Chemical Analysis of the Essential Oil from Siparuna echinata (Kunth) A. DC. (Siparunaceae) of Ecuador and Isolation of the Rare Terpenoid Sipaucin A
Abstract
1. Introduction
2. Results
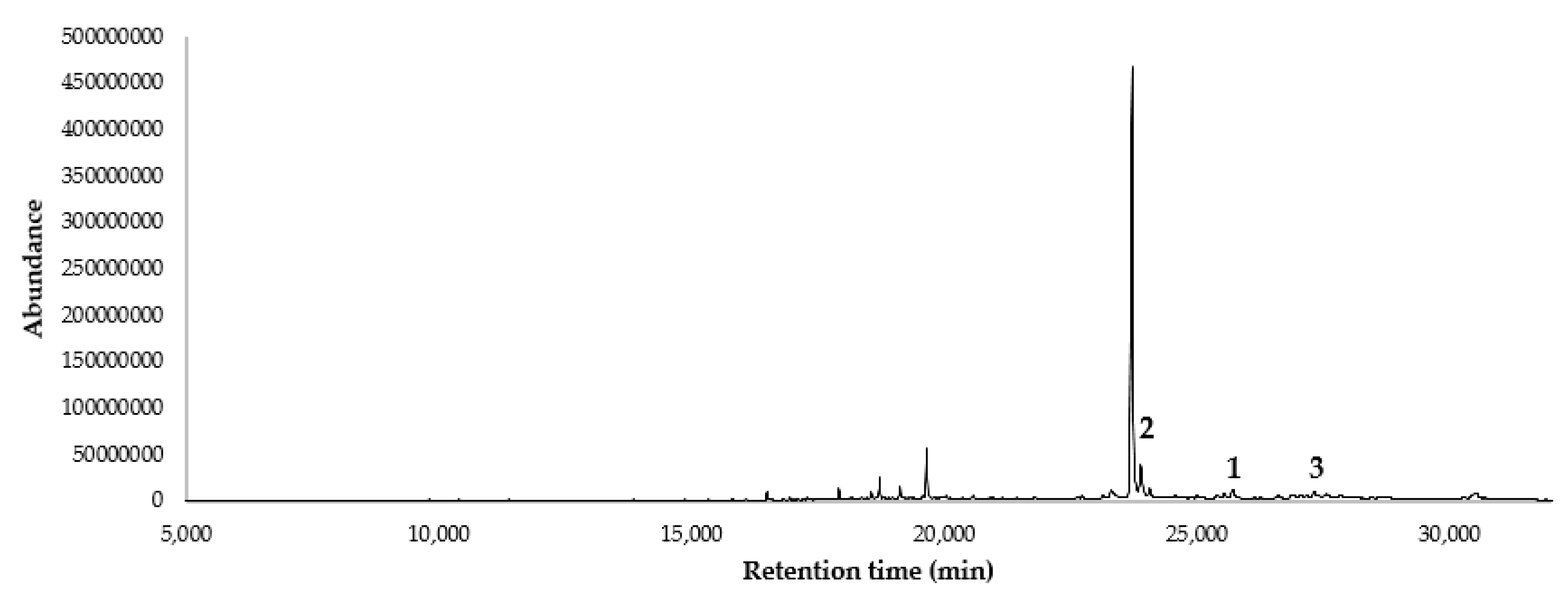
2.1. Characterization of Sipaucin A
2.2. Essential Oil Analysis
Enantioselective GC Analysis
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Plant Material
4.3. Extraction and Isolation of Sipaucin A
4.4. Analysis of the EO
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Worldatlas. 17 Most Ecologically Diverse Countries on Earth. Available online: http://www.worldatlas.com/articles/ecologically-megadiverse-countries-of-the-world.html (accessed on 10 December 2019).
- De la Torre, L.; Navarrete, H.; Muriel, P.; Macías, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador, Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador y Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus. Q. Aarhus Q. Ecuad. 2008, 55. [Google Scholar]
- Panda, S.K.; Mohanta, Y.K.; Padhi, L.; Park, Y.H.; Mohanta, T.K.; Bae, H. Large scale screening of ethnomedicinal plants for identification of potential antibacterial compounds. Molecules 2016, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, P.; Krishnamoorthy, G.; Kannan, S.; Marudhamuthu, M. Bioactive potential of secondary metabolites derived from medicinal plant endophytes. Egypt. J. Basic Appl. Sci. 2018, 5, 303–312. [Google Scholar] [CrossRef]
- Malagón, O.; Ramírez, J.; Andrade, J.M.; Morocho, V.; Armijos, C.; Gilardoni, G. Phytochemistry and ethnopharmacology of the Ecuadorian flora. A Review. Nat. Prod. Commun. 2016, 11, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, S.; Bec, N.; Larroque, C.; Ramírez, J.; Sgorbini, B.; Bicchi, C. Chemical, enantioselective, and sensory analysis of a cholinesterase inhibitor essential oil from Coreopsis triloba S.F. Blake (Asteraceae). Plants 2019, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Tene, V.; Malagón, O.; Finzi, P.V.; Vidari, G.; Armijos, C.; Zaragoza, T. An ethnobotanical survey of medicinal plants used in loja and zamora-chinchipe, ecuador. J. Ethnopharmacol. 2007, 111, 63–81. [Google Scholar] [CrossRef]
- Jenett-Siems, K.; Kraft, C.; Siems, K.; Jakupovic, J.; Solis, P.N.; Gupta, M.P.; Ulrich, B. Sipaucins A–C, sesquiterpenoids from Siparuna pauciflora. Phytochemistry 2003, 63, 377–381. [Google Scholar] [CrossRef]
- Negri, G.; de Santi, D.; Tabach, R. Chemical composition of hydroethanolic extracts from Siparuna guianensis, medicinal plant used as anxiolytics in Amazon region. Braz. J. Pharmacogn. 2012, 22, 1024–1034. [Google Scholar] [CrossRef]
- Renner, S.S.; Won, H. Repeated evolution of dioecy from monoecy in siparunaceae (Laurales). Syst. Biol. 2001, 50, 700–712. [Google Scholar] [CrossRef]
- Marques, C.A.; Leitão, G.G.; Bizzo, H.R.; Peixoto, A.L.; Viera, R.C. Anatomia e análise de óleo essencial das folhas de Hennecartia omphalandra. J. Poisson (Monimiaceae). Braz. J. Pharmacogn. 2009, 19, 95–105. [Google Scholar] [CrossRef]
- González, G.; Archila, M. Anti-bacterial action of extracts and fractions from Siparuna sessiliflora Kunth A. DC (limoncillo). Rev. Cuba Plantas Med. 2012, 17, 65–72. [Google Scholar]
- Leitão, G.G.; Simas, N.K.; Soares, S.; De Brito, A.; Claros, B.; Brito, T.; Delle, F. Chemistry and pharmacology of Monimiaceae: A special focus on Siparuna and Mollinedia. J. Ethnopharmacol. 1999, 65, 87–102. [Google Scholar] [CrossRef]
- Leitão, G.; El-Adji, S.; Araújo, W.; Leitão, S.; Brown, L. Separation of free and glycosylated flavonoids from Siparuna guianensis by gradient and isocratic CCC. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2041–2051. [Google Scholar] [CrossRef]
- Fischer, D.C.H.; Gualda, N.; Bachiega, D.; Carvalho, C.; Lupo, F.; Bonotto, S.V.; Alves, M.O.; Yogi, Á. In vitro screening for antiplasmodial activity of isoquinoline alkaloids from Brazilian plant species. Acta Trop. 2004, 92, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Tempone, A.G.; Treiger, S.; De Andrade, H.F.; De Amorim Gualda, N.C.; Yogi, Á.; Salerno Carvalho, C.; Bachiega, D.; Lupo, F.N.; Bonotto, S.V.; Fischer, D.C.H. Antiprotozoal activity of Brazilian plant extracts from isoquinoline alkaloid-producing families. Phytomedicine 2005, 12, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Kalemba, D.; Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003, 10, 813–829. [Google Scholar] [CrossRef]
- Missouri Botanical Garden. Available online: https://www.tropicos.org/Name/21200125?tab=distribution (accessed on 12 December 2019).
- The New York Botanical Garden. 2005. Available online: http://sweetgum.nybg.org/science/world-flora/monographs-details/?irn=19238 (accessed on 17 December 2019).
- Noorizadeh, H.; Farmany, A. Exploration of linear and nonlinear modeling techniques to predict of retention index of essential oils. J. Chin. Chem. Soc. 2010, 57, 1268–1277. [Google Scholar] [CrossRef]
- Frizzo, C.D.; Serafini, L.A.; Dellacassa, E.; Lorenzo, D.; Moyna, P. Essential oil of baccharis uncinella DC. from southern Brazil. Flavour. Fragr. J. 2001, 16, 286–288. [Google Scholar] [CrossRef]
- Valarezo, E.; Rosales, J.; Morocho, V.; Cartuche, L.; Guaya, D.; Ojeda-Riascos, S.; Armijos, C.; González, S. Chemical composition and biological activity of the essential oil of Baccharis obtusifolia Kunth from Loja, Ecuador. J. Essent. Oil Res. 2015, 27, 212–216. [Google Scholar] [CrossRef]
- Ruiz, S.; Malagón, O.; Zaragoza, T.; Valarezo, E. Composition of the essential oils of Artemisia sodiroi Hieron., Siparuna eggersii Hieron., Tagetes filifolia Lag. and Clinopodium nubigenum (Kunth) Kuntze from Loja Ecuador. J. Essent. Oil Bear Plants 2010, 13, 676–691. [Google Scholar] [CrossRef]
- Neffati, A.; Skandrani, I.; Ben Sghaier, M.; Bouhlel, I.; Kilani, S.; Ghedira, K.; Neffati, M.; Chraief, I.; Hammami, M.; Chekir-Ghedira, L. Chemical composition, mutagenic and antimutagenic activities of essential oils from (Tunisian) Artemisia campestris and Artemisia herba-alba. J. Essent. Oil Res. 2008, 20, 471–477. [Google Scholar]
- Formisano, C.; Rigano, D.; Napolitano, F.; Senatore, F. Volatile constituents of Calamintha origanifolia Boiss. growing wild in Lebanon. Nat. Prod. Commun. 2007, 2, 1253–1256. [Google Scholar] [CrossRef]
- Ferretti, G.; Maggi, F.; Tirillini, B. Essential oil composition of Hypericum richeri Vill. from Italy. Flavour. Fragr. J. 2005, 20, 295–298. [Google Scholar] [CrossRef]
- Naturels, P.; Belkaid, A.B.; Uvelier, L.; Sophia-antipolis, D. Composition and antibacterial activity of Pseudocytisus integrifolius (salisb.) essential oil from Algeria. J. Agric. Food Chem. 2005, 53, 2947–2952. [Google Scholar]
- Valarezo, E.; Arias, A.; Cartuche, L.; Meneses, M.; Ojeda-Riascos, S.; Morocho, V. Biological activity and chemical composition of the essential oil from Chromolaena laevigata (lam.) R.M. King, H. Rob, (Asteraceae) from loja, ecuador. J. Essent. Oil Bear Plants 2016, 19, 384–390. [Google Scholar] [CrossRef]
- Hachicha, S.F.; Skanji, T.; Barrek, S.; Ghrabi, Z.G.; Zarrouk, H. Composition of the essential oil of teucrium ramosissimum desf. (lamiaceae) from tunisia. Flavour. Fragr. J. 2007, 22, 101–104. [Google Scholar] [CrossRef]
- Mancini, E.; Apostolides, N.; Martino, L.; De Feo, V.; Formisano, C.; Rigano, D.; Senatore, F. Chemical composition and phytotoxic effects of essential oils of Salvia hierosolymitana Boiss. and Salvia multicaulis Vahl. var. simplicifolia Boiss. growing wild in Lebanon. Molecules 2009, 14, 4725–4736. [Google Scholar] [CrossRef]
- Feng, T.; Cui, J.; Xiao, Z.; Tian, H.; Yi, F.; Ma, X. Chemical composition of essential oil from the peel of Chinese Torreya grandis Fort. Org. Chem. Int. 2011, 2011, 187372. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 10-1932633219. [Google Scholar]
- Noriega, P.; Guerrini, A.; Sacchetti, G.; Grandini, A.; Ankuash, E.; Manfredini, S. Chemical composition and biological activity of five rain forest. Molecules 2019, 24, 1637. [Google Scholar] [CrossRef]
- Bulow, N.; Konig, W. The role of germacrene D as a precursor in sesquiterpene biosynthesis: Investigations of acid catalyzed, photochemically and thermally induced rearrangements. Phytochemistry 2000, 55, 141–168. [Google Scholar] [CrossRef]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour. Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
- Gilardoni, G.; Ramírez, J.; Montalván, M.; Quinche, W.; León, J.; Benítez, L.; Morocho, V.; Cumbicus, N.; Bicchi, C. Phytochemistry of three ecuadorian lamiaceae: Lepechinia heteromorpha (briq.) epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (kunth) epling. Plants 2019, 8, 1. [Google Scholar] [CrossRef] [PubMed]


| Compounds | DB-5ms | HP-INNOWax | Reference Literature | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LRI a | LRI b | % | σ | LRI a | LRI | % | σ | ||
| α-pinene | 932 | 932 | 24.3 | 2.36 | 1065 | 1076 | 20.3 | 2.69 | [20] |
| camphene | 947 | 946 | 0.9 | 0.18 | 1083 | 1092 | 0.8 | 0.43 | [21] |
| sabinene | 970 | 969 | 1.1 | 0.02 | 1120 | 1125 | 1.4 | 0.07 | [21] |
| β-pinene | 976 | 974 | 21.7 | 1.43 | 1108 | 1103 | 22.7 | 1.70 | [22] |
| β-myrcene | 989 | 988 | 11.3 | 2.34 | 1165 | 1161 | 14.8 | 2.57 | [22] |
| limonene | 1027 | 1024 | 10.0 | 3.55 | 1199 | 1194 | 11.3 | 3.94 | [22] |
| cis-ocimene | 1036 | 1032 | 8.5 | 1.77 | 1235 | 1228 | 8.1 | 1.40 | [23] |
| trans-ocimene | 1045 | 1044 | 8.9 | 5.43 | 1250 | 1244 | 8.4 | 5.10 | [23] |
| perillene | 1098 | 1102 | 0.1 | 0.03 | - | - | - | - | - |
| linalool | 1101 | 1095 | 0.6 | 0.60 | 1549 | 1546 | 0.8 | 1.04 | [22] |
| nopinone | 1138 | 1135 | 0.1 | 0.03 | - | - | - | - | - |
| trans-pinocarveol | 1140 | 1135 | 0.4 | 0.29 | 1643 | 1642 | 0.7 | 0.63 | [24] |
| cis-verbenol | 1146 | 1137 | 0.4 | 0.52 | 1668 | 1663 | 0.5 | 0.69 | [25] |
| myrtenol | 1196 | 1194 | 0.1 | 0.05 | 1789 | 1794 | 0.3 | 0.13 | [26] |
| 6-undecanol | 1284 | 1284 | 0.2 | 0.10 | 1570 | - | - | - | - |
| 2-undecanone | 1294 | 1284 | 0.2 | 0.13 | 1593 | 1598 | 0.4 | 0.60 | [27] |
| decanoic acid | 1376 | 1364 | 0.5 | 0.68 | - | - | - | - | - |
| β-elemene | 1387 | 1389 | 0.2 | 0.13 | - | - | - | - | - |
| trans-caryophyllene | 1415 | 1417 | 0.2 | 0.09 | 1580 | 1580 | 0.3 | 1.30 | [28] |
| α-humulene | 1451 | 1452 | 0.2 | 0.13 | - | - | - | - | - |
| germacrene D | 1477 | 1480 | 1.4 | 0.45 | 1687 | 1680 | 1.2 | 0.22 | [23] |
| β-selinene | 1484 | 1489 | 0.3 | 0.20 | 1703 | 1708 | 0.4 | 0.50 | [29] |
| 2-tridecanone | 1496 | 1495 | 0.4 | 0.22 | 1808 | 1815 | 0.6 | 0.81 | [30] |
| germacrene A | 1502 | 1508 | 0.3 | 0.12 | 1748 | 1744 | 0.7 | 0.98 | [23] |
| germacrene B | 1554 | 1559 | 1.9 | 0.20 | 1814 | 1811 | 2.1 | 0.78 | [31] |
| caryophyllene oxide | 1578 | 1582 | 0.3 | 0.16 | - | - | - | - | - |
| β-eudesmol | 1651 | 1649 | 0.2 | 0.07 | - | - | - | - | - |
| Monoterpene hydrocarbons | 86.7 | 87.8 | |||||||
| Oxygenated monoterpenes | 1.7 | 2.3 | |||||||
| Sesquiterpene hydrocarbons | 4.5 | 4.7 | |||||||
| Oxygenated sesquiterpenes | 0.5 | 0.0 | |||||||
| Others | 1.3 | 1.0 | |||||||
| Total identified | 94.7 | 95.8 | |||||||
| LRIs | Enantiomers | Enantiomeric Distribution (%) | ee (%) |
|---|---|---|---|
| 858 | (+)-α-pinene | 100.0 | 100.0 |
| 896 | (+)-β-pinene | 53.4 | 6.7 |
| 898 | (-)-β-pinene | 46.6 |
| Sipaucin A | Sipaucin B | Sipaucin C | |||
|---|---|---|---|---|---|
| Ion a | Dwell Time (ms) | Ion a | Dwell Time (ms) | Ion a | Dwell Time (ms) |
| 109 | 25 | 91 | 25 | 109 | 25 |
| 293 | 25 | 106 | 25 | 125 | 25 |
| 307 | 25 | 124 | 25 | 180 | 25 |
| 366 (M+) | 25 | 142 | 25 | 293 | 25 |
| 184 | 25 | 366 (M+) | 25 | ||
| 233 | 25 | ||||
| 272 | 25 | ||||
| 277 | 25 | ||||
| 290 | 25 | ||||
| 332 | 25 | ||||
| 350 (M+) | 25 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, J.; Gilardoni, G.; Cumbicus, N.; Morocho, V. Chemical Analysis of the Essential Oil from Siparuna echinata (Kunth) A. DC. (Siparunaceae) of Ecuador and Isolation of the Rare Terpenoid Sipaucin A. Plants 2020, 9, 187. https://doi.org/10.3390/plants9020187
García J, Gilardoni G, Cumbicus N, Morocho V. Chemical Analysis of the Essential Oil from Siparuna echinata (Kunth) A. DC. (Siparunaceae) of Ecuador and Isolation of the Rare Terpenoid Sipaucin A. Plants. 2020; 9(2):187. https://doi.org/10.3390/plants9020187
Chicago/Turabian StyleGarcía, Jessica, Gianluca Gilardoni, Nixon Cumbicus, and Vladimir Morocho. 2020. "Chemical Analysis of the Essential Oil from Siparuna echinata (Kunth) A. DC. (Siparunaceae) of Ecuador and Isolation of the Rare Terpenoid Sipaucin A" Plants 9, no. 2: 187. https://doi.org/10.3390/plants9020187
APA StyleGarcía, J., Gilardoni, G., Cumbicus, N., & Morocho, V. (2020). Chemical Analysis of the Essential Oil from Siparuna echinata (Kunth) A. DC. (Siparunaceae) of Ecuador and Isolation of the Rare Terpenoid Sipaucin A. Plants, 9(2), 187. https://doi.org/10.3390/plants9020187







