Agrobacterium-Mediated Genetic Transformation of the Medicinal Plant Veratrum dahuricum
Abstract
1. Introduction
2. Materials and Methods
2.1. Embryogenic Callus Induction
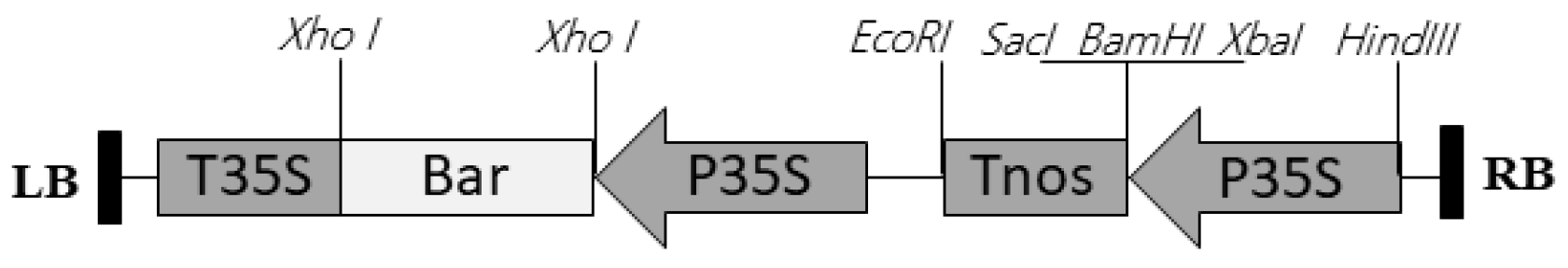
2.2. Transformation and Plant Regeneration
2.3. PCR Analysis
2.4. Reverse Transcription-PCR (RT-PCR) Analysis
2.5. Southern Blot Analysis
2.6. Bar Protein Detection
2.7. Extraction and Targeted HPLC Analysis
2.8. Statistical Analysis
3. Results and Discussion
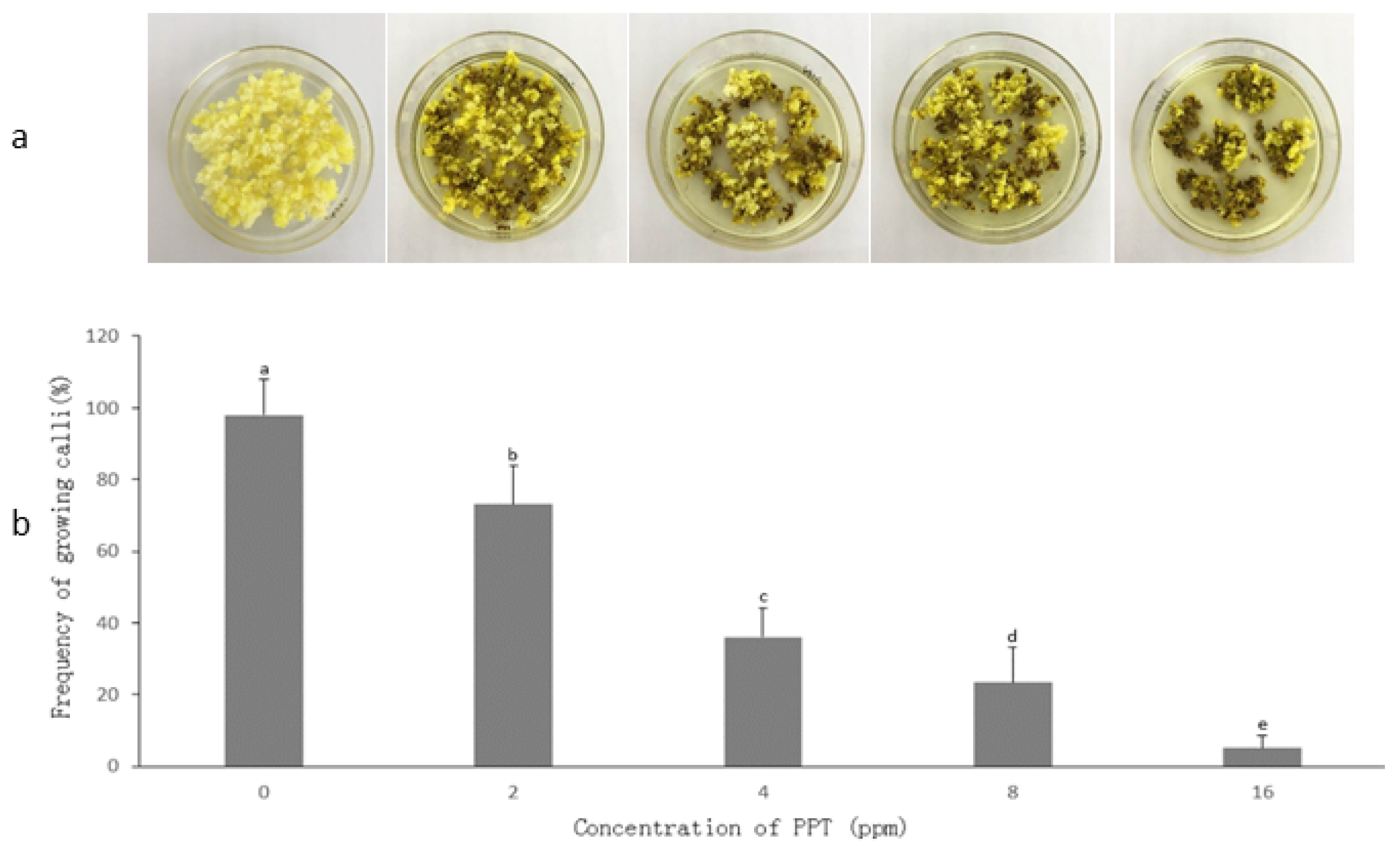
3.1. Embryogenic Callus Induction
3.2. Determination of PPT Concentration for Transgenic Callus Selection
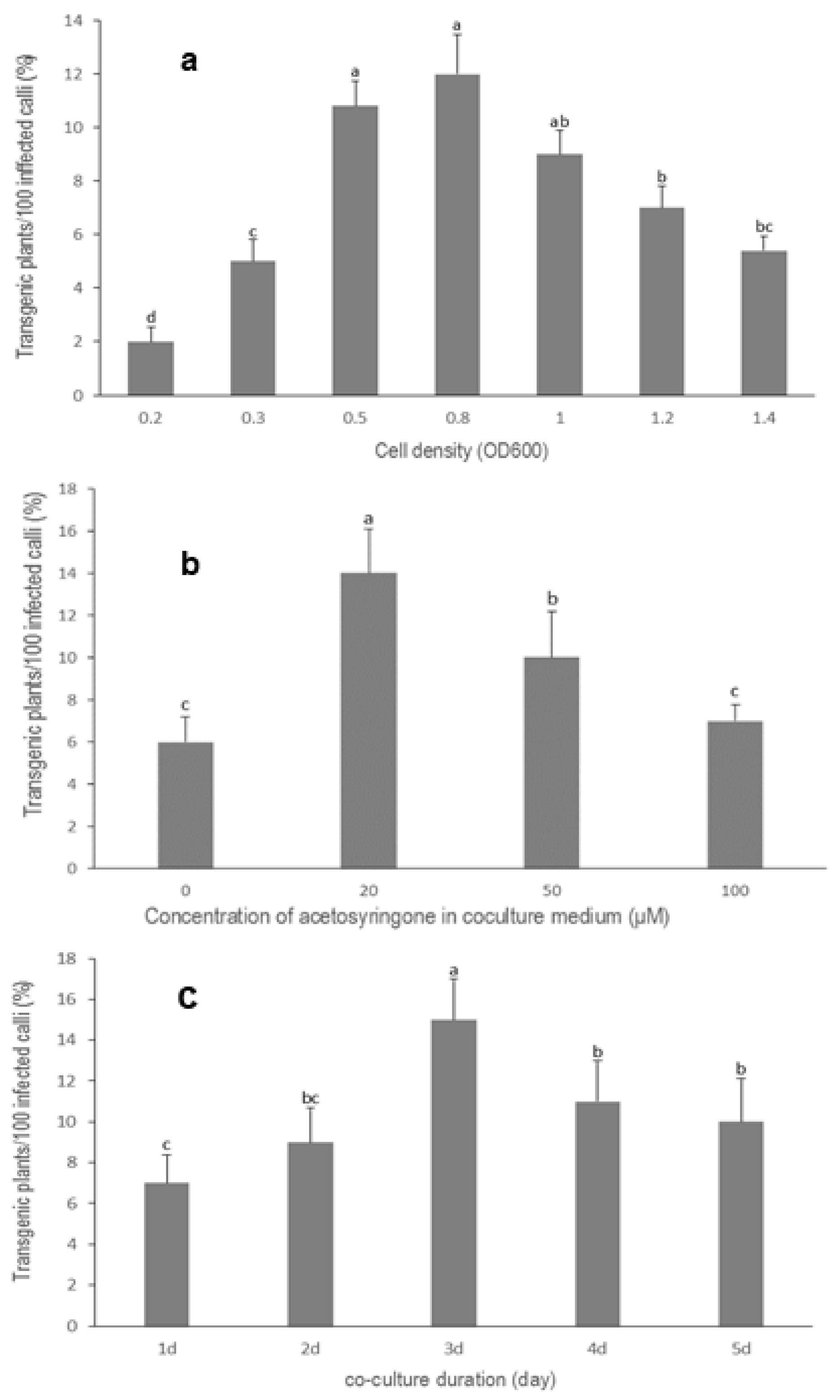
3.3. Optimization of Agrobacterium Cell Density
3.4. Effect of Acetosyringone on Transformation Efficiency
3.5. Influence of Co-Culture Duration on Transformation Efficiency
3.6. Transgenic Callus Selection and Transformed Plant Regeneration
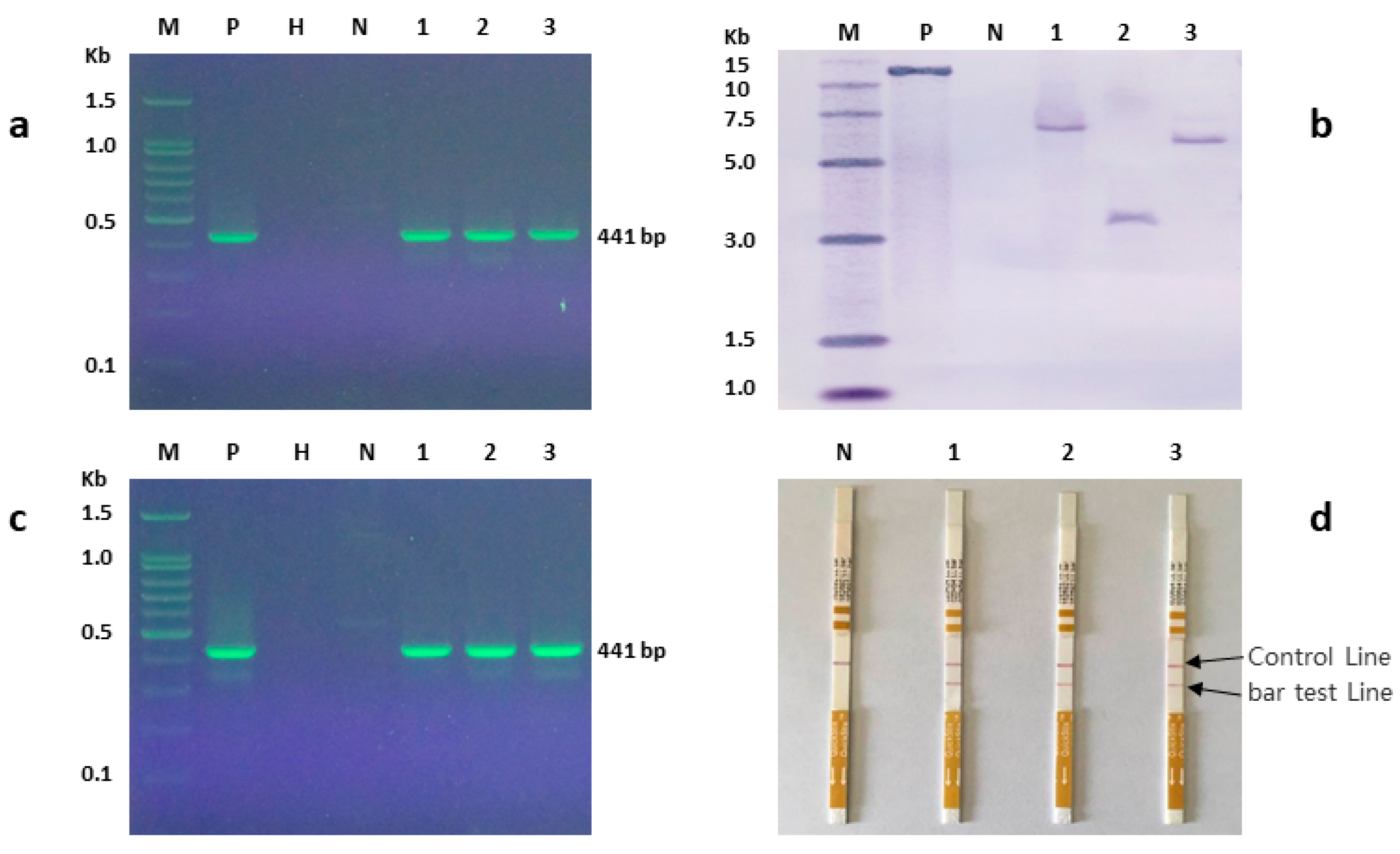
3.7. Molecular Analysis of Putative Transgenic Plants
3.8. Cyclopamine Content of Transgenic Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, Z.J.; Wen, F. Introduction for the pharmacologic action of Veratrum. Chin. Pract. Med. 2016, 20, 282–283. [Google Scholar]
- Zomlefer, W.B.; Whitten, W.M.; Williams, N.H.; Judd, W.S. An overview of Veratrum s.l. (Liliales: Melanthiaceae) and an infrageneric phylogeny based on its sequence data. Syst. Bot. 2003, 28, 250–269. [Google Scholar]
- Keeler, R.F.; Binns, W. Teratogenic compounds of Veratrum californicum (Durand): II. Production of ovine fetal cyclopia by fractions and alkaloid preparations. Can. J. Biochem. 1966, 44, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Keeler, R.F. Teratogenic compounds in Veratrum californicum (Durand) IX. Structure-activity relation. Teratology 1970, 3, 169–173. [Google Scholar] [CrossRef]
- Keeler, R.F. Teratogenic compounds of Veratrum californicum (Durand): X. Cyclopia in rabbits produced by cyclopamine. Teratology 1970, 3, 175–180. [Google Scholar] [CrossRef]
- Taipale, J.; Beachy, P.A. The Hedgehog and Wnt signalling pathways in cancer. Nature 2001, 411, 349–354. [Google Scholar] [CrossRef]
- Watkins, D.N.; Berman, D.M.; Burkholder, S.G.; Wang, B.; Beachy, P.A.; Baylin, S.B. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003, 422, 313–317. [Google Scholar] [CrossRef]
- Bahra, M.; Kamphues, C.; Boas-Knoop, S.; Lippert, S.; Esendik, U.; Schüller, U.; Hartmann, W.; Waha, A.; Neuhaus, P.; Heppner, F. Combination of hedgehog signaling blockage and chemotherapy leads to tumor reduction in pancreatic adenocarcinomas. Pancreas 2012, 41, 222–229. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Behnsawy, H.M.; Shigemura, K.; Meligy, F.Y.; Yamamichi, F.; Yamashita, M.; Haung, W.C.; Li, X.; Miyake, H.; Tanaka, K.; Kawabata, M.; et al. Possible role of sonic hedgehog and epithelial-mesenchymal transition in renal cell cancer progression. Korean J. Urol. 2013, 54, 547–554. [Google Scholar] [CrossRef]
- Gailani, M.R.; Stahle-Backdahl, M.; Leffell, D.J.; Glynn, M.; Zaphiropoulos, P.G.; Pressman, C.; Unden, A.B.; Dean, M.; Brash, D.E.; Bale, A.E.; et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat. Genet. 1996, 14, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Taipale, J.; Chen, J.K.; Cooper, M.K.; Wang, B.; Mann, R.K.; Milenkovic, L.; Scott, M.P.; Beachy, P.A. Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature 2000, 406, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Berman, D.M.; Karhadkar, S.S.; Hallahan, A.R.; Pritchard, J.I.; Eberhart, C.G.; Watkins, D.N.; Chen, J.K.; Cooper, M.K.; Taipale, J.; Olson, J.M.; et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science 2002, 297, 1559–1561. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.L.; Wang, Q.H.; Brown, P.; Peacock, C.; Merchant, A.A.; Brennan, S.; Jones, E.; Mcgovern, K.; Watkins, D.N.; Sakamoto, K.M. Self-renewal of acute lymphocytic leukemia cells is limited by the hedgehog pathway inhibitors cyclopamine and IPI-926. PLoS ONE 2010, 5, e15262. [Google Scholar] [CrossRef]
- Tremblay, M.R.; Nesler, M.; Weatherhead, R.; Castro, A.C. Recent patents for Hedgehog pathway inhibitors for the treatment of malignancy. Expert Opin. Ther. Pat. 2009, 19, 1039–1056. [Google Scholar] [CrossRef]
- Oksman-Caldentey, K.M.; Inze, D. Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 2004, 9, 433–440. [Google Scholar] [CrossRef]
- Ma, R.; Ritala, A.; Oksman-Caldentey, K.M.; Rischer, H. Development of in vitro techniques for the important medicinal plant Veratrum californicum. Planta Med. 2006, 72, 1142–1148. [Google Scholar] [CrossRef]
- Augustin, M.M.; Ruzicka, D.R.; Shukla, A.K.; Augustin, J.M.; Starks, C.M.; O’Neil-Johnson, M.; McKain, M.R.; Evans, B.S.; Barrett, M.D.; Smithson, A.; et al. Elucidating steroid alkaloid biosynthesis in Veratrum californicum: Production of verazine in Sf9 cells. Plant J. 2015, 82, 991–1003. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Müller, A.J.; Grafe, R. Isolation and characterization of cell lines of Nicotiana tabacum lacking nitrate reductase. Mol. Gen. Genet. 1978, 161, 67–76. [Google Scholar] [CrossRef]
- Wang, X.; Han, H. The effect of Potato II medium for triticale anther culture. Plant Sci. Lett. 1984, 36, 237–239. [Google Scholar]
- Telzur, N.; Abbo, S.; Myslabodski, D.; Mizrahi, Y. Modified CTAB procedure for DNA isolation from epiphytic cacti of the genera Hylocereus and Selenicereus (Cactaceae). Plant Mol. Biol. Report. 1999, 17, 249–254. [Google Scholar] [CrossRef]
- Lührs, R.; Lörz, H. Plant regeneration in vitro from embryogenic cultures of spring- and winter-type barley (Hordeum vulgare L.) varieties. Theor. Appl. Genet. 1987, 75, 16–25. [Google Scholar] [CrossRef]
- Ma, R.; Pulli, S. Factors influencing somatic embryogenesis and regeneration ability in somatic tissue culture of spring and winter rye. Agric. Food Sci. 2004, 13, 363–377. [Google Scholar] [CrossRef]
- Armstrong, C.L.; Green, C.E. Establishment and maintenance of friable, embryogenic maize callus and the involvement of l-proline. Planta 1985, 164, 207–214. [Google Scholar] [CrossRef]
- Nhut, D.T. Micropropagation of lily (Lilium longiflorum) via in vitro stem node and pseudo-bulblet culture. Plant Cell Rep. 1998, 17, 913–916. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Sen, S. In vitro response of Scilla siberica. Sci. Hortic. 2002, 95, 51–62. [Google Scholar] [CrossRef]
- Mori, S.; Adachi, Y.; Horimoto, S.; Suzuki, S.; Nakano, M. Callus formation and plant regeneration in various Lilium species and cultivars. In Vitro Cell. Dev. Biol. Plant 2005, 41, 783–788. [Google Scholar] [CrossRef]
- Frame, B.R.; Shou, H.; Chikwamba, R.K.; Zhang, Z.; Xiang, C.; Fonger, T.M.; Pegg, S.E.K.; Li, B.; Dan, S.N.; Pei, D. Agrobacterium tumefaciens-mediated transformation of maize embryos using a standard binary vector system. Plant Physiol. 2002, 129, 13–22. [Google Scholar] [CrossRef]
- Toki, S. Rapid and efficient Agrobacterium-mediated transformation in rice. Plant Mol. Biol. Report. 1997, 15. [Google Scholar] [CrossRef]
- Hu, T.; Metz, S.; Chay, C.; Zhou, H.P.; Biest, N.; Chen, G.; Cheng, M.; Feng, X.; Radionenko, M.; Lu, F.; et al. Agrobacterium-mediated large-scale transformation of wheat (Triticum aestivum L.) using glyphosate selection. Plant Cell Rep. 2003, 21, 1010–1019. [Google Scholar] [CrossRef]
- Paz, M.M.; Martinez, J.C.; Kalvig, A.B.; Fonger, T.M.; Wang, K. Improved cotyledonary node method using an alternative explant derived from mature seed for efficient Agrobacterium-mediated soybean transformation. Plant Cell Rep. 2006, 25, 206–213. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Gu, W.; Cai, T.; Tagliani, L.; Hondred, D.; Bond, D.; Schroeder, S.; Rudert, M.; Pierce, D. High throughput genetic transformation mediated by Agrobacterium tumefaciens in maize. Mol. Breed. 2002, 8, 323–333. [Google Scholar] [CrossRef]
- Kumar, R.; Mamrutha, H.M.; Kaur, A.; Venkatesh, K.; Sharma, D.; Singh, G.P. Optimization of Agrobacterium-mediated transformation in spring bread wheat using mature and immature embryos. Mol. Biol. Rep. 2019, 46, 1845–1853. [Google Scholar] [CrossRef]
- Khan, S.; Fahim, N.; Singh, P.; Rahman, L.U. Agrobacterium tumefaciens mediated genetic transformation of Ocimum gratissimum: A medicinally important crop. Ind. Crops Prod. 2015, 71, 138–146. [Google Scholar] [CrossRef]
- Saha, P.; Datta, K.; Majumder, S.; Sarkar, C.; China, S.P.; Sarkar, S.N.; Sarkar, D.; Datta, S.K. Agrobacterium mediated genetic transformation of commercial jute cultivar Corchorus capsulariscv. JRC 321 using shoot tip explants. Plant Cell Tissue Organ Cult. 2014, 118, 313–326. [Google Scholar] [CrossRef]
- Sheikholeslam, S.N.; Weeks, D.P. Acetosyringone promotes high efficiency transformation of Arabidopsis thaliana explants by Agrobacterium tumefaciens. Plant Mol. Biol. 1987, 8, 291–298. [Google Scholar] [CrossRef]
- Mishra, S.; Sangwan, R.S.; Bansal, S.; Sangwan, N.S. Efficient genetic transformation of Withania coagulans (Stocks) Dunal mediated by Agrobacterium tumefaciens from leaf explants of in vitro multiple shoot culture. Protoplasma 2013, 250, 451–458. [Google Scholar] [CrossRef]
- Markandan, M.; Subramanyam, K.; Ishwarya, R.; Elayaraja, D.; Ganapathi, A. Assessment of factors influencing the tissue culture-independent Agrobacterium-mediated in planta genetic transformation of okra [Abelmoschus esculentus (L.) Moench]. Plant Cell Tissue Organ Cult. 2015, 123, 309–320. [Google Scholar]
- Horn, M.E.; Harkey, R.L.; Vinas, A.K.; Drees, C.F.; Barker, D.K.; Lane, J.R. Use of Hi II-Elite inbred hybrids in agrobacterium-based transformation of maize. In Vitro Cell. Dev. Biol. Plant 2006, 42, 359–366. [Google Scholar] [CrossRef]
- Yang, X.; Yu, X.; Zhou, Z.; Ma, W.; Tang, G. A high-efficiency Agrobacterium tumefaciens mediated transformation system using cotyledonary node as explants in soybean (Glycine max L.). Acta Physiol. Plant. 2016, 38, 60. [Google Scholar] [CrossRef]
- Kaneko, K.; Mitsuhashi, H.; Hirayama, K.; Ohmori, S. 11-Deoxojervine as a precursor for jervine biosynthesis in Veratrum grandiflorum. Phytochemistry 1970, 9, 2497–2501. [Google Scholar] [CrossRef]
- Turner, M.W.; Cruz, R.; Mattos, J.; Baughman, N.; Elwell, J.; Fothergill, J.; Nielsen, A.; Brookhouse, J.; Bartlett, A.; Malek, P.; et al. Cyclopamine bioactivity by extraction method from Veratrum californicum. Bioorg. Med. Chem. 2016, 24, 3752–3757. [Google Scholar] [CrossRef]
- Keeler, R.F.; Binns, W. Teratogenic compounds of Veratrum californicum as a function of plant part, stage, and site of growth. Phytochemistry 1971, 10, 1765–1769. [Google Scholar] [CrossRef]
- Krysan, P.J.; Young, J.C.; Sussman, M.R. T-DNA as an Insertional Mutagen in Arabidopsis. Plant Cell. 1999, 11, 2283–2290. [Google Scholar] [CrossRef]
- Tamura, K.; Kawabayashi, T.; Shikanai, T.; Hara-Nishimura, I. Decreased expression of a gene caused by a T-DNA insertion in an adjacent gene in Arabidopsis. PLoS ONE 2016, 11, e0147911. [Google Scholar] [CrossRef]


| Plant Source | Contents of Steroid Alkaloids (mg/100 g dw) | |||
|---|---|---|---|---|
| Cyclopamine | Jervine | Veratramine | Total | |
| Transgenic plant | 3.89a | 12.79a | 26.54a | 43.22a |
| Non-transformed control plant | 3.83a | 13.56a | 25.04a | 42.43a |
| Wild collected control plant | 4.24a | 11.43a | 23.36a | 39.03a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Yu, Z.; Cai, Q.; Li, H.; Dong, Y.; Oksman-Caldentey, K.-M.; Rischer, H. Agrobacterium-Mediated Genetic Transformation of the Medicinal Plant Veratrum dahuricum. Plants 2020, 9, 191. https://doi.org/10.3390/plants9020191
Ma R, Yu Z, Cai Q, Li H, Dong Y, Oksman-Caldentey K-M, Rischer H. Agrobacterium-Mediated Genetic Transformation of the Medicinal Plant Veratrum dahuricum. Plants. 2020; 9(2):191. https://doi.org/10.3390/plants9020191
Chicago/Turabian StyleMa, Rui, Zhijing Yu, Qinan Cai, Haiyun Li, Yingshan Dong, Kirsi-Marja Oksman-Caldentey, and Heiko Rischer. 2020. "Agrobacterium-Mediated Genetic Transformation of the Medicinal Plant Veratrum dahuricum" Plants 9, no. 2: 191. https://doi.org/10.3390/plants9020191
APA StyleMa, R., Yu, Z., Cai, Q., Li, H., Dong, Y., Oksman-Caldentey, K.-M., & Rischer, H. (2020). Agrobacterium-Mediated Genetic Transformation of the Medicinal Plant Veratrum dahuricum. Plants, 9(2), 191. https://doi.org/10.3390/plants9020191







