Physiological Response of Miscanthus x giganteus to Plant Growth Regulators in Nutritionally Poor Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Experiment Design
2.3. Physiological Parameters
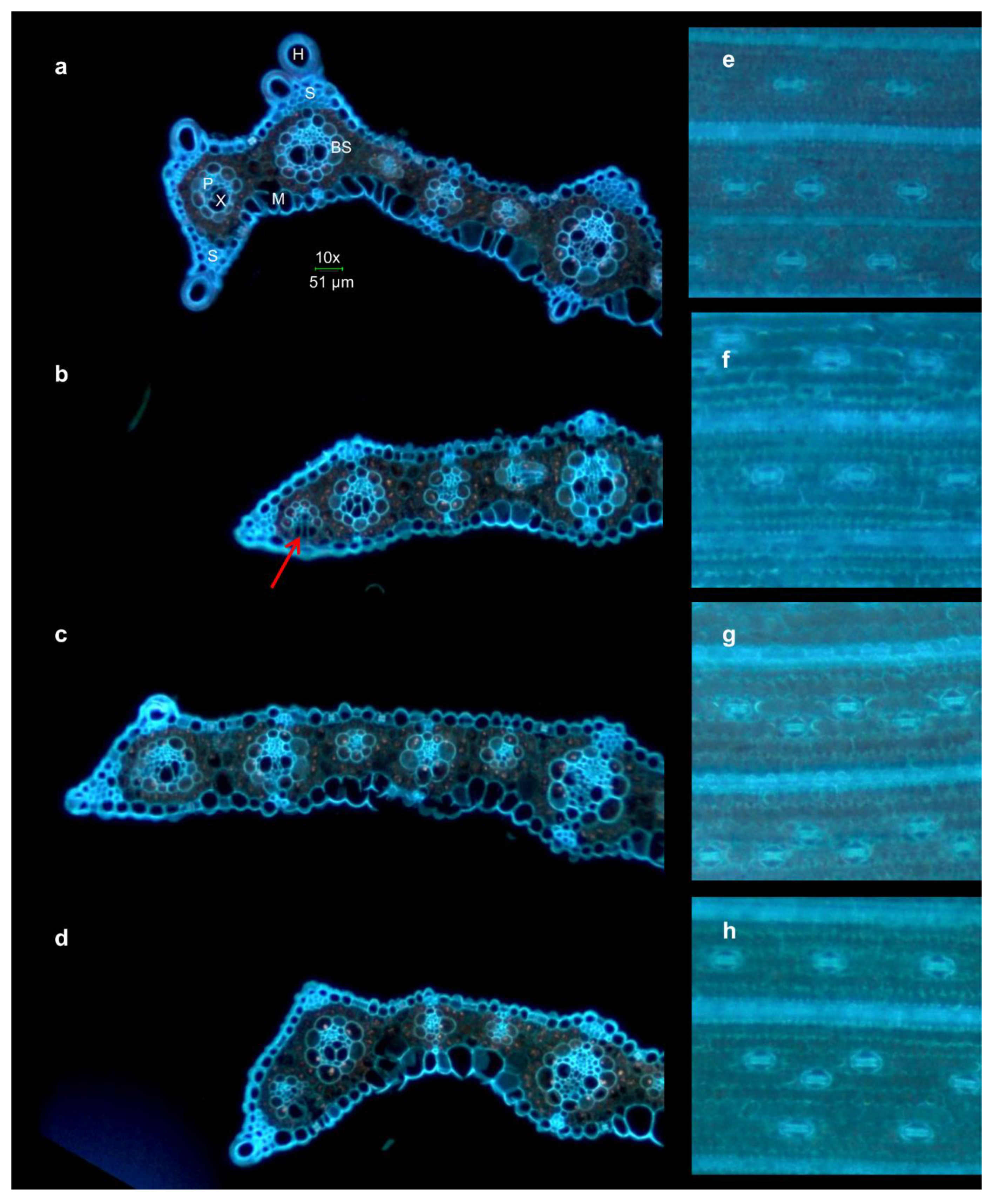
2.4. Microscopy
2.5. Statistical Analysis
3. Results
3.1. Soil Type Effect
3.2. PGRs Treatments Effect
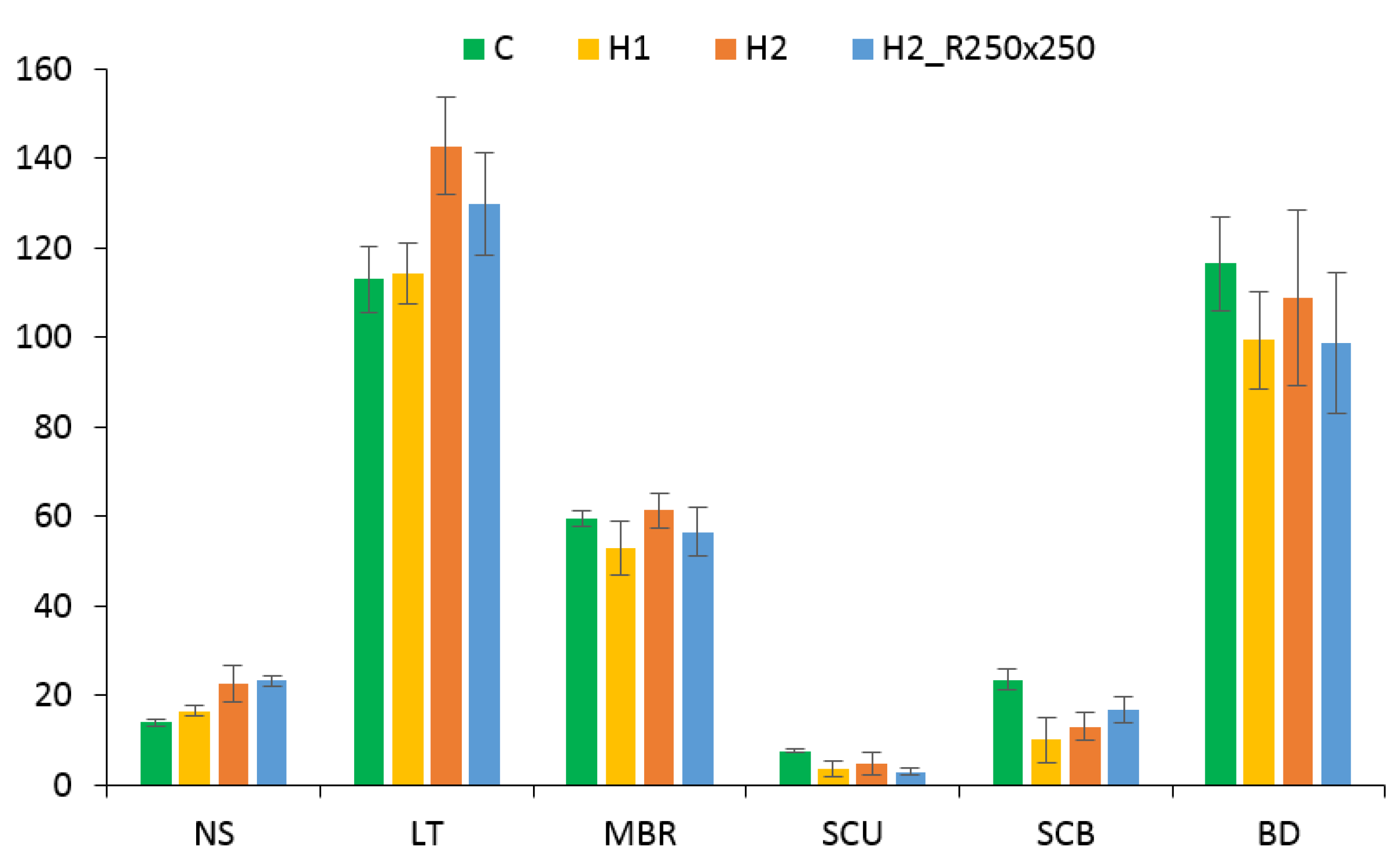
3.3. Effect on Morphology
4. Discussion
4.1. Nutrition Stress
4.2. Physiological Status and Changes in Leaf Fluorescence
4.3. Changes in Leaf Anatomy
4.4. Effect of PGRs
4.5. Recommendations for Miscanthus Cultivation in nutritionally Poor Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A-Abbreviations
| Fo | zero fluorescence, fluorescence level when plastoquinone electron acceptor pool (Qa) is fully oxidized; these acceptors are available to receive light energy due to dark adaptation. |
| Fm | maximum fluorescence, fluorescence level when all electron acceptors are fully reduced, no longer available for photochemistry. |
| Fv | variable fluorescence (Fm − Fo). |
| Fj | fluorescence intensity at 2 ms. |
| Fi | fluorescence intensity at 30 ms. |
| Fk | fluorescence intensity at 300 µs. |
| Fv/Fm | maximum quantum efficiency of Photosystem II; maximum quantum yield of primary photochemistry; typical value for non-stressed plant is 0.85 [69], can be decreased by biotic or abiotic stress; Fv/Fm = (Fm − Fo)/Fm. |
| Tfm | time to achieve maximum fluorescence; shorter Tfm can indicate stress of the plant. |
| M0 | slope at the origin of the fluorescence rise; M0 = 4 (Fk − Fo)/(Fm − Fo). |
| Vj | relative variable fluorescence at 2 ms; Vj = (Fj − Fo)/(Fm − Fo). |
| Vi | relative variable fluorescence at 30 ms; Vi = (Fi − Fo)/(Fm − Fo). |
| TR0/RC | trapping at time zero per RC; TR0/RC = M0/Vj. |
| DI0/RC | dissipation at time zero, per RC; DI0/RC = (ABS/RC) − (TR0/ABS) = M0 (1/Vj) [1/(Fo/Fm)]. |
| ET0/RC | electron transport at time zero per RC; ET0/RC = (M0/Vj) (1 − Vj). |
| RE0/RC | electron flux leading to the reduction of the PS I end acceptor. RE0/RC = M0 (1/Vj) (1 − Vi). |
| ABS/CS0 | absorption at time zero per CS; ABS/CS0 = Fo. |
| TR0/CS0 | trapping at time zero per CS; TR0/CS0 = (TR0/ABS) (ABS/CS0) = Fo [1 − (Fo/Fm)]. |
| DI0/CS0 | dissipation at time zero per CS; DI0/CS0 = (ABS/CS0) − (TR0/CS0) = Fo − {Fo [1 − (Fo/Fm)]}. |
| ETo/CS0 | electron transport at time zero per CS; ET0/CS0 = [1 − (Fo/Fm)] (1 − Vj) Fo. |
| RE0/CS0 | The flux of electrons from QA- to final PSI acceptors per cross section of PSII at time zero; RE0/CS0 = [1 − (F0/Fm)] (1 − Vj) [(1 − Vi)/(1 − Vj)] Fo. |
| ABS/CSm | absorption at maximum time per CS; ABS/CSm = Fm. |
| TR0/CSm | trapping at maximum time per CS; TR0/CSm = (TR0/ABS) (ABS/CSm) = Fm [1 − (Fo/Fm)]. |
| DI0/CSm | dissipation at maximum time per CS; DI0/CSm = (ABS/CSm) − (TR0/CSm) = Fm − {Fm [1 − (Fo/Fm)]}. |
| ETo/CSm | electron transport at maximum time per CS; ET0/CSm = [1 − (Fo/Fm)] (1 − Vj) Fm. |
| RE0/CSm | The flux of electrons from QA- to final PSI acceptors per cross section of PSII at maximum time; RE0/CS0 = [1 − (F0/Fm)] (1 − Vj) [(1 − Vi)/(1 − Vj)] Fm. |
| P.I. | performance index, vitality index; PI abs = {[1 − (Fo/Fm)]/(M0/Vj)} [(Fm − Fo)/Fo] [(1 − Vj)/Vj]. |
References
- United Nations Sustainable development knowledge platform. Available online: https://sustainabledevelopment.un.org/?menu=1300 (accessed on 7 August 2019).
- Gerwin, W.; Repmann, F.; Galatsidas, S.; Vlachaki, D.; Gounaris, N.; Baumgarten, W.; Volkmann, C.; Keramitzis, D.; Kiourtsis, F.; Freese, D. Assessment and quantification of marginal lands for biomass production in Europe using soil-quality indicators. Soil 2018, 4, 267–290. [Google Scholar] [CrossRef]
- Hodkinson, T.R.; Renvoize, S. Nomenclature of Miscanthus xgiganteus (Poaceae). Kew Bull. 2001, 56, 759. [Google Scholar] [CrossRef]
- Dohleman, F.G.; Long, S.P. More productive than maize in the Midwest: How does miscanthus do it? PLANT Physiol. 2009, 150, 2104–2115. [Google Scholar] [CrossRef]
- Christian, D.G.; Riche, A.B.; Yates, N.E. Growth, yield and mineral content of Miscanthus×giganteus grown as a biofuel for 14 successive harvests. Ind. Crops Prod. 2008, 28, 320–327. [Google Scholar] [CrossRef]
- Devrije, T. Pretreatment of Miscanthus for hydrogen production by Thermotoga elfii. Int. J. Hydrogen Energy 2002, 27, 1381–1390. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass and Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Wanat, N.; Austruy, A.; Joussein, E.; Soubrand, M.; Hitmi, A.; Gauthier-Moussard, C.; Lenain, J.-F.; Vernay, P.; Munch, J.C.; Pichon, M. Potentials of Miscanthus×giganteus grown on highly contaminated Technosols. J. Geochemical Explor. 2013, 126–127, 78–84. [Google Scholar] [CrossRef]
- Pogrzeba, M.; Rusinowski, S.; Sitko, K.; Krzyżak, J.; Skalska, A.; Małkowski, E.; Ciszek, D.; Werle, S.; McCalmont, J.P.; Mos, M.; et al. Relationships between soil parameters and physiological status of Miscanthus x giganteus cultivated on soil contaminated with trace elements under NPK fertilisation vs. microbial inoculation. Environ. Pollut. 2017, 225, 163–174. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Mench, M.; Douay, F. Suitability of Miscanthus species for managing inorganic and organic contaminated land and restoring ecosystem services. A review. J. Environ. Manage. 2014, 143, 123–134. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Erickson, L.; Kharchenko, S.; Stefanovska, T. Sustainable land management: Growing Miscanthus in soils contaminated with heavy metals. J. Environ. Prot. (Irvine,. Calif) 2014, 05, 723–730. [Google Scholar] [CrossRef]
- Rusinowski, S.; Krzyżak, J.; Sitko, K.; Kalaji, H.M.; Jensen, E.; Pogrzeba, M. Cultivation of C4 perennial energy grasses on heavy metal contaminated arable land: Impact on soil, biomass, and photosynthetic traits. Environ. Pollut. 2019, 250, 300–311. [Google Scholar] [CrossRef]
- Beale, C.V.; Long, S.P. Can perennial C4 grasses attain high efficiencies of radiant energy conversion in cool climates? Plant, Cell Environ. 1995, 18, 641–650. [Google Scholar] [CrossRef]
- Dohleman, F.G.; Heaton, E.A.; Leakey, A.D.B.; Long, S.P. Does greater leaf-level photosynthesis explain the larger solar energy conversion efficiency of Miscanthus relative to switchgrass? Plant. Cell Environ. 2009, 32, 1525–1537. [Google Scholar] [CrossRef]
- Jiao, X.; Kørup, K.; Andersen, M.N.; Sacks, E.J.; Zhu, X.-G.; Laerke, P.E.; Jørgensen, U. Can miscanthus C 4 photosynthesis compete with festulolium C 3 photosynthesis in a temperate climate? GCB Bioenergy 2017, 9, 18–30. [Google Scholar] [CrossRef]
- Stavridou, E.; Webster, R.J.; Robson, P.R.H. Novel Miscanthus genotypes selected for different drought tolerance phenotypes show enhanced tolerance across combinations of salinity and drought treatments. Ann. Bot. 2019, 124, 653–674. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; Van Der Linden, G.C.; Schwarz, K.U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.L.; Dolstra, O.; Donnison, I.S.; et al. Progress on optimizing miscanthus biomass production for the european bioeconomy: Results of the EU FP7 project OPTIMISC. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Wagner, M.; Mangold, A.; Lask, J.; Petig, E.; Kiesel, A.; Lewandowski, I. Economic and environmental performance of miscanthus cultivated on marginal land for biogas production. GCB Bioenergy 2019, 11, 34–49. [Google Scholar] [CrossRef]
- Nebeská, D.; Pidlisnyuk, V.; Stefanovska, T.; Trögl, J.; Shapoval, P.; Popelka, J.; Černý, J.; Medkow, A.; Kvak, V.; Malinská, H. Impact of plant growth regulators and soil properties on Miscanthus x giganteus biomass parameters and uptake of metals in military soils. Rev. Environ. Health 2019, 34, 283–291. [Google Scholar] [CrossRef]
- Ponomarenko, S.P.; Terek, O.I.; Grytsaenko, Z.M.; Babayants, O.V.; Moiseeva, T.V.; Wenxiu, H.; Eakin, D. Bioregulation of growth and development of plants: Plant growth regulators in crop science. In Bioregulyatsiya mikrobnorastitel’nykh system [Bioregulation of microbial-plant systems]; Ponomarenko, S.P., Iutynska, H.O., Eds.; Nichlava: Kiev, Ukraine, 2010; pp. 251–291, [in Russian]. [Google Scholar]
- Baker, N.R. A possible role for photosystem II in environmental perturbations of photosynthesis. Physiol. Plant. 1991, 81, 563–570. [Google Scholar] [CrossRef]
- Shulaev, V.; Cortes, D.; Miller, G.; Mittler, R. Metabolomics for plant stress response. Physiol. Plant. 2008, 132, 199–208. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Barton, L.; Hemming, B.C. Iron chelation in plants and soil microorganisms; Academic Press: Cambridge, MA, USA, 1993; ISBN 0120798700. [Google Scholar]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. CRC Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll fluorescence analysis: A guide to good practice and understanding some new applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Łukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102. [Google Scholar] [CrossRef]
- Lazár, D. Chlorophyll a fluorescence induction. Biochim. Biophys. Acta - Bioenerg. 1999, 1412, 1–28. [Google Scholar] [CrossRef]
- Butler, W.L. Energy Distribution in the Photochemical Apparatus of Photosynthesis. Annu. Rev. Plant Physiol. 1978, 29, 345–378. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.G. Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteriatle. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Strasser, R.J.; Greppin, H. Primary reactions of photochemistry in higher plants. In Photosynthesis III; Akoyunoglou, G., Ed.; Balaban International Science Services: Philadelphia, PA, USA, 1981; pp. 717–726. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, the Netherlands, 2004; pp. 321–362. [Google Scholar]
- Butler, W.L.; Strasser, R.J. Tripartite model for the photochemical apparatus of green plant photosynthesis. Proc. Natl. Acad. Sci. USA 1977, 74, 3382–3385. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence as a tool in plant physiology. Photosynth. Res. 1984, 5, 139–157. [Google Scholar] [CrossRef]
- Stirbet, A.; Lazár, D.; Kromdijk, J. Govindjee Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Jiang, C.-D.; Wang, X.; Gao, H.-Y.; Shi, L.; Chow, W.S. Systemic regulation of leaf anatomical structure, photosynthetic performance, and high-light tolerance in sorghum. Plant Physiol. 2011, 155, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.B.; Barclay, G.F. Plant Anatomy and Physiology. In Pharmacognosy; Badal, S., Delgoda, R., Eds.; Elsevier: Amsterdam, the Netherlands, 2017; pp. 45–60. ISBN 9780128021040. [Google Scholar]
- Bilska-Kos, A.; Panek, P.; Szulc-Głaz, A.; Ochodzki, P.; Cisło, A.; Zebrowski, J. Chilling-induced physiological, anatomical and biochemical responses in the leaves of Miscanthus × giganteus and maize (Zea mays L.). J. Plant Physiol. 2018, 228, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Brestic, M.; Zivcak, M. PSII fluorescence techniques for measurement of drought and high temperature stress signal in crop plants: Protocols and applications. In Molecular Stress Physiology of Plants; Rout, G., Das, A., Eds.; Springer: New Delhi, India, 2013; pp. 87–131. ISBN 9788132208075. [Google Scholar]
- DSTU. DSTU ISO 11464-2001: Soil quality. Preliminary preparation of samples for physicalchemical analysis; DSTU: Kyiv, Ukraine, 2001. [Google Scholar]
- Zbíral, J.; Čižmárová, E.; Elena, O.; Rychlý, M.; Vilamová, V.; Srnková, J.; Žalmanová, A. Jednotné pracovní postupy: Analýza půd I; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2016; [in Czech]. [Google Scholar]
- Zbíral, J.; Malý, S.; Váňa, M.; Čuhel, J.; Fojtlová, E.; Čižmár, D.; Žalmanová, A.; Srnková, J.; Obdržálková, E. Jednotné pracovní postupy: Analýza půd III; Central Institute for Supervising and Testing in Agriculture: Brno, Czech Republic, 2011; [in Czech]. [Google Scholar]
- Research Institute for Soil and Water Conservation eKatalog BPEJ. Available online: https://bpej.vumop.cz (accessed on 30 March 2019).
- Trögl, J.; Jirková, I.; Zemánková, P.; Pilařová, V.; Dáňová, P.; Pavlorková, J.; Kuráň, P.; Popelka, J.; Křiklavová, L. Estimation of the quantity of bacteria encapsulated in Lentikats Biocatalyst via phospholipid fatty acids content: A preliminary study. Folia Microbiol. (Praha.) 2013, 58, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kuráň, P.; Trögl, J.; Nováková, J.; Pilařová, V.; Dáňová, P.; Pavlorková, J.; Kozler, J.; Novák, F.; Popelka, J. Biodegradation of spilled diesel fuel in agricultural soil: Effect of humates, zeolite, and bioaugmentation. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Kaur, A.; Chaudhary, A.; Kaur, A.; Choudhary, R.; Kaushik, R. Phospholipid fatty acid – A bioindicator of environment monitoring and assessment in soil ecosystem. Curr. Sci. 2005. [Google Scholar] [CrossRef]
- Oukarroum, A.; Saïd, S.; Madidi, E.; Schansker, G.; Strasser, R.J.; El Madidi, S.; Schansker, G. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of ranks in one-criterion variance analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Porter, M.M.; Niksiar, P. Multidimensional mechanics: Performance mapping of natural biological systems using permutated radar charts. PLoS ONE 2018, 13, e0204309. [Google Scholar] [CrossRef]
- Strašil, Z.; Weger, J.; Hutla, P.; Kára, J. The cultivation of Miscanthus determined for energy use (a summary of long-term monitoring). In Biopaliva z pohledu energetiky a vlivu na životní prostředí; eAGRI Ministerstvo zemědělství: Prague, Czech Republic, 2015; pp. 29–41, [in Czech]. [Google Scholar]
- Cadoux, S.; Riche, A.B.; Yates, N.E.; Machet, J.-M. Nutrient requirements of Miscanthus x giganteus: Conclusions from a review of published studies. Biomass Bioenergy 2012, 38, 14–22. [Google Scholar] [CrossRef]
- Gordana, D.; Jelena, M.; Jela, I.; Ivana, P. Influence of fertilization on Miscanthus × giganteus (Greef et Deu) yield and biomass traits in three experiments in Serbia. Plant Soil Environ. 2017, 63, 189–193. [Google Scholar] [CrossRef]
- Jeżowski, S.; Mos, M.; Buckby, S.; Cerazy-Waliszewska, J.; Owczarzak, W.; Mocek, A.; Kaczmarek, Z.; McCalmont, J.P. Establishment, growth, and yield potential of the perennial grass Miscanthus × giganteus on degraded coal mine soils. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.M.F.; Simister, R.; Roberts, L.A.; Timms-Taravella, E.; Cambler, A.B.; Corke, F.M.K.; Han, J.; Ward, R.J.; Buckeridge, M.S.; Gomez, L.D.; et al. Nutrient and drought stress: Implications for phenology and biomass quality in miscanthus. Ann. Bot. 2019, 124, 553–566. [Google Scholar] [CrossRef]
- Guha, A.; Sengupta, D.; Reddy, A.R. Polyphasic chlorophyll a fluorescence kinetics and leaf protein analyses to track dynamics of photosynthetic performance in mulberry during progressive drought. J. Photochem. Photobiol. B 2013, 119, 71–83. [Google Scholar] [CrossRef]
- Strasser, R.J.; Stirbet, A.D. Heterogeneity of photosystem II probed by the numerically simulated chlorophyll a fluorescence rise (O-J-I-P). Math. Comput. Simul. 1998, 48, 3–9. [Google Scholar] [CrossRef]
- Smethurst, C.F.; Garnett, T.; Shabala, S. Nutritional and chlorophyll fluorescence responses of lucerne (Medicago sativa) to waterlogging and subsequent recovery. Plant Soil 2005, 270, 31–45. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Oukarroum, A.; Alexandrov, V.; Kouzmanova, M.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Allakhverdiev, S.I.; Goltsev, V. Identification of nutrient deficiency in maize and tomato plants by in vivo chlorophyll a fluorescence measurements. Plant Physiol. Biochem. 2014, 81, 16–25. [Google Scholar] [CrossRef]
- Gindel, I. Stomata constellation in the leaves of cotton, maize and wheat plants as a function of soil moisture and environment. Physiol. Plant. 1969, 22, 1143–1151. [Google Scholar] [CrossRef]
- Vaithilingam, C.B.M. Effect of potash on sclerenchyma thickness and silica content in rice. Indian Potash J. 1976, 1, 17–23. [Google Scholar]
- Beaton, J.D.; Sekhon, G.S. Potassium Nutrition of Wheat and Other Small Grains. In Potassium in Agriculture; Munson, R.D., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1985. [Google Scholar]
- Sharma, S.R.; Kolte, S.J. Effect of soil-applied NPK fertilizers on severity of black spot disease (Alternaria brassicae) and yield of oilseed rape. Plant Soil 1994, 167, 313–320. [Google Scholar] [CrossRef]
- Pitman, W.D.; Holt, E.C.; Conrad, B.E.; Bashaw, E.C. Histological differences in moisture-stressed and nonstressed kleingrass forage. Crop Sci. 1983, 23, 793. [Google Scholar] [CrossRef]
- Makbul, S.; Saruhan Güler, N.; Durmuş, N. Changes in anatomical and physiological parameters of soybean under drought stress. Turk J Bot 2011, 35, 369–377. [Google Scholar] [CrossRef]
- Kharytonov, M.; Pidlisnyuk, V.; Stefanovska, T.; Babenko, M.; Martynova, N.; Rula, I. The estimation of Miscanthus×giganteus’ adaptive potential for cultivation on the mining and post-mining lands in Ukraine. Environ. Sci. Pollut. Res. 2019, 26, 2974–2986. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Schwarz, K.-U.; Awty-Carroll, D.; Iurato, A.; Meyer, H.; Greef, J.; Gwyn, J.; Mos, M.; Ashman, C.; Hayes, C.; et al. Breeding strategies to improve Miscanthus as a sustainable source of biomass for bioenergy and biorenewable products. Agronomy 2019, 9, 673. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef]

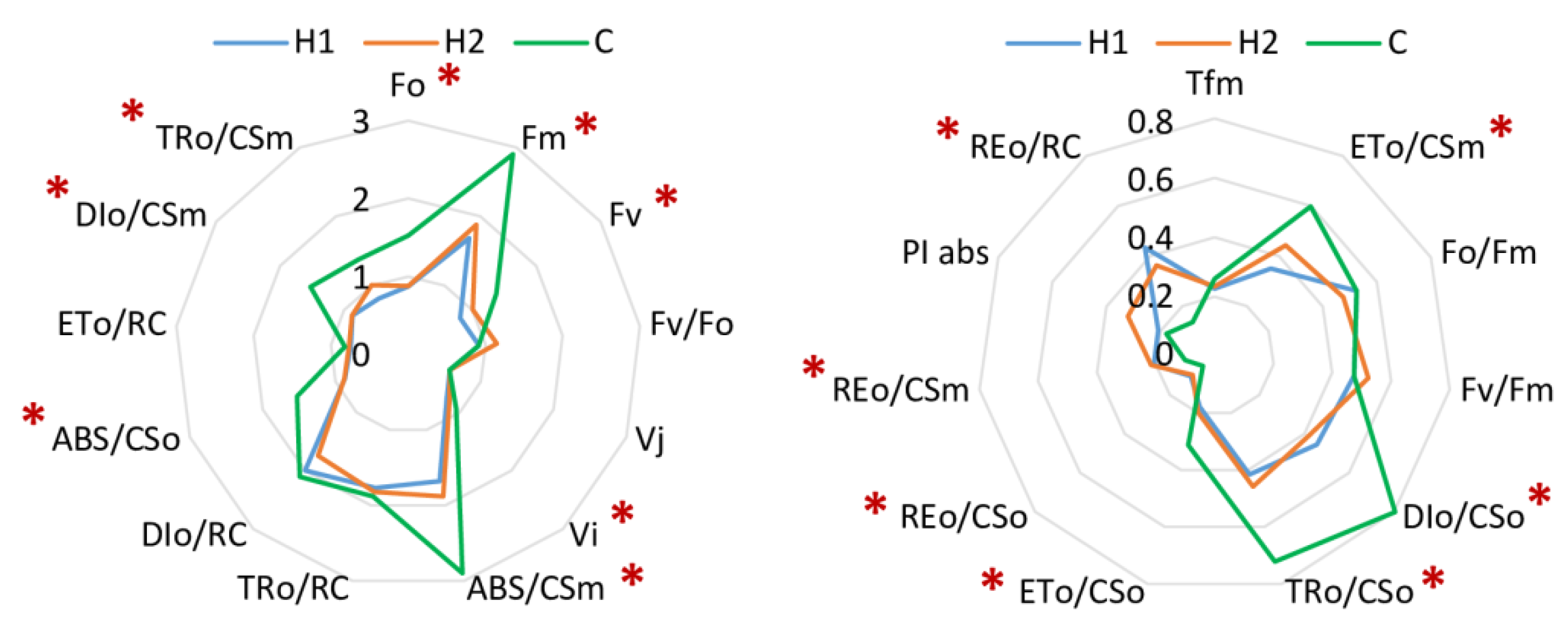
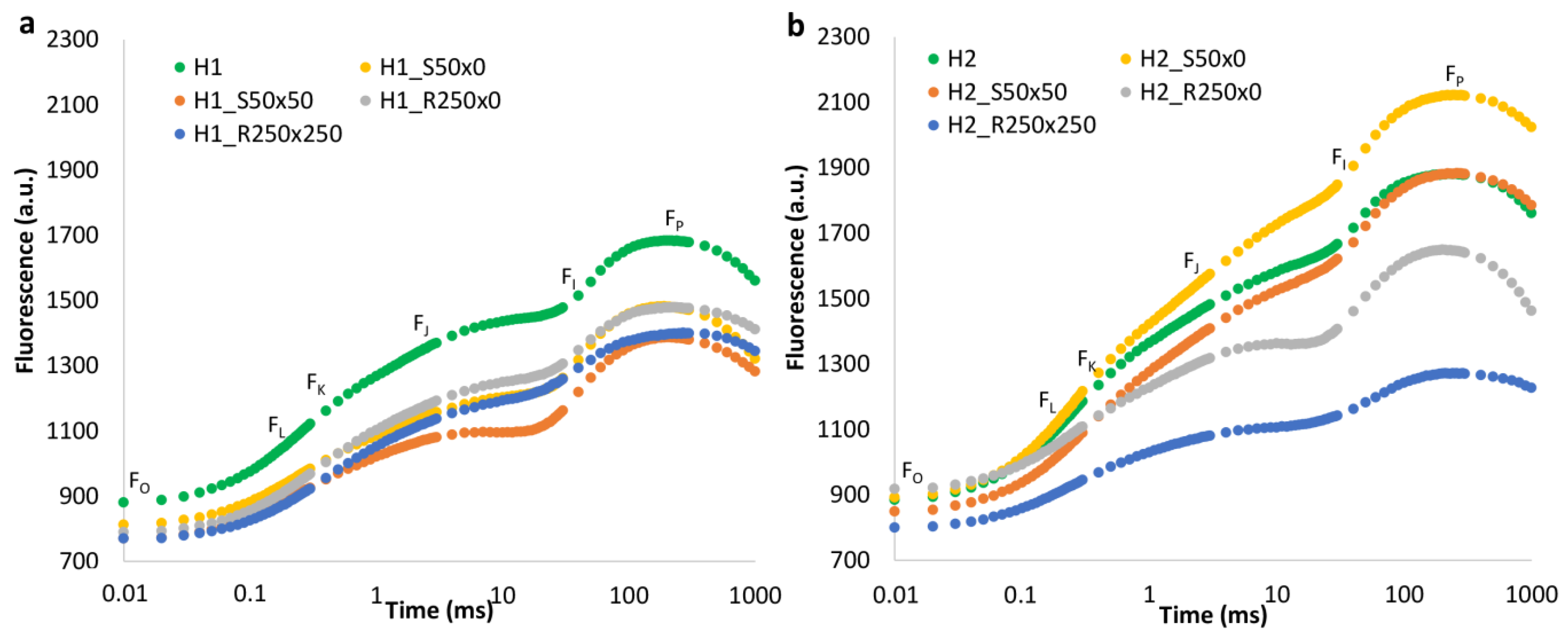
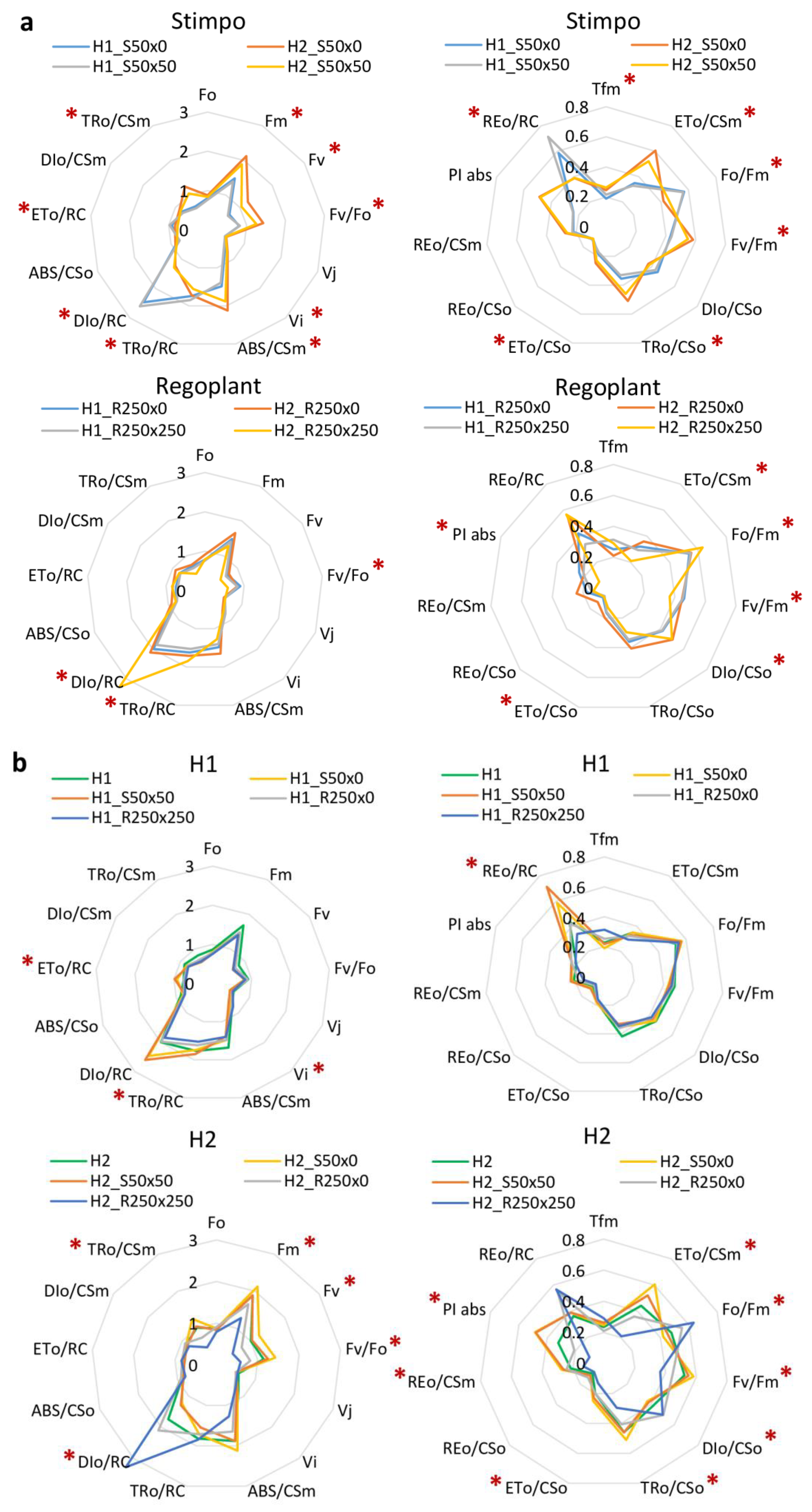
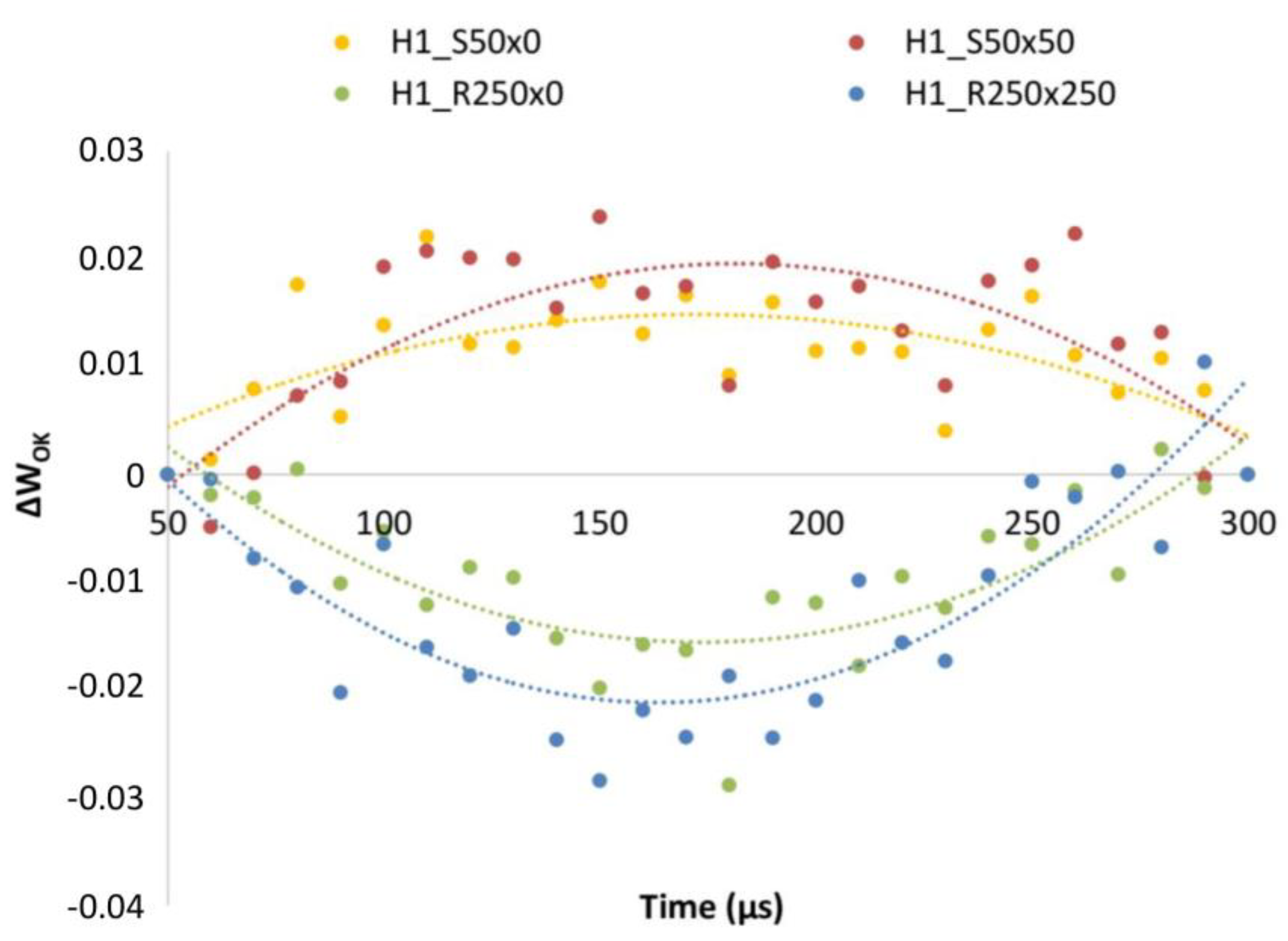

| Parameter | H1 | H2 | C |
|---|---|---|---|
| pH (H2O) | 5.89 | 6.30 | 7.58 |
| Available P [mg/kg] | 24 | 49 | 1227 |
| Available K [mg/kg] | 44 | 57 | 3620 |
| Available Ca [mg/kg] | 74 | 365 | 12,917 |
| Available Mg [mg/kg] | 19 | 40 | 1757 |
| Ntot [%] | 0.02 | 0.02 | 1.52 |
| S [%] | 0.33 | 0.19 | 1.21 |
| Humus [%] | 1.32 | 0.77 | 33.11 |
| PLFAtot [mg/kg] | 3.11 ± 0.69 | 2.31 ± 0.62 | 22.30 ± 3.27 |
| G+/G− PLFA | 0.49 ± 0.12 | 0.31 ± 0.04 | 0.84 ± 0.04 |
| F/B PLFA | 0.14 ± 0.03 | 0.18 ± 0.13 | 0.08 ± 0.01 |
| Respiration [nmol/min/g] | 2.16 ± 0.51 | 0.86 ± 0.46 | 22.13 ± 4.97 |
| PGR Title | Stimpo | Regoplant |
|---|---|---|
| Standard | TU U 20.2-31168762-005:2012 | TU U 20.2-31168762-006:2012 |
| Description | Balanced composition of biologically active compounds: analogues of phytohormones, amino acids, fatty acids, oligosaccharides, microelements, and bioprotective compounds | Balanced composition of biologically active compounds: analogues of phytohormones, amino acids, fatty acids, oligosaccharides, chitosan, microelements, and bioprotective compounds |
| Composition | Emistim C: Cylindrocarpon obtusiusculum-(auxin phytohormones, cytokinin nature, saturated and unsaturated fatty acids, amino acids), carbohydrates, ion biogenic microelements | Emistim C: Cylindrocarpon obtusiusculum - (auxin phytohormones, cytokinin nature, saturated and unsaturated fatty acids, amino acids), carbohydrates, ion biogenic microelements |
| Microbial pesticide “Actofit, 0.2% к.e.”: Natural complex Aversectin C, a product of vital activity of actinobacterium Streptomyces avermytilis | Microbial pesticide “Actofit, 0.2% к.e.”: Natural complex Aversectin C, a product of vital activity of actinobacterium Streptomyces avermytilis | |
| Microelements: Acid boron, Copper sulfuric acid (II) 5-water, ammonium, molybdenum acid, Manganese (II) chloride 4-water, Potassium iodide | “Reakom”: Composition of biogenic microelements (microfertilizer universal on the basis of micronutrient complexonates) | |
| K, Na, Fe, Zn, Mn, Cu, Mg, Ca, Co | K, Fe, Zn, Mn, Cu, Mg, Ca, S, Mo, B, N | |
| Ethanol | Brilliant green | |
| Purified water | Potassium salt of 1-naphthylacetic acid C12H9KO2 | |
| Ethanol | ||
| Purified water |
| Variant Label | Soil | PGR | PGR Concentration [mL/10 L] | Number of Pots | |
|---|---|---|---|---|---|
| Soaking | Spraying | ||||
| C | Compost | - | - | - | 3 |
| H1 | Hradcany 1 | Water | 0 | - | 3 |
| H2 | Hradcany 2 | Water | 0 | - | 3 |
| H1_S50x0 | Hradcany 1 | Stimpo | 50 | - | 3 |
| H2_S50x0 | Hradcany 2 | Stimpo | 50 | - | 3 |
| H1_S50x50 | Hradcany 1 | Stimpo | 50 | 50 | 3 |
| H2_S50x50 | Hradcany 2 | Stimpo | 50 | 50 | 3 |
| H1_R250x0 | Hradcany 1 | Regoplant | 250 | - | 2 |
| H2_R250x0 | Hradcany 2 | Regoplant | 250 | - | 2 |
| H1_R250x250 | Hradcany 1 | Regoplant | 250 | 250 | 2 |
| H2_R250x250 | Hradcany 2 | Regoplant | 250 | 250 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malinská, H.; Pidlisnyuk, V.; Nebeská, D.; Erol, A.; Medžová, A.; Trögl, J. Physiological Response of Miscanthus x giganteus to Plant Growth Regulators in Nutritionally Poor Soil. Plants 2020, 9, 194. https://doi.org/10.3390/plants9020194
Malinská H, Pidlisnyuk V, Nebeská D, Erol A, Medžová A, Trögl J. Physiological Response of Miscanthus x giganteus to Plant Growth Regulators in Nutritionally Poor Soil. Plants. 2020; 9(2):194. https://doi.org/10.3390/plants9020194
Chicago/Turabian StyleMalinská, Hana, Valentina Pidlisnyuk, Diana Nebeská, Anna Erol, Andrea Medžová, and Josef Trögl. 2020. "Physiological Response of Miscanthus x giganteus to Plant Growth Regulators in Nutritionally Poor Soil" Plants 9, no. 2: 194. https://doi.org/10.3390/plants9020194
APA StyleMalinská, H., Pidlisnyuk, V., Nebeská, D., Erol, A., Medžová, A., & Trögl, J. (2020). Physiological Response of Miscanthus x giganteus to Plant Growth Regulators in Nutritionally Poor Soil. Plants, 9(2), 194. https://doi.org/10.3390/plants9020194







