Agro-Morphological, Yield and Quality Traits and Interrelationship with Yield Stability in Quinoa (Chenopodium quinoa Willd.) Genotypes under Saline Marginal Environment
Abstract
1. Introduction
2. Material and Methods
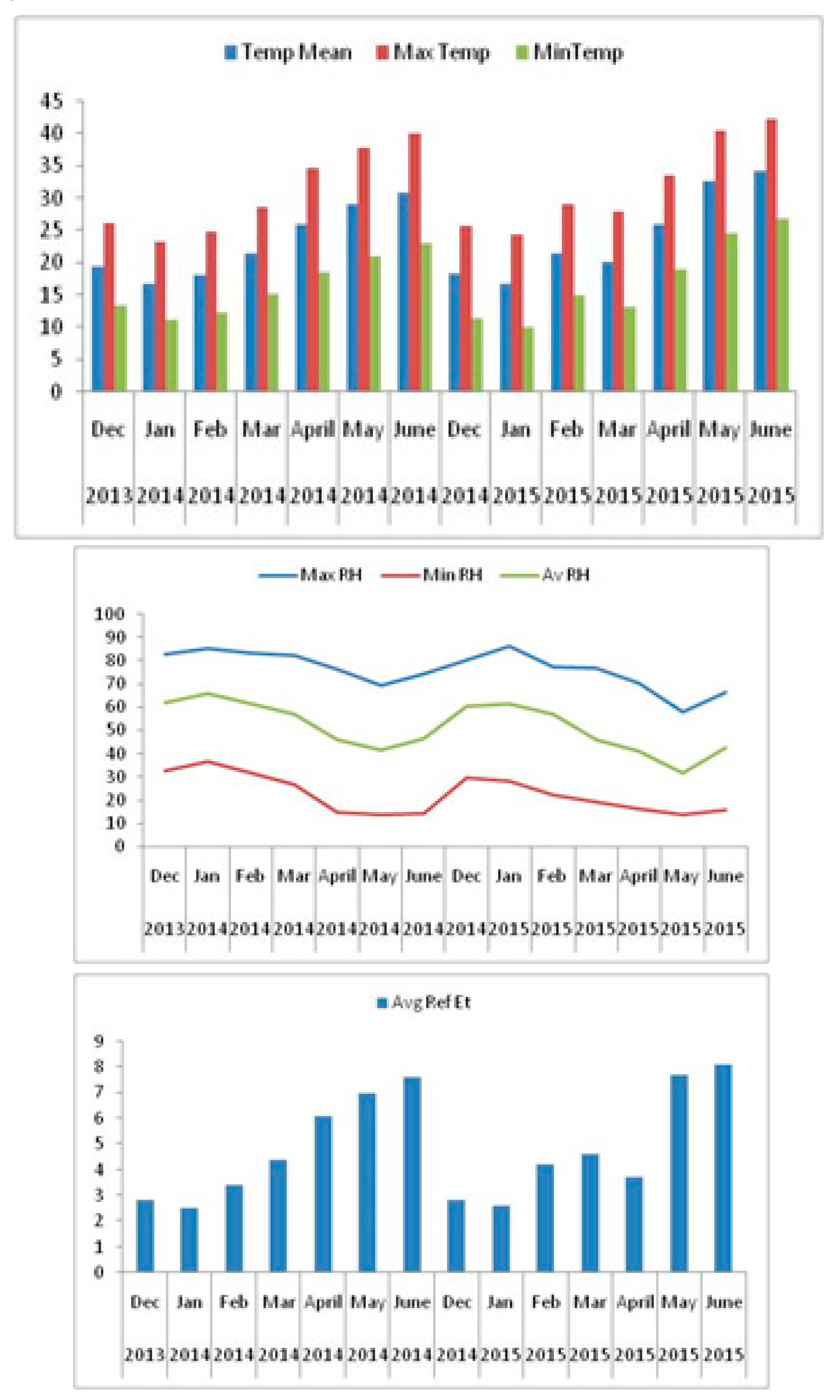
2.1. Experimental Site
2.2. Field Soil Physiological and Chemical Analysis
2.3. Land Preparation, Sowing, Plant Growth and Agronomic Practices
2.4. Irrigation System and Treatment Application
2.5. Crop Phenology and Morphological Measurements
2.6. Harvesting, Biomass and Yield Traits
2.7. Leaf Carbon (%) and Nitrogen Contents (%) Analysis
2.8. Harvest Index (%)
2.9. Grain Protein Contents Measurements
2.10. Statistical Analysis
3. Results
3.1. The Effect of Treatments and Genotypes on Growth Parameters
3.2. Saline Water Irrigation Impact on Carbon (C%) and Nitrogen (N%) and C/N Ratios
3.3. Seed Yield and Harvest Index
3.4. Grain Protein Contents
3.5. Yield Stability Trend in Quinoa Genotypes
3.6. Correlations between Seed Yield, Agro-physiological and Yield Attributes
4. Discussion
Lessons from Different Stress Levels: Trade-off between Survival and Growth
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Dakheel, A.J.; Hussain, M.I. Genotypic variation for salinity tolerance in Cenchrus ciliaris L. Front. Plant Sci. 2016, 7, 1090. [Google Scholar] [CrossRef]
- Bateman, A.; Lewandrowski, W.; Stevens, J.; Muñoz-Rojas, M. Ecophysiological indicators to assess drought responses of arid zone native seedlings in reconstructed soils. Land Degrad. Dev. 2016, 29, 984–993. [Google Scholar] [CrossRef]
- Hussain, M.I.; Farooq, M.; Muscolo, A.; Rehman, A. Crop diversification and saline water irrigation as potential strategies to save freshwater resources and reclamation of marginal soils—A review. Environ. Sci. Poll. Res. 2020, 27, 28695–28729. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xue, X.; Zhou, L.; Guo, J. Combating aeolian desertification in Northern China. Land Degrad. Dev. 2015, 26, 118–132. [Google Scholar] [CrossRef]
- Ambalam, K. United Nations Convention to Combat Desertification: Issues and Challenges. E-Int. Rel. 2014, 30. [Google Scholar]
- Gelfand, I.; Sahajpal, R.; Zhang, X.; Izaurralde, R.C.; Gross, K.L.; Robertson, G.P. Sustainable bioenergy production from marginal lands in the US Midwest. Nature 2013, 493, 514–517. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Abdelfattah, M.A.; Khedr, A.H.A. Relationships between landforms, soil characteristics and dominant xerophytes in the hyper-arid northern United Arab Emirates. J. Arid Environ. 2015, 117, 28–36. [Google Scholar] [CrossRef]
- Hussain, M.I.; Al-Dakheel, A.J.; Reigosa, M.J. Genotypic differences in agro-physiological, biochemical and isotopic responses to salinity stress in quinoa (Chenopodium quinoa Willd.) plants: Prospects for salinity tolerance and yield stability. Plant Physiol. Biochem. 2018, 129, 411–420. [Google Scholar] [CrossRef]
- Golos, P.J.; Dixon, K.W.; Erickson, T.E. Plant recruitment from the soil seed bank depends on topsoil stockpile age, height, and storage history in an arid environment. Restor. Ecol. 2016, 24, S53–S61. [Google Scholar] [CrossRef]
- Hussain, M.I.; Al-Dakheel, A.J. Using Alternate Water Resources for Cultivation of Salt Tolerant Perennial Grasses under Marginal Environment; TROPENTAG, Management of Land Use Systems for Enhanced Food Security-Conflicts, Controversies and Resolutions: Berlin, Germany, 2015; pp. 16–18. [Google Scholar]
- Hussain, M.I.; Lyra, D.A.; Farooq, M.; Nikoloudakis, N.; Khalid, N. Salt and drought stresses in safflower: A Review. Agron. Sustain. Dev. 2016, 36, 4. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pando, L.R.; Álvarez-Castro, R.; Eguiluz-De La Barra, A. Effect of salt stress on Peruvian germplasm of Chenopodium quinoa Willd.: A promising crop. J. Agron. Crop Sci. 2010, 196, 391–396. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Gallardo, M.; Hilal, M.; Rosa, M.; Prado, F.E. Physiological responses of quinoa (Chenopodium quinoa Willd.) to drought and waterlogging stresses: Dry matter partitioning. Bot. Studies. 2009, 50, 35–42. [Google Scholar]
- Lewandrowski, W.; Erickson, T.E.; Dixon, K.W.; Stevens, J.C. Increasing the germination envelope under water stress improves seedling emergence in two dominant grass species across different pulse rainfall events. J. Appl. Ecol. 2016, 54, 997–1007. [Google Scholar] [CrossRef]
- Hussain, M.I.; Al-Dakheel, A.J. Effect of salinity stress on phenotypic plasticity, yield stability and signature of stable isotopes of carbon and nitrogen in Safflower. Environ. Sci. Pollut. Res. 2018, 25, 23685–23694. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Iqbal, M.; Shakeel, A.; Ul-Allah, S.; Hussain, M.; Rehman, A.; Zafar, Z.U.; Ashraf, M. Genetic basis of ion exclusion in salinity stressed wheat: Implications in improving crop yield. Plant Growth Regul. 2020, 92, 479–496. [Google Scholar] [CrossRef]
- Al-Dakheel, A.J.; Hussain, M.I.; Al-Gailani, A.Q.M. Impact of irrigation water salinity on agronomical and quality attributes of Cenchrus ciliaris L. accessions. Agric. Water Manag. 2015, 159, 148–154. [Google Scholar] [CrossRef]
- Koyro, H.W.; Khan, M.A.; Lieth, H. Halophytic crops: A resource for the future to reduce the water crisis? Emir. J. Food Agric. 2011, 23, 1–16. [Google Scholar] [CrossRef]
- Koyro, H.W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa abiotic stress responses: A review. Plants 2018, 7, 106. [Google Scholar] [CrossRef]
- Ruiz, K.B.; Aloisi, I.; Del Duca, S.; Canelo, V.; Torrigiani, P.; Silva, H.; Biondi, S.S.V. coastal ecotypes of quinoa: Salinity responses in Chilean landraces from contrasting habitats. Plant Physiol. Biochem. 2016, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Peterson, A.J.; Morris, C.F.; Murphy, K.M. Quinoa seed quality response to sodium chloride and sodium sulfate salinity. Front. Plant Sci. 2016, 7, 790. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 2009, 60, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218. [Google Scholar] [CrossRef]
- Rojas, W.; Soto, J.L.; Pinto, M.; Jäger, M.; Padulosi, S.; Granos, A. Avances, Logros y Experiencias Desarrolladas en Quinua, Cañahua y Amaranto en Bolivia; Bioversity International: Roma, Italia, 2010; p. 178. [Google Scholar]
- Jacobsen, S.E.; Monteros, C.; Christiansen, J.L.; Bravo, L.A.; Corcuera, L.J.; Mujica, A. Plant responses of quinoa (Chenopodium quinoa Willd.) to frost at various phenological stages. Eur. J. Agron. 2005, 22, 131–139. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Monteros, C.; Corcuera, L.J.; Bravo, L.A.; Christiansen, J.L.; Mujica, A. Frost resistance mechanisms in quinoa (Chenopodium quinoa Willd.). Eur. J. Agron. 2007, 26, 471–475. [Google Scholar] [CrossRef]
- Razzaghi, F.; Jacobsen, S.E.; Jensen, C.R.; Andersen, M.N. Ionic and photosynthetic homeostasis in quinoa challenged by salinity and drought–mechanisms of tolerance. Func. Plant Biol. 2015, 42, 136–148. [Google Scholar] [CrossRef]
- Koyro, H.W.; Eisa, S.S. Effect of salinity on composition, viability and germination of seeds of Chenopodium quinoa Willd. Plant Soil 2008, 302, 79–90. [Google Scholar] [CrossRef]
- Babatunde, R.O.; Olungunju, F.I.; Fakayode, S.B.; Sola-Ojo, F.E. Prevalence and determinants of malnutrition among under five children of farming households in Kwara State, Nigeria. J. Agric. Sci. 2011, 3, 173–181. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Mujica, A.; Jensen, C.R. The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev. Int. 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Brakez, M.; Harrouni, M.C.; Tachbibi, N.; Daoud, S. Comparative effect of NaCl and seawater on germination of quinoa seed (Chenopodium quinoa Willd.). Emir. J. Food Agric. 2014, 26, 1091–1096. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Jacobsen, S.E.; Akhtar, S.S.; Muscolo, A. Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants 2014, 6, plu047. [Google Scholar] [CrossRef] [PubMed]
- Burt, R. Soil Survey Laboratory Methods Manual; Soil Survey Investigation Report No. 42, Version 4.0; U.S. Department of Agriculture, Natural Resources Conservation Service, National Soil Survey Center: Lincoln, NE, USA, 2004.
- Walkley, A.; Black, I. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bertero, H.D.; de la Vega, A.J.; Correa, G.; Jacobsen, S.E.; Mujica, A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crop. Res. 2004, 89, 299–318. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid, sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hussain, M.I.; Reigosa, M.J. Allelochemical stress inhibits growth, leaf water relations, PSII photochemistry, non-photochemical fluorescence quenching and heat energy dissipation in three C3 perennial species. J. Exp. Bot. 2011, 62, 4533–4545. [Google Scholar] [CrossRef]
- Roemer, T. Sind die ertragsreichen Sorten ertragssicherer? Mitteilung Deutsche Landwirtschafts-Gesellschaft. DGL-Mitt 1917, 32, 87–89. [Google Scholar]
- Wricke, G. Uber eine Methode zur Erfassung der okologischen Streubreite in Feldversuchen. Z. Pflanzenzuchtg 1962, 47, 92–96. [Google Scholar]
- Cocozza, C.; Pulvento, C.; Lavini, A.; Riccardi, M.; d’Andria, R.; Tognetti, R. Effects of increasing salinity stress and decreasing water availability on ecophysiological traits of Quinoa (Chenopodium quinoa Willd.) grown in a Mediterranean-type agroecosystem. J. Agron. Crop Sci. 2013, 199, 229–240. [Google Scholar] [CrossRef]
- Roman, V.J.; den Toom, L.A.; Gamiz, C.C.; van der Pijl, N.; Visser, R.G.; van Loo, E.N.; van der Linden, C.G. Differential responses to salt stress in ion dynamics, growth and seed yield of European quinoa varieties. Environ. Exp. Bot. 2020, 177, 104146. [Google Scholar] [CrossRef]
- Ali, Y.; Aslam, Z.; Ashraf, M.Y.; Tahir, G.R. Effect of salinity on chlorophyll concentration, leaf area, yield and yield components of rice genotypes grown under saline environment. Int. J. Environ. Sci. Tech. 2004, 3, 221–225. [Google Scholar] [CrossRef]
- Hirich, A.; Choukr-Allah, R.; Jacobsen, S.E. Deficit irrigation and organic compost improve growth and yield of quinoa and pea. J. Agron. Crop Sci. 2014, 200, 390–398. [Google Scholar] [CrossRef]
- Shabala, S.; Hariadi, Y.; Jacobsen, S.-E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J. Plant Physiol. 2013, 170, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Adolf, V.I.; Shabala, S.; Andersen, M.N.; Razzaghi, F.; Jacobsen, S.E. Varietal differences of quinoa’s tolerance to saline conditions. Plant Soil 2012, 357, 117–129. [Google Scholar] [CrossRef]
- Bonales-Alatorre, E.; Pottosin, I.; Shabala, L.; Chen, Z.-H.; Zeng, F.; Jacobsen, S.-E.; Shabala, S. Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in a halophyte species, Chenopodium quinoa. Int. J. Mol. Sci. 2013, 14, 9267–9285. [Google Scholar] [CrossRef]
- Long Nguyen, V. Effects of salinity stress on growth and yield of quinoa (Chenopodium quinoa Willd.) at flower initiation stages. Viet. J. Agric. Sci. 2016, 14, 321–327. [Google Scholar]
- Yousfi, S.; Serret, M.D.; Márquez, A.J.; Voltas, J.; Araus, J.L. Combined use of δ13C, δ18O and δ15N tracks nitrogen metabolism and genotypicadaptation of durum wheat to salinity and water deficit. N. Phytol. 2012, 194, 230–244. [Google Scholar] [CrossRef]
- Hussain, M.I.; El-Keblawy, A.; Mitterand Tsombou, F. Leaf age, canopy position, and habitat affect the carbon isotope discrimination and water-use efficiency in three C3 leguminous Prosopis species from a hyper-arid climate. Plants 2019, 8, 402. [Google Scholar] [CrossRef]
- Hussain, M.I.; Al-Shamsi, N.S.; El-Keblawy, A. Physiological responses of the xerohalophyte Suaeda vermiculata to salinity in its hyper-arid environment. Flora 2020, 273, 151705. [Google Scholar]
- Hussain, M.I.; Elnaggar, A.; El-Keblawy, A. Eco-physiological adaptations of Salsola drummondii to soil salinity: Role of reactive oxygen species, ion homeostasis, carbon isotope signatures and anti-oxidant feedback. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2020, 1–20. [Google Scholar] [CrossRef]
- Eisa, S.; Hussein, S.; Geissler, N.; Koyro, H.W. Effect of NaCl salinity on water relations, photosynthesis and chemical composition of Quinoa (Chenopodium quinoa Willd.) as a potential cash crop halophyte. Aust. J. Crop Sci. 2012, 6, 357–368. [Google Scholar]
- Jacobsen, S.E. The worldwide potential of quinoa (Chenopodium quinoa Willd.). Food Rev. Int. 2003, 19, 167–177. [Google Scholar] [CrossRef]
- Blum, A. Effective use of water (EUW) and not wateruse efficiency (WUE) is the target of crop yield improvement under drought stress. Field Crop. Res. 2009, 112, 119–123. [Google Scholar] [CrossRef]
- Aloisi, I.; Parrotta, L.; Ruiz, K.B.; Landi, C.; Bini, L.; Cai, G.; Biondi, S.; Del Duca, S. New insight into quinoa seed quality under salinity: Changes in proteomic and amino acid profiles, phenolic content, and antioxidant activity of protein extracts. Front. Plant Sci. 2016, 7, 656. [Google Scholar] [CrossRef]
- Schmöckel, S.M.; Lightfoot, D.J.; Razali, R.; Tester, M.; Jarvis, D.E. Identification of putative transmembrane proteins involved in salinity tolerance in Chenopodium quinoa by integrating physiological data, RNAseq, and SNP analyses. Front. Plant Sci. 2017, 8, 1023. [Google Scholar] [CrossRef]
- Orsini, F.; Accorsi, M.; Gianquinto, G.; Dinelli, G.; Antognoni, F.; Carrasco, K.B.R.; Martinez, E.A.; Alnayef, M.; Marotti, I.; Bosi, S.; et al. Beyond the ionic and osmotic response to salinity in Chenopodium quinoa: Functional elements of successful halophytism. Funct. Plant Biol. 2011, 38, 818–831. [Google Scholar] [CrossRef]

| Parameters | Values | |
|---|---|---|
| Pre-Sowing 2013 | Post-Harvest 2014 | Post-Harvest 2014 |
| - | 10 dS m−1 | 20 dS m−1 |
| Sand (%) | 97.60 | 97.60 |
| Silt (%) | 2.20 | 2.20 |
| Clay (%) | 0.20 | 0.20 |
| Soil textural class | Sand | Sand |
| Ece dS m−1 | 2.04 | 4.10 |
| pHs | 7.04 | 7.31 |
| Total N mg kg−1 | 52.00 | 51.59 |
| P mg kg−1 | 41.51 | 46.74 |
| K mg kg−1 | 45.95 | 41.61 |
| %Organic matter | 1.46 | 1.32 |
| S. No. | Code | Germination Line | Source | Origin | Status | Seed Color |
|---|---|---|---|---|---|---|
| 2 | Q 18 | C. quinoa PI614886 | USDA | Maule, Chile | Cultivated | Yellow |
| 4 | Q 21 | C. quinoa PI614889 | USDA | Bio-Bio, Chile | Cultivated | Orche |
| 5 | Q 22 | C. quinoa PI634918 | USDA | Chile | // | Yellow |
| 8 | Q 29 | C. quinoa PI634925 | USDA | Chile | // | Yellow |
| 11 | AMES 13761 | C. quinoa | USDA | USA | - | - |
| 12 | NSL 106398 | C. quinoa | USDA | USA | - | - |
| Variables | Plant Height (cm) | Number of Branches/Plant | No. of Panicles/Plant | Average Panicles Length (cm) | Plant Dry Biomass (t/ha) | Leaf N% | Leaf C% | C:N Ratio | Grain Protein Content | SY (t/ha) | HI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1–0 (Control) | 102.55a | 16.88a | 14.56a | 18.23a | 6.86b | 1.41a | 28.46a | 13.73a | 8.87c | 2.63a | 37.56a |
| S2–10 dS m−1 | 84.22b | 14.72b | 12.39c | 18.57a | 6.64b | 1.61a | 26.54b | 7.94b | 10.06b | 1.46b | 24.59b |
| S3–20 dS m−1 | 69.44c | 15.33b | 13.5b | 18.85a | 6.71a | 1.75a | 25.97b | 8.4b | 10.98a | 1.06c | 14.88c |
| Genotype (G) | 0.00 | 0.29 | 0.05 | 0.22 | 0.00 | 0.00 | 0.265 | 0.105 | 0.000 | 0.04 | 0.012 |
| Treatment (T) | 0.00 | 0.35 | 0.35 | 0.88 | 0.36 | 0.00 | 0.00 | 0.00 | 0.00116 | 0.00 | 0.00 |
| G × T interaction | 0.79 | 0.296 | 0.35 | 0.48 | 0.37 | 0.00 | 0.91 | 0.85 | 0 | 0.02 | 0.016 |
| Variables | Plant Height (cm) | Number of Branches/Plant | No. of Panicles/Plant | Average Panicles Length (cm) | Plant Dry Biomass (t/ha) | Leaf N% | Leaf C% | C:N Ratio | Grain Protein Content | SY (t/ha) | HI |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypes (G) | - | - | - | - | - | - | - | - | - | - | - |
| Q 18 | 74.88d | 19.0a | 17.44a | 17.0b | 6.74b | 1.58b | 27.05a | 9.88c | 9.89b | 1.27b | 20.19c |
| Q 21 | 87.0b | 15.33b | 12.88c | 19.89a | 9.0a | 1.43c | 27.82a | 8.04cd | 8.96c | 2.3a | 26.98b |
| Q 22 | 73.33d | 14.11c | 12.22c | 17.12b | 9.01a | 1.45c | 26.45b | 11.97a | 9.12b | 1.53b | 19.05c |
| Q 29 | 79.88c | 15.66b | 14.22b | 18.78a | 6.28b | 1.4c | 26.76b | 10.33b | 8.76c | 1.72b | 26.04b |
| AMES 13761 | 120.44a | 15b | 10.56d | 20.78a | 5.91c | 1.47c | 27.16a | 10.56b | 9.19b | 1.77b | 31.8a |
| NSL 106398 | 77.0c | 14.78c | 13.56b | 17.74b | 5.45c | 2.23a | 26.74b | 9.35c | 13.9a | 1.69b | 30a |
| S. No. | Genotypes Name | Mi | S2i | W2i |
|---|---|---|---|---|
| 1 | Q 18 | 1.274 | 1.019 | 1.677 |
| 2 | Q 21 | 1.082 | 3.461 | 2.080 |
| 3 | Q 22 | 1.919 | 2.310 | 2.470 |
| 4 | Q 29 | 1.494 | 2.591 | 2.349 |
| 5 | AMES 13761 | 1.398 | 2.659 | 5.362 |
| 6 | NSL 106398 | 2.577 | 2.540 | 9.586 |
| Traits | SY | PH | NOB | NOP | APL | TB | HI | N% | C% | CN Ratio | Protein |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SY | 1 | - | - | - | - | - | - | - | - | - | - |
| PH | 0.46 ** | 1 | - | - | - | - | - | - | - | - | - |
| NOB | −0.001 | 0.212 | 1 | - | - | - | - | - | - | - | - |
| NOI | −0.039 | 0.034 | 0.832 ** | 1 | - | - | - | - | - | - | - |
| AIL | −0.118 | 0.414 ** | 0.267 ** | 0.223 ** | 1 | - | - | - | - | - | - |
| TB | 0.209 | −0.071 | −0.026 | 0.08 | 0.058 | 1 | - | - | - | - | - |
| HI | 0.837 ** | 0.503 ** | −0.012 | −0.107 | −0.167 | −0.268 ** | 1 | - | - | - | - |
| N% | −0.311 ** | −0.201 | 0.136 | 0.244 ** | 0.043 | −0.174 | −0.249 ** | 1 | - | - | - |
| C% | 0.637 ** | 0.422 ** | 0.176 | 0.141 | −0.031 | 0.025 | 0.637 ** | −0.219 ** | 1 | - | - |
| CN Ratio | 0.119 | 0.264 ** | 0.051 | 0.019 | 0.01 | −0.153 | 0.186 | −0.277 ** | 0.182 | 1 | - |
| Protein | −0.311 ** | −0.201 | 0.136 | 0.244 ** | 0.043 | −0.174 | −0.249 ** | 0.000 ** | −0.219 ** | −0.277 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.I.; Muscolo, A.; Ahmed, M.; Asghar, M.A.; Al-Dakheel, A.J. Agro-Morphological, Yield and Quality Traits and Interrelationship with Yield Stability in Quinoa (Chenopodium quinoa Willd.) Genotypes under Saline Marginal Environment. Plants 2020, 9, 1763. https://doi.org/10.3390/plants9121763
Hussain MI, Muscolo A, Ahmed M, Asghar MA, Al-Dakheel AJ. Agro-Morphological, Yield and Quality Traits and Interrelationship with Yield Stability in Quinoa (Chenopodium quinoa Willd.) Genotypes under Saline Marginal Environment. Plants. 2020; 9(12):1763. https://doi.org/10.3390/plants9121763
Chicago/Turabian StyleHussain, M. Iftikhar, Adele Muscolo, Mukhtar Ahmed, Muhammad Ahsan Asghar, and Abdullah J. Al-Dakheel. 2020. "Agro-Morphological, Yield and Quality Traits and Interrelationship with Yield Stability in Quinoa (Chenopodium quinoa Willd.) Genotypes under Saline Marginal Environment" Plants 9, no. 12: 1763. https://doi.org/10.3390/plants9121763
APA StyleHussain, M. I., Muscolo, A., Ahmed, M., Asghar, M. A., & Al-Dakheel, A. J. (2020). Agro-Morphological, Yield and Quality Traits and Interrelationship with Yield Stability in Quinoa (Chenopodium quinoa Willd.) Genotypes under Saline Marginal Environment. Plants, 9(12), 1763. https://doi.org/10.3390/plants9121763








