Molecular Events Involved in Fruitlet Abscission in Litchi
Abstract
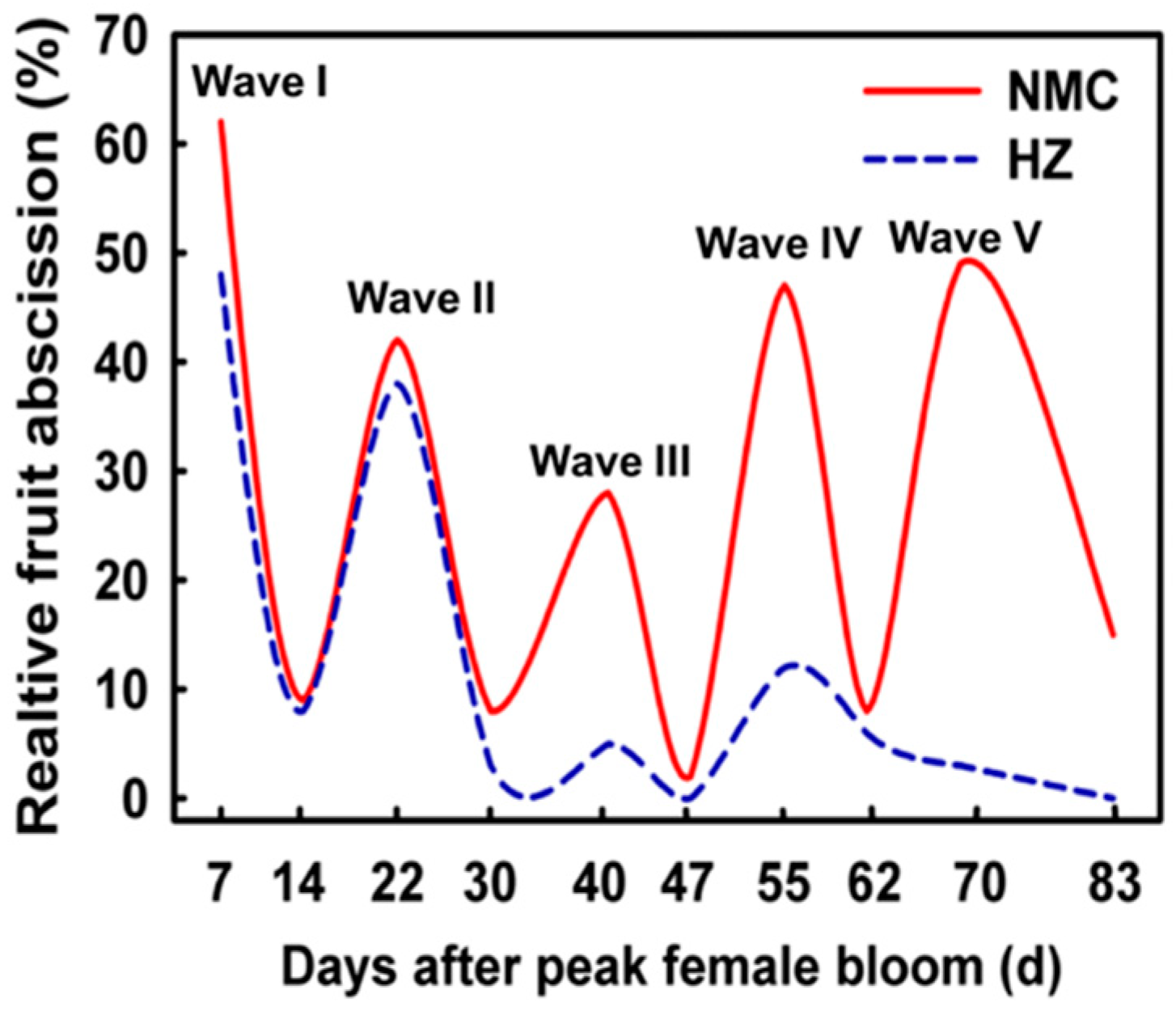
1. Introduction
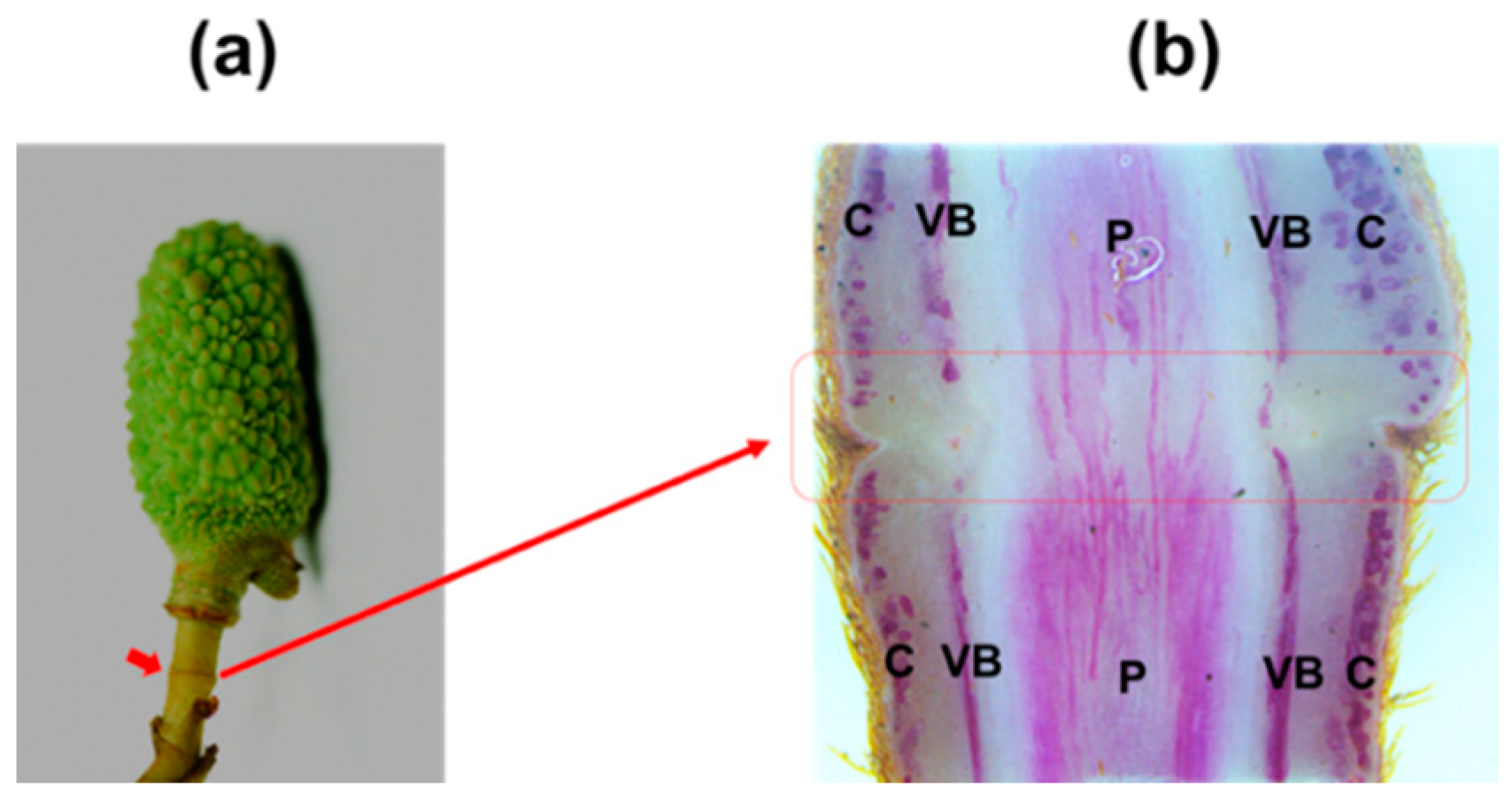
2. Reduced Lignified Cells at the Fruitlet AZ in Litchi
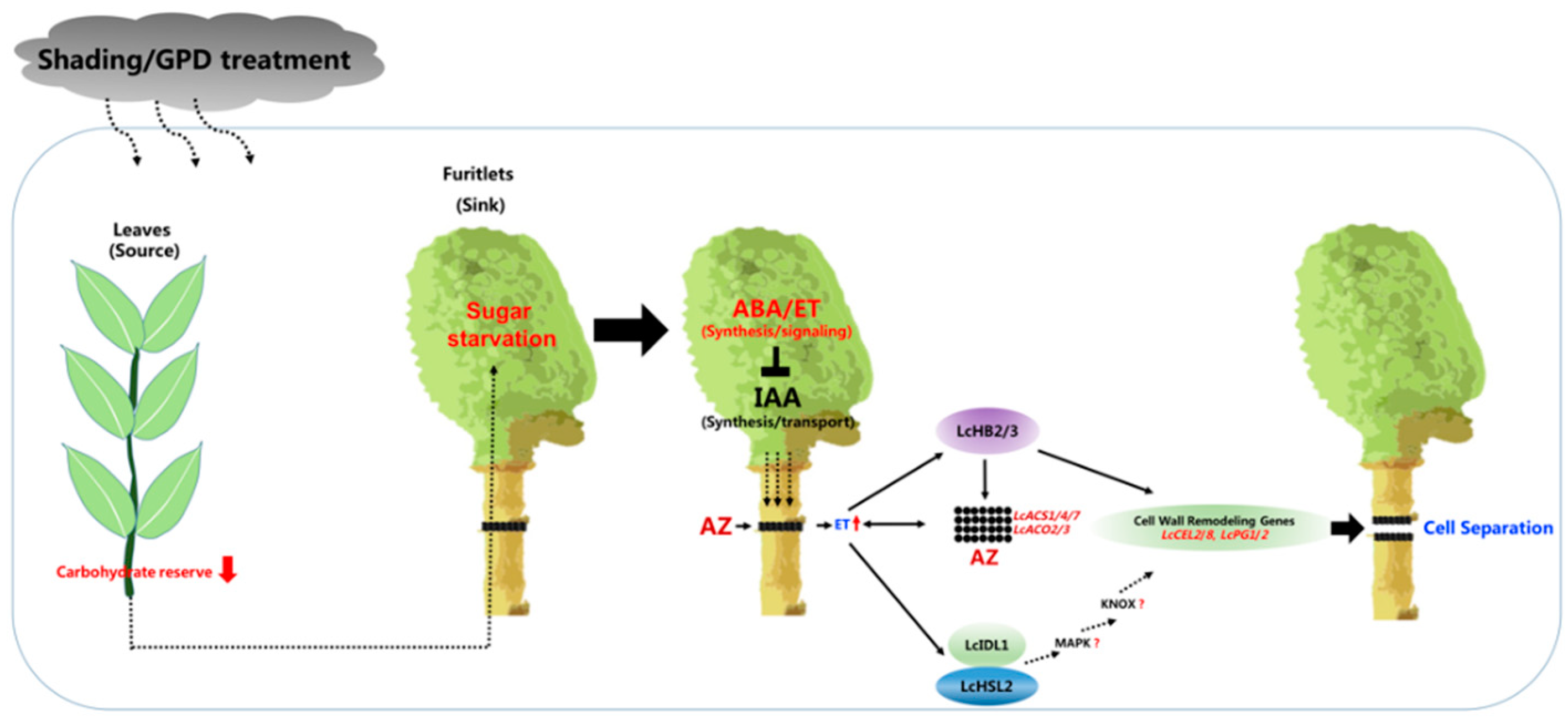
3. The Possible Molecular Events Associated with Fruitlet Abscission within the Fruitlet
4. The Possible Molecular Events Associated with Fruitlet Abscission within the AZ
5. HD-Zip Family Transcription Factors LcHB2/3 Are Involved in the Fruitlet Abscission in Litchi
6. The Involvement of IDA-HAE/HSL2 Signaling Module in the Fruitlet Abscission in Litchi
7. Conclusions and Future Perspectives
- I.
- To validate whether the activation of AZ is remotely controlled by the fruit as the abscission signals were presumably transmitted from the fruit organ to the AZ through the peduncle.
- II.
- To further investigate whether the IDA-HAE/HSL2 pathway is conserved in litchi since key components of this pathway such as SOMATIC EMBRYOGENESIS RECEPTOR KINASES (SERKs), mitogen-activated protein kinase (MAPKs), and KNOTTED1-LIKE HOMEODOMAIN proteins (KNOXs) have not been identified yet (Figure 2).
- III.
- To elucidate whether a direct link exists between the ethylene signaling and the activation of LcIDL1-LcHSL2 pathway. In fact, that IDA-HAE/HSL2 module can act downstream of ethylene signaling in control of abscission has been suggested not only in litchi but also in other plant species such as tomato, soybean, and oil palm.
Author Contributions
Funding
Conflicts of Interest
References
- Estornell, L.H.; Agusti, J.; Merelo, P.; Talon, M.; Tadeo, F.R. Elucidating mechanisms underlying organ abscission. Plant Sci. 2013, 199, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Butenko, M.A.; Patterson, S.E.; Grini, P.E.; Stenvik, G.E.; Amundsen, S.S.; Mandal, A.; Aalen, R.B. Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell 2003, 15, 2296–2307. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Larue, C.T.; Chevalier, D.; Wang, H.; Jinn, T.L.; Zhang, S.; Walker, J.C. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 15629–15634. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.L.; Alling, R.M.; Hammerstad, M.; Aalen, R.B. Control of organ abscission and other cell separation processes by evolutionary conserved peptide signaling. Plants 2019, 8, 225. [Google Scholar] [CrossRef]
- Meng, X.; Zhou, J.; Tang, J.; Li, B.; de Oliveira, M.; Chai, J.; He, P.; Shan, L. Ligand-induced receptor-like kinase complex regulates floral organ abscission in Arabidopsis. Cell Rep. 2016, 14, 1330–1338. [Google Scholar] [CrossRef]
- Jinn, T.L.; Stone, J.M.; Walker, J.C. HAESA, an Arabidopsis leucine-rich repeat receptor kinase, controls floral organ abscission. Genes Dev. 2000, 14, 108–117. [Google Scholar]
- Patharkar, O.R.; Walker, J.C. Floral organ abscission is regulated by a positive feedback loop. Proc. Natl. Acad. Sci. USA 2015, 112, 2906–2911. [Google Scholar] [CrossRef]
- Qi, W.E.; Chen, H.B.; Li, W.W.; Zhang, H.J. Development situation, trend and suggestions of Chinese litchi industry. Guangdong Agric. Sci. 2016, 43, 173–179. [Google Scholar]
- Li, J.G.; Huang, H.B.; Huang, X.M. A revised division of the developmental Stages in litchi fruit. Acta Hortic. Sin. 2003, 3, 307–310. [Google Scholar]
- Li, J.G.; Wang, Z.H. The effect of second male flowers on the fruitlet abscission during flowering in litchi. South China Fruits 1999, 3, 27–28. [Google Scholar]
- Yuan, R.C. Improvement of fruit-set in litchi chinensis sonn. through regulation of source-sink relationships. J. South China Agric. Univ. 1992, 13, 136–141. [Google Scholar]
- Yuan, R.C.; Huang, H.B. Regulation of root and shoot growth and fruit-dorp of young litchi trees by trunk girdling in view of source-sink relationships. J. Fruit Sci. 1993, 10, 195–198. [Google Scholar]
- Yuan, W.Q.; Huang, X.M.; Wang, H.C.; Li, J.G.; Chen, H.B.; Yin, J.H. Seasonal changes in carbon nutrition reserve in Nuomici litchi trees and tts relation to fruit load. Acta Hortic. Sin. 2009, 36, 1568–1574. [Google Scholar]
- Hieke, S.; Menzel, C.M.; Doogan, V.J.; Lüdders, P. The relationship between yield and assimilate supply in lychee (Litchi chinensis Sonn.). J. Hortic. Sci. Biotechnol. 2002, 77, 326–332. [Google Scholar] [CrossRef]
- Kuang, J.F.; Wu, J.Y.; Zhong, H.Y.; Li, C.Q.; Chen, J.Y.; Lu, W.J.; Li, J.G. Carbohydrate stress affecting fruitlet abscission and expression of genes related to auxin signal transduction pathway in litchi. Int. J. Mol. Sci. 2012, 13, 16084–16103. [Google Scholar] [CrossRef]
- Li, C.Q.; Wang, Y.; Huang, X.M.; Li, J.; Wang, H.C.; Li, J.G. An improved fruit transcriptome and the identification of the candidate genes involved in fruit abscission induced by carbohydrate stress in litchi. Front. Plant Sci. 2015, 6, 439. [Google Scholar] [CrossRef]
- Yuan, R.C.; Huang, H.B. Litchi fruit abscission: Its patterns, effect of shading and relation to endogenous abscisic acid. Sci. Hortic. 1988, 36, 281–292. [Google Scholar] [CrossRef]
- Xiang, X.; Qiu, Y.P.; Zhang, Z.W. Endogenous Hormones in the Fruit of Litichi chinensis cv. Nuomici Relating to Furit Abscission. J. Fruit Sci. 1995, 12, 88–92. [Google Scholar]
- Qiu, Y.; Xu, X.; Wang, B.; Zhang, Z.; Peiyuan, A.Y. Endogenous hormone balance in three types of litchi fruit and their fruit set mechanism. J. Fruit Sci. 1998, 1, 39–43. [Google Scholar]
- Lv, L.X.; Yu, X.L.; Ye, M.Z.; Xiao, Z.T. The mehcanism of embryo development in litchi. J. Fujian Agric. For. Univ. 1989, 18, 149–155. [Google Scholar]
- Lv, L.X.; Chen, R.M.; Chen, J.L. Observation of embryo development in litchi. Subtrop. Plant Sci. 1985, 1–5. [Google Scholar]
- Qiu, Y.P.; Zhang, Z.W. The relationship between fruit development and abscission in litchi. Guangdong Agric. Sci. 1993, 1, 17–19. [Google Scholar]
- Stern, R.A.; Kigel, J.; Tomer, E.; Gazit, S. ‘Mauritius’ lychee fruit development and reduced abscission after treatment with the auxin 2,4,5-TP. Am. Soc. Hortic. Sci. 1995, 120, 65–70. [Google Scholar] [CrossRef]
- Mitra, S.K.; Pereira, L.S.; Pathak, P.K.; Majumdar, D. Fruit abscission pattern of lychee cultivars. Acta Hort. 2005, 665, 215–218. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Xu, H.Y.; Wang, H.C.; Hu, G.B.; Li, J.G.; Chen, H.B.; Huang, X.M. A comparison of the costs of flowering in ‘Feizixiao’ and ‘Baitangying’ litchi. Sci. Hortic. 2012, 148, 118–125. [Google Scholar] [CrossRef]
- Ma, X.; Li, C.Q.; Huang, X.M.; Wang, H.C.; Wu, H.; Zhao, M.L.; Li, J.G. Involvement of HD-ZIP I transcription factors LcHB2 and LcHB3 in fruitlet abscission by promoting transcription of genes related to the biosynthesis of ethylene and ABA in litchi. Tree Physiol. 2019, 39, 1600–1613. [Google Scholar]
- Li, C.; Wang, Y.; Huang, X.; Li, J.; Wang, H.; Li, J. De novo assembly and characterization of fruit transcriptome in Litchi chinensis. Sonn and analysis of differentially regulated genes in fruit in response to shading. BMC Genom. 2013, 14, 552. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Ying, P.; Ma, W.; Li, J. Genome-wide digital transcript analysis of putative fruitlet abscission related genes regulated by ethephon in litchi. Front. Plant Sci. 2015, 6, 502. [Google Scholar] [CrossRef]
- Li, C.; Zhao, M.; Ma, X.; Wen, Z.; Ying, P.; Peng, M.; Ning, X.; Xia, R.; Wu, H.; Li, J. The HD-Zip transcription factor LcHB2 regulates litchi fruit abscission through the activation of two cellulase genes. J. Exp. Bot. 2019, 70, 5189–5203. [Google Scholar] [CrossRef]
- Peng, G.; Wu, J.; Lu, W.; Li, J. A polygalacturonase gene clustered into clade E involved in lychee fruitlet abscission. Sci. Hortic. 2013, 150, 244–250. [Google Scholar] [CrossRef]
- Ying, P.; Li, C.; Liu, X.; Xia, R.; Zhao, M.; Li, J. Identification and molecular characterization of an IDA-like gene from litchi, LcIDL1, whose ectopic expression promotes floral organ abscission in Arabidopsis. Sci. Rep. 2016, 6, 37135. [Google Scholar] [CrossRef] [PubMed]
- Sexton, R.; Roberts, J.A. Cell biology of abscission. Annu. Rev. Plant Biol. 1982, 33, 133–162. [Google Scholar] [CrossRef]
- Zanchin, A.; Marcato, C.; Trainotti, L.; Casadoro, G.; Rascio, N. Characterization of abscission zones in the flowers and fruits of peach [Prunus persica (L.) Batsch]. New Phytol. 2006, 129, 345–354. [Google Scholar] [CrossRef]
- Szymkowiak, E.J.; Irish, E.E. Interactions between jointless and wild-type tomato tissues during development of the pedicel abscission zone and the inflorescence meristem. Plant Cell 1999, 11, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Tadeo, F.R.; Cercós, M.; Colmenero-Flores, J.M.; Iglesias, D.J.; Talon, M. Molecular physiology of development and quality of Citrus. Adv. Bot. Res. 2008, 47, 147–223. [Google Scholar]
- Nakano, T.; Kimbara, J.; Fujisawa, M.; Kitagawa, M.; Ihashi, N.; Maeda, H.; Kasumi, T.; Ito, Y. MACROCALYX and JOINTLESS interact in the transcriptional regulation of tomato fruit abscission zone development. Plant Physiol. 2012, 158, 439–450. [Google Scholar] [CrossRef]
- Roppolo, D.; Geldner, N. Membrane and walls: Who is master, who is servant? Curr. Opin. Plant Biol. 2012, 15, 608–617. [Google Scholar] [CrossRef]
- Hofhuis, H.; Moulton, D.; Lessinnes, T.; Routier-Kierzkowska, A.L.; Bomphrey, R.J.; Mosca, G.; Reinhardt, H.; Sarchet, P.; Gan, X.; Tsiantis, M.; et al. Morphomechanical innovation drives explosive seed dispersal. Cell 2016, 166, 222–233. [Google Scholar] [CrossRef]
- Merelo, P.; Agusti, J.; Arbona, V.; Costa, M.L.; Estornell, L.H.; Gomez-Cadenas, A.; Coimbra, S.; Gomez, M.D.; Perez-Amador, M.A.; Domingo, C.; et al. Cell wall remodeling in abscission zone cells during ethylene-promoted fruit abscission in citrus. Front. Plant Sci. 2017, 8, 126. [Google Scholar]
- Zhu, H.; Dardick, C.D.; Beers, E.P.; Callanhan, A.M.; Xia, R.; Yuan, R.C. Transcriptomics of shading-induced and NAA-induced abscission in apple (Malus domestica) reveals a shared pathway involving reduced photosynthesis, alterations in carbohydrate transport and signaling and hormone crosstalk. BMC Plant Biol. 2011, 11, 138. [Google Scholar] [CrossRef]
- Denisov, Y.; Glick, S.; Zviran, T.; Ish-Shalom, M.; Levin, A.; Faigenboim, A.; Cohen, Y.; Irihimovitch, V. Distinct organ-specific and temporal expression profiles of auxin-related genes during mango fruitlet drop. Plant Physiol Biochem. 2017, 115, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Dal Cin, V.; Danesin, M.; Boschetti, A.; Dorigoni, A.; Ramina, A. Ethylene biosynthesis and perception in apple fruitlet abscission (Malus domestica L. Borck). J. Exp. Bot. 2005, 56, 2995–3005. [Google Scholar]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B.; et al. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Eccher, G.; Begheldo, M.; Boschetti, A.; Ruperti, B.; Botton, A. Roles of ethylene production and ethylene receptor expression in regulating apple fruitlet abscission. Plant Physiol. 2015, 169, 125–137. [Google Scholar] [CrossRef]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant. Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef]
- Botton, A.; Ruperti, B. The Yes and No of the ethylene involvement in abscission. Plants 2019, 8, 187. [Google Scholar] [CrossRef]
- Xie, R.; Ge, T.; Zhang, J.; Pan, X.; Ma, Y.; Yi, S.; Zheng, Y. The molecular events of IAA inhibiting citrus fruitlet abscission revealed by digital gene expression profiling. Plant Physiol. Biochem. 2018, 130, 192–204. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R.; et al. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef]
- Nakano, T.; Fujisawa, M.; Shima, Y.; Ito, Y. The AP2/ERF transcription factor SlERF52 functions in flower pedicel abscission in tomato. J. Exp. Bot. 2014, 65, 3111–3119. [Google Scholar] [CrossRef]
- Okushima, Y.; Mitina, I.; Quach, H.L.; Theologis, A. AUXIN RESPONSE FACTOR 2 (ARF2): A pleiotropic developmental regulator. Plant J. 2005, 43, 29–46. [Google Scholar] [CrossRef]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Zeng, Z.H.; Chen, C.J.; Li, C.; Xia, R.; Li, J.G. Genome-wide characterization of the auxin response factor (ARF) gene family of litchi (Litchi chinensis Sonn.): Phylogenetic analysis, miRNA regulation and expression changes during fruit abscission. PeerJ 2019, 7, e6677. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Hall, B.D.; Bennett, A.B. Antisense suppression of tomato endo-1,4-β-glucanase Cel2 mRNA accumulation increases the force required to break fruit abscission zones but does not affect fruit softening. Plant Mol. Biol. 1999, 40, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.K.; Lewandowski, D.J.; Nairn, C.J.; Brown, G.E. Endo-1,4-β-glucanase gene expression and cell wall hydrolase activities during abscission in Valencia orange. Physiol. Plant. 1998, 102, 217–225. [Google Scholar] [CrossRef]
- Gonzalez-Carranza, Z.H.; Elliott, K.A.; Roberts, J.A. Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 3719–3730. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.Z.; Lu, F.; Imsabai, W.; Meir, S.; Reid, M.S. Silencing polygalacturonase expression inhibits tomato petiole abscission. J. Exp. Bot. 2008, 59, 973–979. [Google Scholar] [CrossRef]
- Singh, A.P.; Tripathi, S.K.; Nath, P.; Sane, A.P. Petal abscission in rose is associated with the differential expression of two ethylene-responsive xyloglucan endotransglucosylase/hydrolase genes, RbXTH1 and RbXTH2. J. Exp. Bot. 2011, 62, 5091–5103. [Google Scholar] [CrossRef]
- Ruberti, I.; Sessa, G.; Lucchetti, S.; Morelli, G. A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 1991, 10, 1787–1791. [Google Scholar] [CrossRef]
- Ariel, F.D.; Manavella, P.A.; Dezar, C.A.; Chan, R.L. The true story of the HD-Zip family. Trends Plant Sci. 2007, 12, 419–426. [Google Scholar] [CrossRef]
- Butenko, M.A.; Wildhagen, M.; Albert, M.; Jehle, A.; Kalbacher, H.; Aalen, R.B.; Felix, G. Tools and strategies to match peptide-ligand receptor pairs. Plant Cell 2014, 26, 1838–1847. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, Z.H.; Yuan, Y.; Li, J.G.; Zhao, M.L. Identification and characterization of HAESA-Like genes involved in the fruitlet abscission in litchi. Int. J. Mol. Sci. 2019, 20, 5945. [Google Scholar] [CrossRef] [PubMed]
- Sto, I.M.; Orr, R.J.; Fooyontphanich, K.; Jin, X.; Knutsen, J.M.; Fischer, U.; Tranbarger, T.J.; Nordal, I.; Aalen, R.B. Conservation of the abscission signaling peptide IDA during Angiosperm evolution: Withstanding genome duplications and gain and loss of the receptors HAE/HSL2. Front. Plant Sci. 2015, 6, 931. [Google Scholar] [CrossRef] [PubMed]
- Estornell, L.H.; Wildhagen, M.; Perez-Amador, M.A.; Talon, M.; Tadeo, F.R.; Butenko, M.A. The IDA Peptide Controls Abscission in Arabidopsis and Citrus. Front. Plant Sci. 2015, 6, 1003. [Google Scholar] [CrossRef] [PubMed]
- Tucker, M.L.; Yang, R. IDA-like gene expression in soybean and tomato leaf abscission and requirement for a diffusible stelar abscission signal. AoB Plants 2012, 2012, pls035. [Google Scholar] [CrossRef] [PubMed]
- Tranbarger, T.J.; Domonhedo, H.; Cazemajor, M.; Dubreuil, C.; Fischer, U.; Morcillo, F. The PIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION enhances Populus leaf and Elaeis guineensis fruit abscission. Plants 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed]
| Fruitlet Age | Treatments | Tissue Examined | DEGs Number |
|---|---|---|---|
| 30 dpa | Shading | Fruitlet | 1039 |
| 35 dpa | GPD | Fruitlet/AZ | 2771 |
| 25 dpa | ET | AZ | 2730 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, J. Molecular Events Involved in Fruitlet Abscission in Litchi. Plants 2020, 9, 151. https://doi.org/10.3390/plants9020151
Zhao M, Li J. Molecular Events Involved in Fruitlet Abscission in Litchi. Plants. 2020; 9(2):151. https://doi.org/10.3390/plants9020151
Chicago/Turabian StyleZhao, Minglei, and Jianguo Li. 2020. "Molecular Events Involved in Fruitlet Abscission in Litchi" Plants 9, no. 2: 151. https://doi.org/10.3390/plants9020151
APA StyleZhao, M., & Li, J. (2020). Molecular Events Involved in Fruitlet Abscission in Litchi. Plants, 9(2), 151. https://doi.org/10.3390/plants9020151






