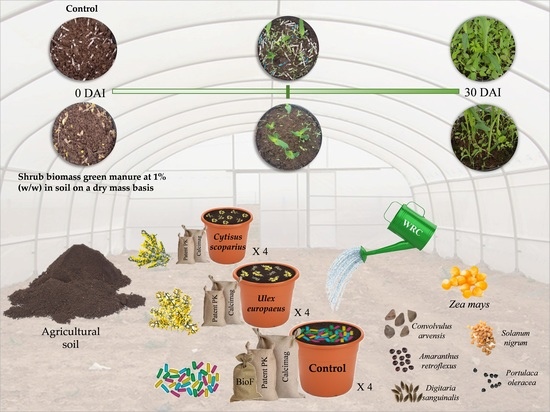
The Phytotoxic Potential of the Flowering Foliage of Gorse (Ulex europaeus) and Scotch Broom (Cytisus scoparius), as Pre-Emergent Weed Control in Maize in a Glasshouse Pot Experiment
Abstract
1. Introduction
2. Results
2.1. Effects of Gorse and Scotch Broom Foliage Used as Soil Amendments on Weeds and Maize
2.2. Effects on Soil Community-Level Physiological Profile
2.3. Effects on Soil Physicochemical Parameters
3. Discussion
4. Materials and Methods
4.1. Soil and Plant Materials Description
4.2. Soil Amendment with Shrub Foliage in Pot Experiments
4.2.1. Assessing the Effects of Gorse and Scotch Broom Foliage on Weeds and Maize
4.2.2. BIOLOG Ecoplates for Soil Community-Level Physiological Profile (CLPP)
4.2.3. Measuring Post-Trial Soil Physicochemical Parameters
4.3. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Qasem, J.R.; Foy, C.L. Weed Allelopathy, Its Ecological Impacts and Future Prospects. J. Crop Prod. 2001, 4, 43–119. [Google Scholar] [CrossRef]
- Xuan, T.D.; Shinkichi, T.; Khanh, T.D.; Chung, I.M. Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: An overview. Crop Prot. 2005, 24, 197–206. [Google Scholar] [CrossRef]
- Narwal, S.S. Allelopathy in ecological sustainable organic agriculture. Allelopath. J. 2010, 25, 51–72. [Google Scholar]
- De Albuquerque, M.B.; Dos Santos, R.C.; Lima, L.M.; MeloFilho, P.D.A.; Nogueira, R.J.M.C.; Da Câmara, C.A.G.; Ramos, A.D.R. Allelopathy, an alternative tool to improve cropping systems. A review. Agron. Sustain. Dev. 2011, 31, 379–395. [Google Scholar] [CrossRef]
- Tabaglio, V.; Marocco, A.; Schulz, M. Allelopathic cover crop of rye for integrated weed control in sustainable agroecosystems. Ital. J. Agron. 2013, 8, e5. [Google Scholar] [CrossRef]
- Álvarez-Iglesias, L.; Puig, C.G.; Revilla, P.; Reigosa, M.J.; Pedrol, N. Faba bean (Vicia faba L.) as green manure for field weed control in maize. Weed Res. 2018, 58, 437–449. [Google Scholar] [CrossRef]
- Cherr, C.M.; Scholberg, J.M.S.; Mcsorley, R. Green manure approaches to crop production: A synthesis. Agron. J. 2006, 98, 302–319. [Google Scholar] [CrossRef]
- Fujii, Y. Screening and future exploitation of allelopathic plants as alternative herbicides with special reference to hairy vetch. J. Crop Prod. 2001, 4, 257–275. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.B.; Eleftherohorinos, I.G.; Lithourgidis, A.S. Allelopathic potential of winter cereal cover crop mulches on grass weed suppression and sugarbeet development. Crop Sci. 2006, 46, 1682–1691. [Google Scholar] [CrossRef]
- Kruidhof, H.M.; Bastiaans, L.; Kropff, M.J. Ecological weed management by cover cropping: Effects on weed growth in autumn and weed establishment in spring. Weed Res. 2008, 48, 492–502. [Google Scholar] [CrossRef]
- Bezuidenhout, S.R.; Reinhardt, C.F.; Whitwell, M.I. Cover crops of oats, stooling rye and three annual ryegrass cultivars influence maize and Cyperus esculentus growth. Weed Res. 2012, 52, 153–160. [Google Scholar] [CrossRef]
- Dhima, K.V.; Vasilakoglou, I.B.; Gatsis, T.D.; Panou-Philotheou, E.; Eleftherohorinos, I.G. Effects of aromatic plants incorporated as green manure on weed and maize development. Field Crop Res. 2009, 110, 235–241. [Google Scholar] [CrossRef]
- Sodaeizadeh, H.; Rafieiolhossaini, M.; Van Damme, P. Herbicidal activity of a medicinal plant, Peganum harmala L., and decomposition dynamics of its phytotoxins in the soil. Ind. Crop Prod. 2010, 31, 385–394. [Google Scholar] [CrossRef]
- Puig, C.G.; Álvarez-Iglesias, L.; Reigosa, M.J.; Pedrol, N. Eucalyptus globulus Leaves incorporated as Green Manure for Weed Control in Maize. Weed Sci. 2013, 61, 154–161. [Google Scholar] [CrossRef]
- Puig, C.G.; Revilla, P.; Barreal, M.E.; Reigosa, M.J.; Pedrol, N. On the suitability of Eucalyptus globulus green manure for field weed control. Crop Prot. 2019, 121, 57–65. [Google Scholar] [CrossRef]
- Souza-Alonso, P.; Puig, C.G.; Pedrol, N.; Freitas, H.; Rodríguez-Echeverría, S.; Lorenzo, P. Exploring the use of residues from the invasive Acacia sp. for weed control. Renew. Agric. Food Syst. 2018, 35, 1–12. [Google Scholar] [CrossRef]
- de Galicia, X. Primeira Revisión do Plan Forestal de Galicia; Documento Previo; Dirección Xeral de Ordenación e Produción Forestal, Consellería do Medio Rural: Santiago de Compostela, Spain, 2016. [Google Scholar]
- Pérez, S.; Renedo, C.J.; Ortiz, A.; Delgado, F.; Fernández, I. Energy potential of native shrub species in northern Spain. Renew. Energy 2014, 62, 79–83. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- CAB International. 2020. Available online: https://www.cabi.org/isc (accessed on 17 January 2020).
- Pardo-Muras, M.; Puig, C.G.; López-Nogueira, A.; Cavaleiro, C.; Pedrol, N. On the bioherbicide potential of Ulex europaeus and Cytisus scoparius: Profiles of volatiles organic compounds and their phytotoxic effects. PLoS ONE 2018, 13, e0205997. [Google Scholar] [CrossRef]
- Pardo-Muras, M.; Puig, C.G.; Souto, C.; Pedrol, N. Water-soluble phenolic acids and flavonoids involved in the bioherbicidal potential of Ulex europaeus and Cytisus scoparius. S. Afr. J. Bot. (Submitted).
- Pardo-Muras, M.; Puig, C.G.; Pedrol, N. Cytisus scoparius and Ulex europaeus produce volatile organic compounds with powerful synergistic herbicidal effects. Molecules 2019, 24, 4539. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K. Factors affecting phytotoxic activity of allelochemicals in soil. Weed Biol. Manag. 2004, 4, 1–7. [Google Scholar] [CrossRef]
- Duke, S.O. Allelopathy: Current status of research and future of the discipline: A commentary. Allelop. J. 2010, 25, 17–30. [Google Scholar]
- Inderjit Dakshini, K.M.M. Allelopathic effect of Pluchea lanceolata (Asteraceae) on characteristics of four soils and tomato and mustard growth. Am. J. Bot. 1994, 81, 799–804. [Google Scholar] [CrossRef]
- Isidrón, M.P.; Allaert, K.; Isla, L.H.; Suárez, N.; García, S.T.; Navarro, C.P.; García, M.R. Determinación de la actividad alelopática de extractos vegetales sobre algunos hongos fitopatógenos del suelo. Centro Agrícola 2003, 30, 64–68. [Google Scholar]
- Quispe, F.E.; Ruíz, R.E.; Isidrón, M.P.; Cupull Santana, R.; García, M.R. Efecto alelopático de Tagetes erecta L. y Terminalia catappa L. sobre Rhizoctonia solani (Kühn). Centro Agrícola 2010, 37, 89–92. [Google Scholar]
- Meissle, M.; Mouron, P.; Musa, T.; Bigler, F.; Pons, X.; Vasileiadis, V.; Otto, S.; Anitchi, D.; Kiss, J.; Palinkas, Z.; et al. Pests, pesticide use and alternative options in European maize production: Current status and future prospects. J. Appl. Entomol. 2010, 134, 357–375. [Google Scholar] [CrossRef]
- Labrada, R.; Caseley, J.C.; Parker, C. Manejo de Malezas Para Países en Desarrollo, 1st ed.; FAO: Roma, Italy, 1996; p. 120. [Google Scholar]
- Kruidhof, H.M.; Gallandt, E.R.; Haramoto, E.R.; Bastiaans, L. Selective weed suppression by cover crop residues: Effects of seed mass and timing of species’ sensitivity. Weed Res. 2011, 51, 177–186. [Google Scholar] [CrossRef]
- Puig, C.G.; Gonçalves, R.F.; Valentão, P.; Andrade, P.B.; Reigosa, M.J.; Pedrol, N. The Consistency Between Phytotoxic Effects and the Dynamics of Allelochemicals Release from Eucalyptus globulus Leaves Used as Bioherbicide Green Manure. J. Chem. Ecol. 2018, 44, 658–670. [Google Scholar] [CrossRef]
- Gallandt, E.R. How can we target the weed seedbank? Weed Sci. 2006, 54, 588–596. [Google Scholar] [CrossRef]
- Hanifi, S.; El Hadrami, I. Phytotoxicity and fertilising potential of olive mill wastewaters for maize cultivation. Agron. Sustain. Dev. 2008, 28, 313–319. [Google Scholar] [CrossRef]
- Al Hamdi, B.; Inderjit; Olofsdotter, M.; Streibig, J.C. Laboratory bioassay for phytotoxicity: An example from wheat straw. Agron. J. 2011, 93, 43–48. [Google Scholar]
- Domínguez, J.; Gómez-Brandón, M.; Martínez-Cordeiro, H.; Lores, M. Bioconversion of Scotch broom into a high quality organic fertiliser: Vermicomposting as sustainable option. Waste Manag. Res. 2018, 36, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Pedrol, N.; Puig, C.G.; Souza, P.; Forján, R.; Vega, F.A.; Asensio, V.; González, L.; Cerqueira, B.; Covelo, E.F.; Andrade, L. Soil fertility and spontaneous revegetation in lignite spoil banks under different amendments. Soil Tillage Res. 2010, 110, 134–142. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef]
- Inderjit; van der Putten, W.H. Impacts of soil microbial communities on exotic plant invasions. Trends Ecol Evol. 2010, 25, 512–519. [Google Scholar] [CrossRef]
- Elfstrand, S.; Båth, B.; Mårtensson, A. Influence of various forms of green manure amendment on soil microbial community composition, enzyme activity and nutrient levels in leek. Appl. Soil Ecol. 2007, 36, 70–82. [Google Scholar] [CrossRef]
- Huang, X.; Xue, D.; Xue, L. Changes in soil microbial functional diversity and biochemical characteristics of tree peony with amendment of sewage sludge compost. Environ. Sci. Pollut. Res. 2015, 22, 11617–11625. [Google Scholar] [CrossRef]
- Blum, U. Allelopathic interactions involving phenolic acids. J. Nematol. 1996, 28, 259–267. [Google Scholar]
- Sampietro, D.A.; Vattuone, M.A. Sugarcane straw and its phytochemicals as growth regulators of weed and crop plants. Plant Growth Regul. 2006, 48, 21–27. [Google Scholar] [CrossRef]
- Souto, X.C.; Chiapusio, G.; Pellissier, F. Relationships between phenolics and soil microorganisms in spruce forests: Significance for natural regeneration. J. Chem. Ecol. 2000, 26, 2025–2034. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Eyakub Ali, M.; Zhang, W.; Duan, Y.; Li, D. Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment. PLoS ONE 2018, 13, e0203812. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, A.; Rayns, F. Sort out Your Soil: A Practical Guide to Green Manures; Cotswold Grass Seeds Direct: Moreton-in-Marsh, UK, 2011; pp. 1–21. [Google Scholar]
- Sims, C.; Finnoff, D.; Shogren, J.F. Bioeconomics of invasive species: Using real options theory to integrate ecology, economics, and risk management. Food Secur. 2016, 8, 61–70. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Matloob, A.; Mahajan, G.; Aslam, F.; Florentine, S.K.; Jha, P. Emerging Challenges and Opportunities for Education and Research in Weed Science. Front. Plant Sci. 2017, 8, 1537. [Google Scholar] [CrossRef] [PubMed]
- Neve, P.; Barney, J.N.; Buckley, Y.; Cousens, R.D.; Graham, S.; Jordan, N.R.; Lawton-Rauh, A.; Liebman, M.; Mesgaran, M.B.; Schut, M.; et al. Reviewing research priorities in weed ecology, evolution and management: A horizon scan. Weed Res. 2018, 58, 250–258. [Google Scholar] [CrossRef]
- Olsen, R.S.; Cole, V.C.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Circular No. 939; USDA: Washington, DC, USA, 1954; pp. 4–6.
- Peech, M.; Alexander, L.T.; Dean, L.A.; Reed, J.F. Methods of Soil Analysis for Soil Fertility Investigations; Circular No. 757; USDA: Washington, DC, USA, 1947; pp. 7–11.
- Wuest, S.B.; Albrecht, S.L.; Skirvin, K.W. Crop residue position and interference with wheat seedling development. Soil Tillage Res. 2000, 55, 175–182. [Google Scholar] [CrossRef]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef]
- Garland, J.L. Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol. Biochem. 1996, 28, 213–221. [Google Scholar] [CrossRef]
- Zak, J.C.; Willig, M.R.; Moorhead, D.L.; Wildman, H.G. Functional diversity of microbial communities: A quantitative approach. Soil Biol. Biochem. 1994, 26, 1101–1108. [Google Scholar] [CrossRef]
| Control | Gorse | Sig. | Control | Scotch Broom | Sig. | ||
|---|---|---|---|---|---|---|---|
| General effects on weeds | |||||||
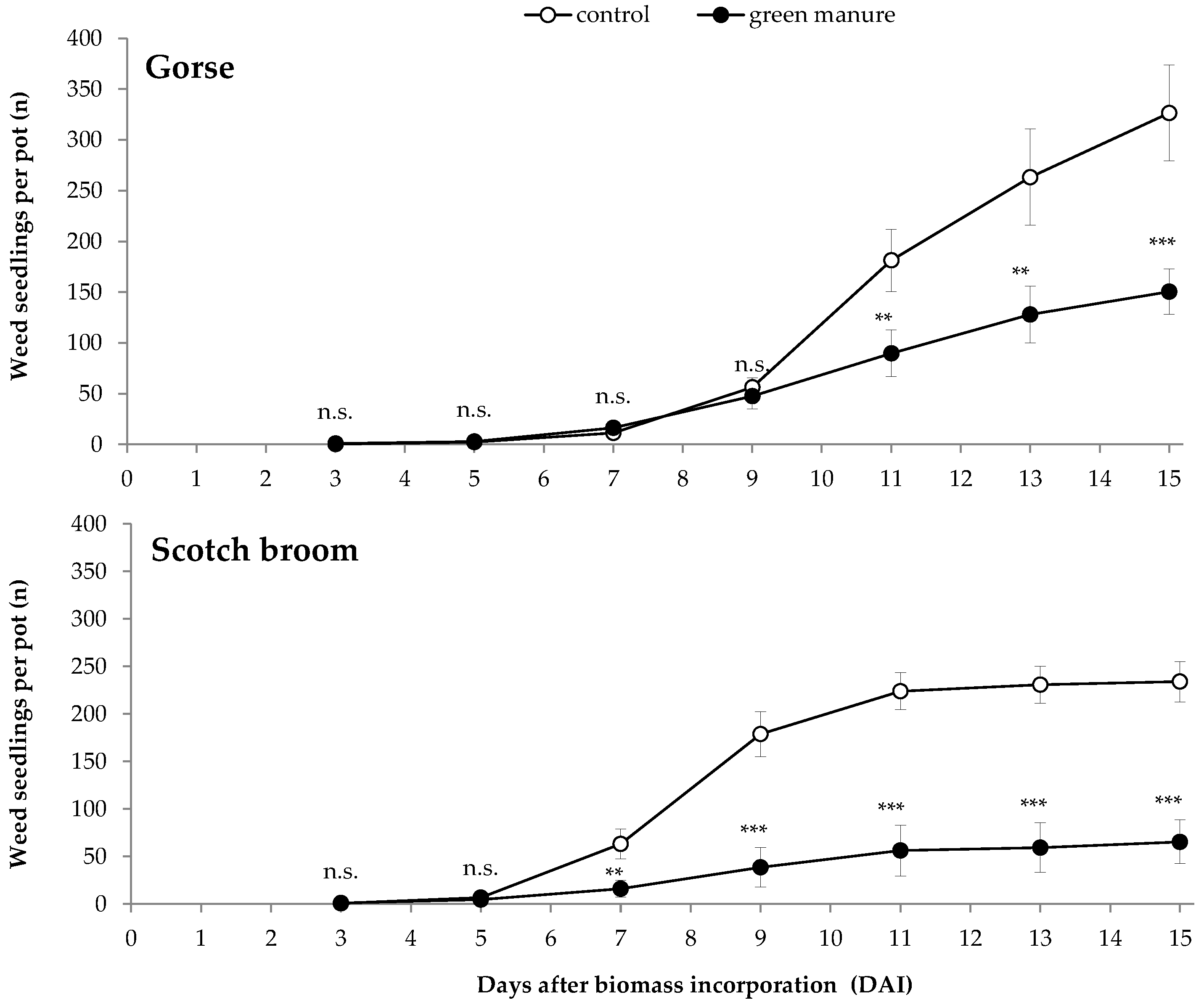
| Seedlings per pot (n) | 322.00 ± 23.48 | 218.25 ± 19.77 | − − − | 191.50 ± 18.41 | 77.50 ± 14.75 | − − − | |
| Dicotyledon seedlings per pot (n) | 289.00 ± 23.85 | 169.25 ± 8.14 | − − − | 158.25 ± 19.45 | 56.50 ± 13.40 | − − − | |
| Monocotyledon seedlings per pot (n) | 33.00 ± 2.83 | 49.00 ± 11.80 | + | 33.25 ± 2.22 | 21.00 ± 2.45 | − − − | |
| Weed biomass (mg) | 1968.63 ± 384.93 | 1228.58 ± 294.21 | − | 2140.40 ± 377.39 | 984.93 ± 279.93 | − − | |
| Dicotyledons biomass (mg) | 1917.38 ± 369.47 | 1119.18 ± 266.93 | − | 1913.55 ±352.90 | 557.83 ± 230.32 | − − − | |
| Monocotyledons biomass (mg) | 51.25 ± 17.88 | 109.40 ± 47.87 | n.s. | 226.85 ± 67.08 | 427.10 ± 455.06 | n.s. | |
| Effects on weed species | |||||||
| Plants per pot (n) | |||||||
| Convolvulus arvensis | 1.25 ± 0.96 | 2.00 ± 0.82 | n.s. | 2.25 ± 0.96 | 0.50 ± 0.58 | − | |
| Amaranthus retroflexus | 113.25 ± 6.85 | 77.50 ± 6.81 | − − − | 80.25 ± 14.22 | 22.75 ± 8.42 | − − − | |
| Solanum nigrum | 11.75 ± 4.72 | 10.50 ± 1.73 | n.s. | 10.00 ± 2.16 | 11.00 ± 4.55 | n.s. | |
| Portulaca oleracea | 9.25 ± 2.50 | 11.50 ± 3.70 | n.s. | 6.75 ± 0.96 | 3.00 ± 1.63 | − − | |
| Digitaria sanguinalis | 33.00 ± 2.83 | 48.75 ± 11.32 | + | 32.50 ± 2.65 | 20.50 ± 3.32 | − − − | |
| Other dicotyledons | 153.50 ± 23.10 | 67.75 ± 3.50 | − − | 59.00 ± 6.68 | 19.25 ± 5.32 | − − − | |
| Other monocotyledons | 0.00 ± 0.00 | 0.25 ± 0.50 | n.s. | 0.75 ± 0.96 | 0.50 ± 1.00 | n.s. | |
| Plant height (cm) | |||||||
| Convolvulus arvensis | 8.48 ± 6.57 | 6.50 ± 1.00 | n.s. | 11.35 ± 1.69 | 5.25 ± 6.10 | n.s. | |
| Amaranthus retroflexus | 10.38 ± 0.64 | 7.70 ± 0.69 | − − − | 11.65 ± 0.31 | 7.55 ± 1.67 | − − − | |
| Solanum nigrum | 5.59 ± 1.78 | 4.78 ± 0.64 | n.s. | 7.48 ± 1.11 | 7.93 ± 1.03 | n.s. | |
| Portulaca oleracea | 1.87 ± 0.20 | 1.34 ± 0.23 | − | 1.78 ± 0.23 | 1.48 ± 0.47 | n.s. | |
| Digitaria sanguinalis | 7.28 ± 0.84 | 5.93 ± 0.38 | − | 13.10 ± 1.75 | 11.00 ± 1.89 | n.s. | |
| Other dicotyledons | 16.43 ± 2.16 | 12.63 ± 0.82 | − | 15.98 ± 1.00 | 11.68 ± 2.76 | − | |
| Other monocotyledons | 0.00 ± 0.00 | 7.13 ± 14.25 | n.s. | 11.70 ± 21.57 | 11.03 ± 22.05 | n.s. | |
| Aerial biomass per pot (mg) | |||||||
| Convolvulus arvensis | 15.90 ± 12.04 | 17.55 ± 6.80 | n.s. | 38.27 ± 16.68 | 10.65 ± 12.31 | − | |
| Amaranthus retroflexus | 370.75 ± 109.78 | 238.02 ± 71.74 | n.s. | 665.97 ± 109.50 | 137.80 ± 90.32 | − − − | |
| Solanum nigrum | 30.30 ± 16.21 | 34.37 ± 8.50 | n.s. | 52.00 ± 12.68 | 128.37 ± 68.99 | n.s. | |
| Portulaca oleracea | 1.82 ± 1.02 | 4.37 ± 1.87 | n.s. | 3.42 ± 0.95 | 1.82 ± 0.87 | − | |
| Digitaria sanguinalis | 51.25 ± 17.88 | 93.77 ± 20.74 | + | 194.67 ± 61.60 | 142.25 ± 51.89 | n.s. | |
| Other dicotyledons | 1489.60 ± 305.89 | 824.85 ± 199.03 | − − | 1.15 ± 0.26 | 0.28 ± 0.14 | − − − | |
| Other monocotyledons | 0.00 ± 0.00 | 15.62 ± 31.25 | n.s. | 32.17 ± 64.21 | 36.90 ± 73.80 | n.s. |
| Variable | Control | Gorse | Sig. | Control | Scotch Broom | Sig. | |
|---|---|---|---|---|---|---|---|
| Maize seedlings per pot (n) | 2.00 ± 1.41 | 2.75 ± 1.26 | n.s. | 3.50 ± 0.58 | 3.67 ± 1.16 | n.s. | |
| Root length (cm) | 31.79 ± 4.42 | 31.68 ± 6.17 | n.s. | 46.88 ± 7.03 | 46.03 ± 2.67 | n.s. | |
| Plant height (cm) | 43.49 ± 2.58 | 40.74 ± 4.69 | n.s. | 59.85 ± 2.56 | 57.09 ± 1.98 | n.s. | |
| Root biomass per plant (g) | 0.74 ± 0.04 | 0.85 ± 0.12 | n.s. | 0.95 ± 0.06 | 1.08 ± 0.16 | n.s. | |
| Aerial biomass per plant (g) | 0.54 ± 0.09 | 0.48 ± 0.13 | n.s. | 1.00 ± 0.15 | 1.09 ± 0.24 | n.s. | |
| Total biomass per plant (g) | 1.29 ± 0.13 | 1.33 ± 0.23 | n.s. | 1.95 ± 0.19 | 2.17 ± 0.39 | n.s. | |
| SLA (m2 kg−1) | 56.66 ± 2.71 | 60.50 ± 3.56 | n.s. | 59.26 ± 6.04 | 56.26 ± 1.58 | n.s. | |
| Leaf area (cm2) | 34.93 ± 5.55 | 29.29 ± 4.72 | n.s. | 40.03 ± 2.82 | 47.52 ± 7.59 | n.s. | |
| Maize yield (% of total harvest) | 50.17 ± 6.15 | 58.11 ± 11.42 | n.s. | 50.10 ± 7.58 | 70.28 ± 5.25 | + + | |
| Shoot: root ratio | 0.73 ± 0.09 | 0.56 ± 0.11 | − | 1.06 ± 0.13 | 1.01 ± 0.12 | n.s. | |
| rWUE (g L−1) | 0.34 ± 0.06 | 0.30 ± 0.10 | n.s. | 0.57 ± 0.09 | 0.59 ± 0.13 | n.s. |
| Gorse | Scotch Broom | |||||
|---|---|---|---|---|---|---|
| Control Soil | Soil Amendment | Sig. | Control Soil | Soil Amendment | Sig. | |
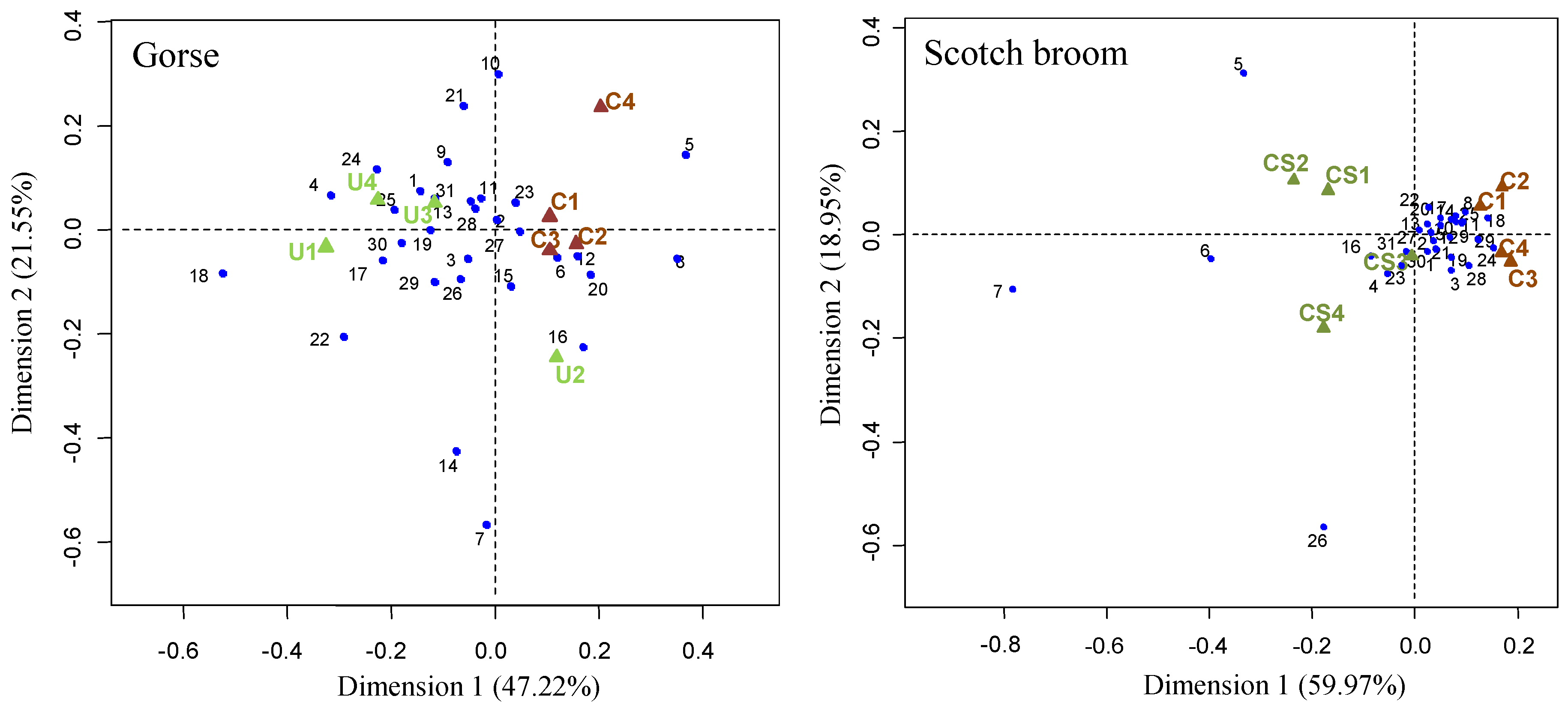
| Richness | 28.00 ± 1.41 | 30.00 ± 0.82 | + | 29.50 ± 1.29 | 30.75 ± 0.50 | n.s. |
| Diversity | 3.12 ± 0.05 | 3.20 ± 0.02 | + | 3.25 ± 0.03 | 3.30 ± 0.02 | + |
| Evenness | 0.97 ± 0.01 | 0.99 ± 0.01 | + | 0.98 ± 0.01 | 0.99 ± 0.01 | + |
| Variable | Control | Gorse | Sig. | Control | Scotch broom | Sig. | |
|---|---|---|---|---|---|---|---|
| pH | 6.86 ± 0.03 | 6.70 ± 0.08 | − | 6.86 ± 0.03 | 6.73 ± 0.29 | n.s. | |
| Soil EC (dS m−1) | 0.15 ± 0.03 | 0.16 ± 0.02 | n.s. | 0.13 ± 0.03 | 0.15 ± 0.02 | n.s. | |
| Organic matter (%) | 5.70 ± 0.12 | 5.58 ± 0.33 | n.s. | 5.25 ± 0.47 | 5.63 ± 0.05 | n.s. | |
| Total N (%) | 0.29 ± 0.02 | 0.30 ± 0.02 | n.s. | 0.30 ± 0.05 | 0.28 ± 0.04 | n.s. | |
| Total C (%) | 3.27 ± 0.15 | 3.38 ± 0.23 | n.s. | 3.32 ± 0.59 | 3.10 ± 0.35 | n.s. | |
| P (mg kg−1) | 63.75 ± 1.89 | 68.00 ± 5.58 | + | 67.50 ± 5.26 | 65.00 ± 4.83 | n.s. | |
| Ca/Mg | 5.50 ± 0.58 | 5.00 ± 0.00 | n.s. | 5.25 ± 0.50 | 6.25 ± 0.50 | + | |
| K/Mg | 0.60 ± 0.00 | 0.70 ± 0.08 | + | 0.63 ± 0.05 | 0.73 ± 0.10 | n.s. | |
| CECe | 12.72 ± 0.76 | 10.80 ± 0.26 | − − | 12.09 ± 0.91 | 12.27 ± 2.22 | n.s. | |
| Exchangeable cations (cmol (+) kg−1): | |||||||
| Ca2+ | 9.58 ± 0.67 | 7.58 ± 0.15 | − − − | 9.00 ± 0.78 | 9.25 ± 2.05 | n.s. | |
| Mg2+ | 1.72 ± 0.07 | 1.60 ± 0.04 | − | 1.66 ± 0.07 | 1.55 ± 0.17 | n.s. | |
| K+ | 1.02 ± 0.06 | 1.12 ± 0.10 | n.s. | 1.04 ± 0.06 | 1.11 ± 0.11 | n.s. | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo-Muras, M.; Puig, C.G.; Souza-Alonso, P.; Pedrol, N. The Phytotoxic Potential of the Flowering Foliage of Gorse (Ulex europaeus) and Scotch Broom (Cytisus scoparius), as Pre-Emergent Weed Control in Maize in a Glasshouse Pot Experiment. Plants 2020, 9, 203. https://doi.org/10.3390/plants9020203
Pardo-Muras M, Puig CG, Souza-Alonso P, Pedrol N. The Phytotoxic Potential of the Flowering Foliage of Gorse (Ulex europaeus) and Scotch Broom (Cytisus scoparius), as Pre-Emergent Weed Control in Maize in a Glasshouse Pot Experiment. Plants. 2020; 9(2):203. https://doi.org/10.3390/plants9020203
Chicago/Turabian StylePardo-Muras, María, Carolina G. Puig, Pablo Souza-Alonso, and Nuria Pedrol. 2020. "The Phytotoxic Potential of the Flowering Foliage of Gorse (Ulex europaeus) and Scotch Broom (Cytisus scoparius), as Pre-Emergent Weed Control in Maize in a Glasshouse Pot Experiment" Plants 9, no. 2: 203. https://doi.org/10.3390/plants9020203
APA StylePardo-Muras, M., Puig, C. G., Souza-Alonso, P., & Pedrol, N. (2020). The Phytotoxic Potential of the Flowering Foliage of Gorse (Ulex europaeus) and Scotch Broom (Cytisus scoparius), as Pre-Emergent Weed Control in Maize in a Glasshouse Pot Experiment. Plants, 9(2), 203. https://doi.org/10.3390/plants9020203