. [Google Scholar] [CrossRef]
- Chai, Y.; Ma, S.; Zeng, X.; E, S.; Che, Z.; Li, L.; Duan, R.; Su, S. Long-term fertilization effects on soil organic carbon stocks in the irrigated desert soil of NW China. J. Plant Nutr. Soil Sci. 2015, 178, 622–630. [Google Scholar] [CrossRef]
- Li, X.G.; Li, F.M.; Rengel, Z.; Wang, Z.F. Cultivation effects on temporal changes of organic carbon and aggregate stability in desert soils of Hexi Corridor region in China. Soil Tillage Res. 2006, 91, 22–29. [Google Scholar] [CrossRef]
- Su, Y.Z.; Zhao, W.Z.; Su, P.X.; Zhang, Z.H.; Wang, T.; Ram, R. Ecological effects of desertification control and desertified land reclamation in an oasis-desert ecotone in an arid region: A case study in Hexi Corridor, northwest China. Ecol. Eng. 2007, 29, 117–124. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-term manure and fertilizer effects on soil organic matter fractions and microbes under a wheat-maize cropping system in northern China. Geoderma 2009, 149, 318–324. [Google Scholar] [CrossRef]
- Wang, S.; Nan, Z.; Liu, X.; Zhang, G.; Zhao, Z. Availability and speciation of Cu, Zn, and Pb added to irrigated desert soil. Polish J. Environ. Stud. 2010, 19, 865–869. [Google Scholar]
- Sootahar, M.K.; Zeng, X.; Su, S.; Wang, Y.; Bai, L.; Zhang, Y.; Li, T.; Zhang, X. The effect of fulvic acids derived from different materials on changing properties of albic black soil in the Northeast Plain of China. Molecules 2019, 24, 1535. [Google Scholar] [CrossRef]
- Khan, R.U.; Khan, M.Z.; Khan, A.; Saba, S.; Hussain, F.; Jan, I.U. Effect of humic acid on growth and crop nutrient status of wheat on two different soils. J. Plant Nutr. 2018, 41, 453–460. [Google Scholar] [CrossRef]
- Gerke, J. Concepts and misconceptions of humic substances as the stable part of soil organic matter: A review. Agronomy 2018, 8, 76. [Google Scholar] [CrossRef]
- Bi, D.; Yuan, G.; Wei, J.; Xiao, L.; Feng, L.; Meng, F. A Soluble Humic Substance for the Simultaneous Removal of Cadmium and Arsenic from Contaminated Soils. Int. J. Environ. Res. Public Health 2019, 16, 4999. [Google Scholar] [CrossRef] [PubMed]
- Albers, C.N.; Banta, G.T.; Hansen, P.E.; Jacobsen, O.S. Effect of different humic substances on the fate of diuron and its main metabolite 3,4-dichloroaniline in soil. Environ. Sci. Technol. 2008, 42, 8687–8691. [Google Scholar] [CrossRef]
- Arslan, E.; Obek, E.; Kirbag, S.; Ipek, U. Determination of the Effect of Compost on Soil Microorganisms. Int. J. Sci. Technol. 2008, 3, 151–159. [Google Scholar]
- Sharif, M.; Khattak, R.A.; Sarir, M.S. Effect of different levels of lignitic coal derived humic acid on growth of maize plants. Commun. Soil Sci. Plant Anal. 2002, 33, 3567–3580. [Google Scholar] [CrossRef]
- Piccolo, A.; Mbagwu, J.S.C. Role of Hydrophobic Components of Soil Organic Matter in Soil Aggregate Stability. Soil Sci. Soc. Am. J. 1999, 63, 1801–1810. [Google Scholar] [CrossRef]
- Piccolo, A.; Conte, P.; Spaccini, R.; Chiarella, M. Effects of some dicarboxylic acids on the association of dissolved humic substances. Biol Fertil. Soils 2003, 37, 255–259. [Google Scholar] [CrossRef]
- Vaughan, D.; Ord, B.G. Uptake and incorporation of14C-labelled soil organic matter by roots of Pisum sativum L. J. Exp. Bot. 1981, 32, 679–687. [Google Scholar] [CrossRef]
- Dobbss, L.B.; Medici, L.O.; Peres, L.E.P.; Pino-Nunes, L.E.; Rumjanek, V.M.; Façanha, A.R.; Canellas, L.P. Changes in root development of Arabidopsis promoted by organic matter from oxisols. Ann. Appl. Biol. 2007, 151, 199–211. [Google Scholar] [CrossRef]
- Oyinlola, E.Y.; Jinadu, S.A. Growth, Yield and Nutrient Concentrations of Tomato as Affected by Soil Texures and Nitrogen. Asian J. Agric. Res. 2012, 6, 39–45. [Google Scholar]
- Moody, P.W.; Aitken, R.L. Soil acidification under some tropical agricultural systems. Aust. J. Soil Res. 1997, 35, 163–174. [Google Scholar] [CrossRef]
- Mayhew, L. Humic Substances in Biological Agriculture. Acres 2004, 34, 54–61. [Google Scholar]
- Pratt, P. Methods of soil analysis. Part 2. In Chemical and Microbiological Properties; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1022–1030. [Google Scholar]
- Boguta, P.; D’Orazio, V.; Sokołowska, Z.; Senesi, N. Effects of selected chemical and physicochemical properties of humic acids from peat soils on their interaction mechanisms with copper ions at various pHs. J. Geochem. Explor. 2016, 168, 119–126. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K.; Doran, J.W. Aggregation and Soil Organic Matter Accumulation in Cultivated and Native Grassland Soils. Soil Sci. Soc. Am. J. 1998, 62, 1367–1377. [Google Scholar] [CrossRef]
- Cunha-Santino, M.B.; Bianchini, I. Humic substances mineralization: The variation of pH, electrical conductivity and optical density. Acta Limnol. Bras. 1999, 11, 65–78. [Google Scholar]
- Ullah, M.A.; Aamir, S.S.; Haider, H.; Adil, B.; Mahmood, I.A.; Badar-uz-Zaman; Hyder, S.I. Effect of salinity, humic acid, biozote and vermicompost on soil physicochemical properties and olive plants species. J. Agric. Sci. Pract. 2018, 3, 27–32. [Google Scholar] [CrossRef]
- Horie, T.; Yoshida, K.; Nakayama, H.; Yamada, K.; Oiki, S.; Shinmyo, A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J. 2001, 27, 129–138. [Google Scholar] [CrossRef]
- Khaleda, L.; Park, H.J.; Yun, D.J.; Jeon, J.R.; Kim, M.G.; Cha, J.Y.; Kim, W.Y. Humic acid confers HIGH-affinity K+ transporter 1-mediated salinity stress tolerance in arabidopsis. Mol. Cells 2017, 40, 966–975. [Google Scholar]
- Delfine, S.; Tognetti, R.; Desiderio, E.; Alvino, A. Effect of foliar application of N and humic acids on growth and yield of durum wheat. Agron. Sustain. Dev. 2005, 25, 183–191. [Google Scholar] [CrossRef]
- Bermudez, D.; Juarez, M.; Sanchez-Andreu, J.; Jorda, J.D. Role of eddha and humic acids on the solubility of soil phosphorus. Commun. Soil Sci. Plant Anal. 1993, 24, 673–683. [Google Scholar] [CrossRef]
- Wang, X.J.; Wang, Z.Q.; Li, S.G. The effect of humic acids on the availability of phosphorus fertilizers in alkaline soils. Soil Use Manag. 1995, 11, 99–102. [Google Scholar] [CrossRef]
- Cimrin, K.M.; Yilmaz, I. Humic acid applications to lettuce do not improve yield but do improve phosphorus availability. Acta Agric. Scand. Sect. B Soil Plant Sci. 2005, 55, 58–63. [Google Scholar] [CrossRef]
- Imbufe, A.U.; Patti, A.F.; Burrow, D.; Surapaneni, A.; Jackson, W.R.; Milner, A.D. Effects of potassium humate on aggregate stability of two soils from Victoria, Australia. Geoderma 2005, 125, 321–330. [Google Scholar] [CrossRef]
- Tan, Z.; Lal, R.; Owens, L.; Izaurralde, R.C. Distribution of light and heavy fractions of soil organic carbon as related to land use and tillage practice. Soil Tillage Res. 2007, 92, 53–59. [Google Scholar] [CrossRef]
- Janzen, H.H.; Campbell, C.A.; Brandt, S.A.; Lafond, G.P. Light-Fraction Organic Matter in Soils from Long-Term Crop Rotations. Soil Sci. Soc. Am. J. 1992, 56, 1799–1806. [Google Scholar] [CrossRef]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Study of free and occluded particulate organic matter in soils by solid state 13c cp/mas nmr spectroscopy and scanning electron microscopy. Aust. J. Soil Res. 1994, 32, 285–309. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Carbon and Nitrogen Dynamics of Soil Organic Matter Fractions from Cultivated Grassland Soils. Soil Sci. Soc. Am. J. 2010, 58, 123. [Google Scholar] [CrossRef]
- Hassink, J. Density fractions of soil macroorganic matter and microbial biomass as predictors of C and N mineralization. Soil Biol. Biochem. 1995, 27, 1099–1108. [Google Scholar] [CrossRef]
- Golchin, A.; Clarke, P.; Oades, J.M.; Skjemstad, J.O. The effects of cultivation on the composition of organic matter and structural stability of soils. Aust. J. Soil Res. 1995, 33, 975–993. [Google Scholar] [CrossRef]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Structural and dynamic properties of soil organic matter as reflected by13c natural abundance, pyrolysis mass spectrometry and solid-state13c nmr spectroscopy in density fractions of an oxisol under forest and pasture. Aust. J. Soil Res. 1995, 33, 59–76. [Google Scholar] [CrossRef]
- Wander, M. Soil Organic Matter Fractions and Their Relevance to Soil Function; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9780203496374. [Google Scholar]
- Crow, S.E.; Swanston, C.W.; Lajtha, K.; Brooks, J.R.; Keirstead, H. Density fractionation of forest soils: Methodological questions and interpretation of incubation results and turnover time in an ecosystem context. Biogeochemistry 2007, 85, 69–90. [Google Scholar] [CrossRef]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions—A review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Whalen, J.K.; Bottomley, P.J.; Myrold, D.D. Carbon and nitrogen mineralization from light- and heavy-fraction additions to soil. Soil Biol. Biochem. 2000, 32, 1345–1352. [Google Scholar] [CrossRef]
- Anderson, D.W.; Paul, E.A. Organo-mineral complexes and their study by radiocarbon dating. Soil Sci. Soc. Am. J. 1984, 48, 298–301. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Muscolo, A.; Vianello, A. Physiological effects of humic substances on higher plants. Soil Biol. Biochem. 2002, 34, 1527–1536. [Google Scholar] [CrossRef]
- Arjumend, T.; Abbasi, M.K.; Rafique, E. Effects of lignite-derived Humic acid on some selected soil properties, growth and nutrient uptake of wheat (Triticum aestivum L.) grown under greenhouse conditions. Pak. J. Bot. 2015, 47, 2231–2238. [Google Scholar]
- Pettit, R.E. Organic matter, humus, humate, humic acid, fulvic acid and humin: Their importance in soil fertility and plant health. In Proceeding of the IEEE Geoscience and Remote Sensing Symposium (IGARSS 2014), Quebec, QC, Canada, 13–18 July 2014; pp. 1–5. [Google Scholar]
- Guo, Y.; Halfter, U.; Ishitani, M.; Zhu, J.K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 2001, 13, 1383–1399. [Google Scholar] [CrossRef]
- Eshwar, M.; Srilatha, M.; Bhanu Rekha, K.; Harish Kumar Sharma, S.; Eshwar, C.M. Effect of humic substances (humic, fulvic acid) and chemical fertilizers on nutrient uptake, dry matter production of aerobic rice (Oryza sativa L.). J. Pharmacogn. Phytochem. 2017, 6, 1063–1066. [Google Scholar]
- Khaled, H.; Fawy, H.A. Effect of Different Levels of Humic Acids on the Nutrient Content, Plant Growth, and Soil Properties under Conditions of Salinity. Soil Water Res. 2011, 6, 21–29. [Google Scholar] [CrossRef]
- Çelik, H.; Katkat, A.V.; Aşik, B.B.; Turan, M.A. Effects of humus on growth and nutrient uptake of maize under saline and calcareous soil conditions. Zemdirbyste 2010, 97, 15–22. [Google Scholar]
- Tan, K. Soil Sampling, Preparation, and Analysis; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Nelson, D.W.; Sommers, L. Total carbon, organic carbon, and organic matter. In Mthods of Soil Analysis Part 3; Wiley: Hoboken, NJ, USA, 1996; pp. 961–1010. [Google Scholar]
- Parkinson, J.A.; Allen, S.E. A wet oxidation procedure suitable for the determination of nitrogen and mineral nutrients in biological material. Commun. Soil Sci. Plant Anal. 1975, 6, 1–11. [Google Scholar] [CrossRef]

| FA | Oganic–Inorganic Degree Compound (g/kg) | Orgnanic–Inorganic Composite (g/kg) | ||||
|---|---|---|---|---|---|---|
| Mollisols | Aridisols | Vertisols | Mollisols | Aridisols | Vertisols | |
| CK | 71.85 ± 5.41 a | 46.9 ± 1.36 c | 53.0 ± 1.39 c | 0.30 ± 0.03 a | 0.14 ± 0.006 b | 0.497 ± 0.03 a |
| PSFA | 44.7 ± 2.42 b | 61.3 ± 1.04 b | 65.2 ± 1.24 b | 0.22 ± 0.008 b | 0.22 ± 0.009 a | 0.432 ± 0.04 ab |
| NLFA | 49.7 ± 0.65 a | 61.7 ± 2.3 b | 74.6 ± 1.42 a | 0.28 ± 0.01 ab | 0.22 ± 0.006 a | 0.422 ± 0.02 b |
| PLFA | 61.9 ± 5.3 a | 68.8 ± 0.86 a | 68.4 ± 1.78 b | 0.34 ± 0.02 a | 0.21 ± 0.009 a | 0.452 ± 0.01 ab |
| FA | Thousand Grain Weight (g) | Spike Grain Weight (g) | ||||
|---|---|---|---|---|---|---|
| Mollisols | Aridisols | Vertisols | Mollisols | Aridisols | Vertisols | |
| CK | 49.7 ± 1.9 a | 55.79 ± 2.55 a | 53.4 ± 0.12 a | 99 ± 6.55 b | 76.7 ± 5.57 ab | 138.4 ± 8.23 a |
| PSFA | 50.5 ± 2.0 a | 47.0 ± 1.89 b | 47.3 ± 1.12 b | 123.2 ± 6.63 a | 30.3 ± 5.5 b | 124.2 ± 8.85 ab |
| NLFA | 46.5 ± 0.57 a | 51.1 ± 1.27 ab | 46.2 ± 188 b | 97.2 ± 4.63 b | 87.1 ± 5.73 a | 126.8 ± 5.23 ab |
| PLFA | 47.2 ± 1.21 a | 47.2 ± 1.49 b | 50.1 ± 1.15 a | 110.4 ± 8.20 ab | 68.5 ± 9.21 ab | 76.7 ± 8.45 b |
| FA | Plant Height (cm) | Plant Biomass (g) | ||||
|---|---|---|---|---|---|---|
| Mollisols | Aridisols | Vertisols | Mollisols | Aridisols | Vertisols | |
| CK | 78.2.0 ± 0.91 b | 68.9.5 ± 1.26 b | 76.7 ± 1.78 b | 160.6 ± 1.75 d | 153.3 ± 4.37 a | 207.4 ± 1.73 b |
| PSFA | 87.0 ± 2.65 a | 71.2 ± 1.65 b | 84 ± 1.35 a | 194.2 ± 4.08 b | 82.6 ± 1.42 b | 178.3 ± 6.40 c |
| NLFA | 84.2 ± 0.75 ab | 88.7 ± 1.11 a | 88.2 ± 1.18 a | 179.6 ± 3.89 c | 166.3 ± 7.29 a | 253.0 ± 3.76 a |
| PLFA | 81.6 ± 1.17 b | 74.3 ± 1.49 b | 82.7 ± 3.03 a | 206.3 ± 1.84 a | 161.1 ± 9.16 a | 210.4 ± 1.8 2 b |
| Soil | EC us/cm | pH | OM g/kg | CEC cmol/kg | AN mg/kg | AP mg/kg | AK mg/kg | TN g/kg | TP g/kg | TK g/kg | Textural Class |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mollisols | 30.1 | 5.2 | 8.4 | 21.6 | 66.0 | 0.44 | 78.0 | 0.70 | 0.39 | 20.4 | Silty clay |
| Aridisols | 2063 | 8.4 | 2.2 | 4.6 | 19.0 | 2.4 | 525.3 | 0.20 | 0.39 | 20.1 | Sandy Loam |
| Vertisols | 132 | 7.9 | 11.7 | 25.2 | 52.0 | 1.19 | 186.6 | 0.83 | 0.39 | 17.3 | Clay loam |
| FA Type | N | C | H | S |
|---|---|---|---|---|
| % | ||||
| PSFA | 5.39 | 25.31 | 5.75 | 8.47 |
| NLFA | 10.29 | 52.476 | 9.74 | 14.84 |
| PLFA | 10.78 | 50.61 | 11.56 | 16.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar Sootahar, M.; Zeng, X.; Wang, Y.; Su, S.; Soothar, P.; Bai, L.; Kumar, M.; Zhang, Y.; Mustafa, A.; Ye, N. The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures. Plants 2020, 9, 205. https://doi.org/10.3390/plants9020205
Kumar Sootahar M, Zeng X, Wang Y, Su S, Soothar P, Bai L, Kumar M, Zhang Y, Mustafa A, Ye N. The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures. Plants. 2020; 9(2):205. https://doi.org/10.3390/plants9020205
Chicago/Turabian StyleKumar Sootahar, Mahendar, Xibai Zeng, Yanan Wang, Shiming Su, Permanand Soothar, Lingyu Bai, Mukesh Kumar, Yang Zhang, Adnan Mustafa, and Ning Ye. 2020. "The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures" Plants 9, no. 2: 205. https://doi.org/10.3390/plants9020205
APA StyleKumar Sootahar, M., Zeng, X., Wang, Y., Su, S., Soothar, P., Bai, L., Kumar, M., Zhang, Y., Mustafa, A., & Ye, N. (2020). The Short-Term Effects of Mineral- and Plant-Derived Fulvic Acids on Some Selected Soil Properties: Improvement in the Growth, Yield, and Mineral Nutritional Status of Wheat (Triticum aestivum L.) under Soils of Contrasting Textures. Plants, 9(2), 205. https://doi.org/10.3390/plants9020205







