Development of a Rapid Selection System for Salt-Resistant Mutants of Nicotiana benthamiana through Protoplast Culture after Gamma Irradiation
Abstract
1. Introduction
2. Results and Discussion
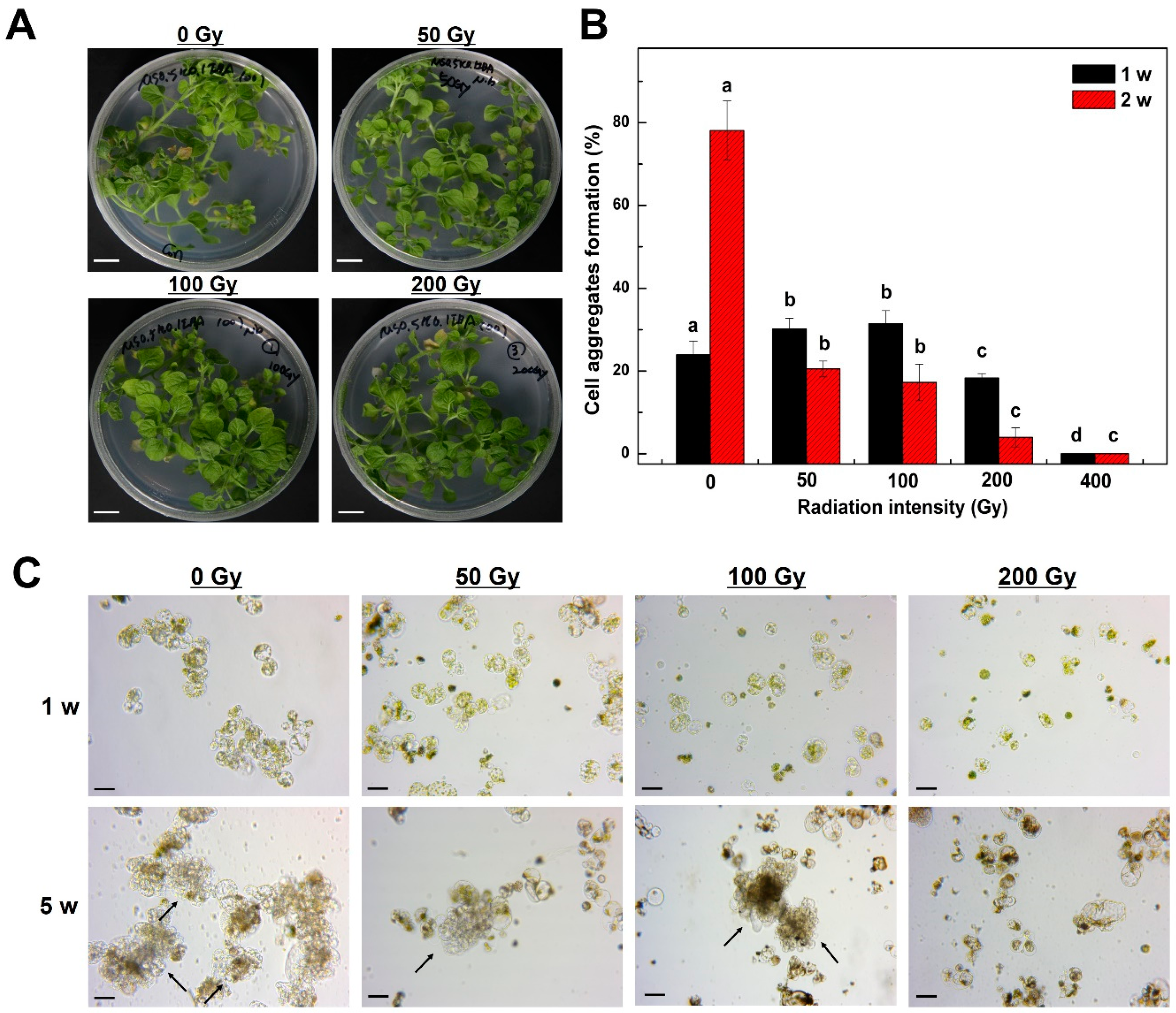
2.1. Mitotic Division of Tobacco Protoplast-Derived Cells According to Radiation Intensity
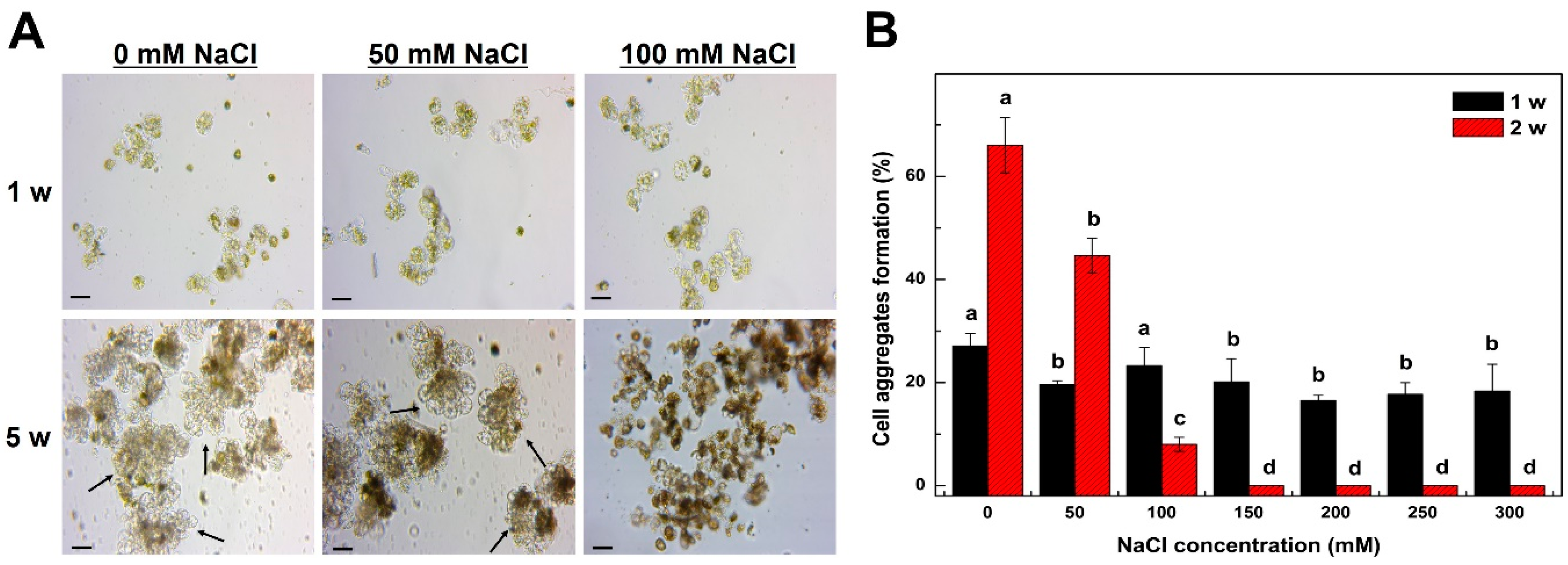
2.2. Mitotic Division of Tobacco Protoplast-Derived Cells According to NaCl Treatment Concentration
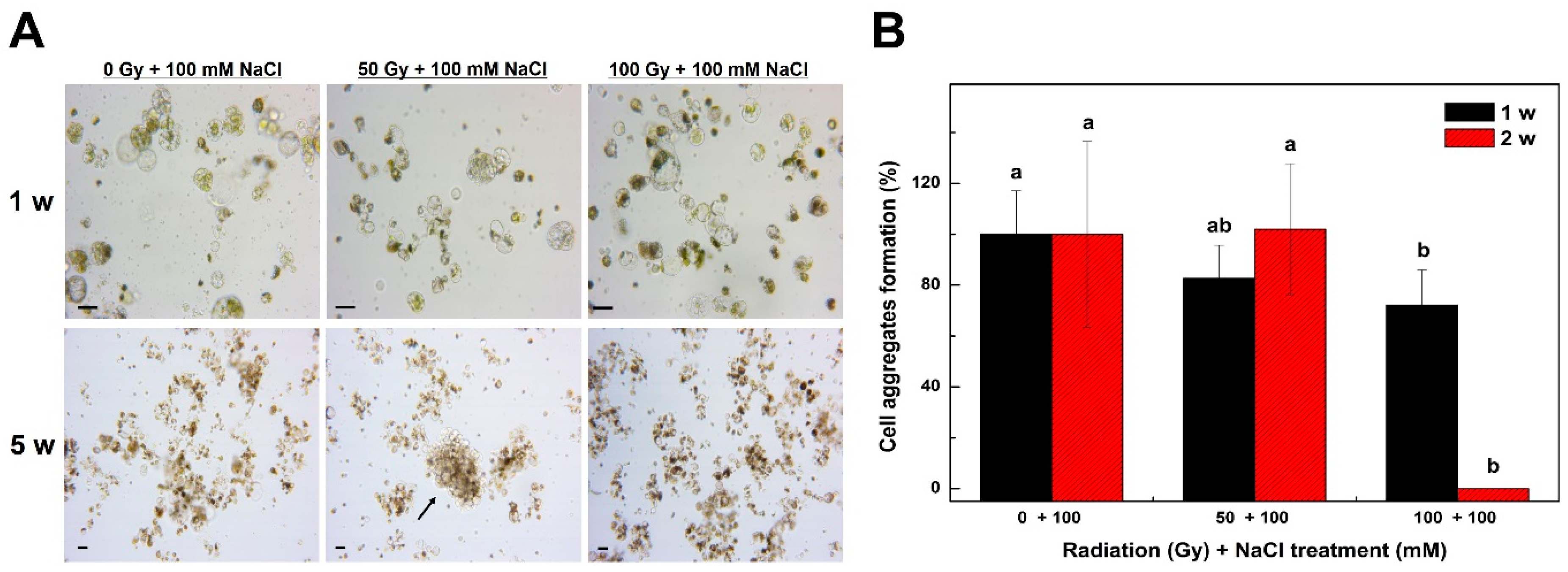
2.3. Establishment of Optimal Conditions for Selection of a Salt-Resistant Tobacco Radiation Mutant
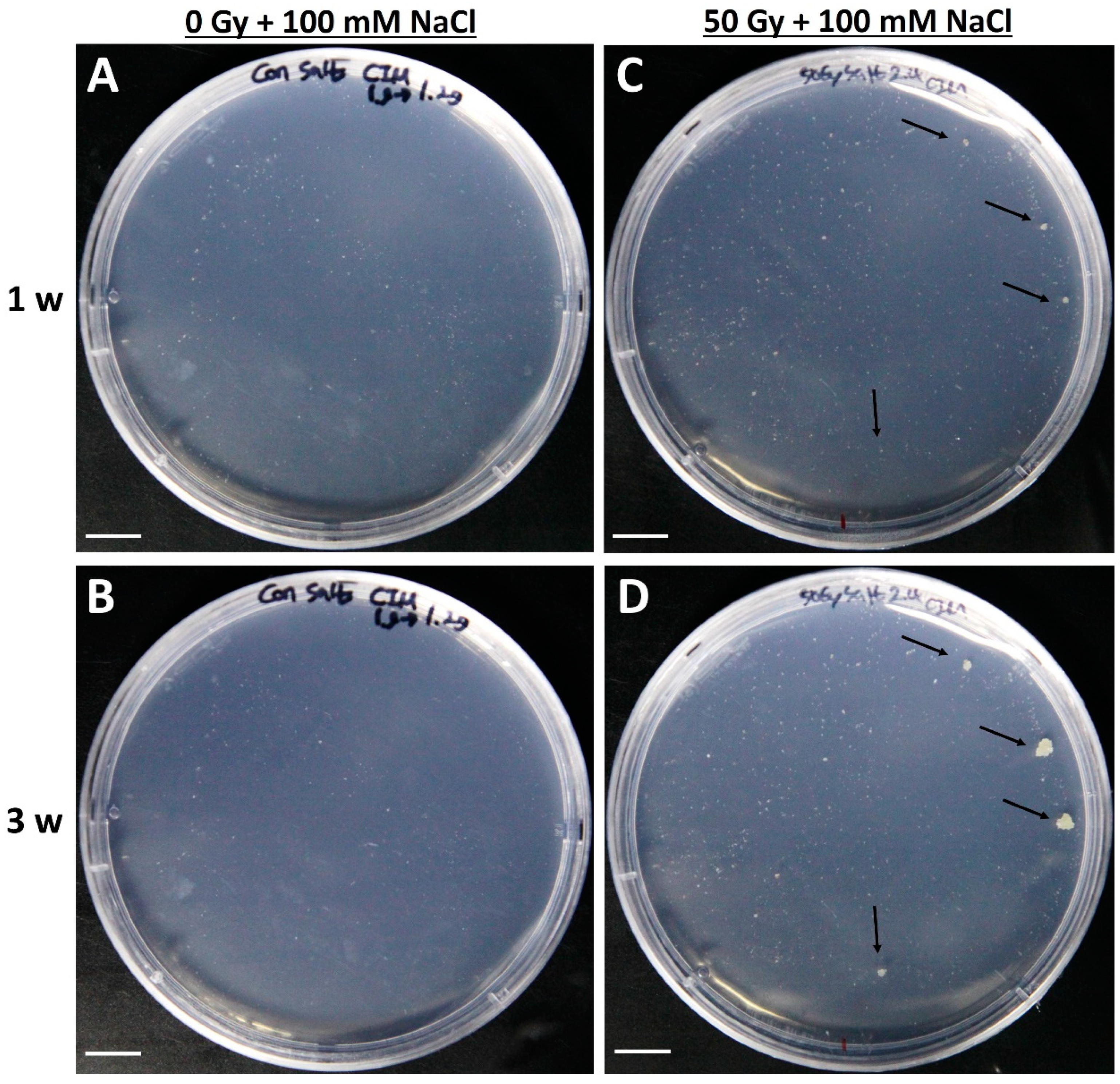
2.4. Selection of Salt-Resistant Calli Derived from Tobacco Protoplasts and Their Proliferation
2.5. Adventitious Shoot Formation of Salt-Resistant Callus Derived from Tobacco Protoplasts
2.6. Salt Resistance Test of Young Tobacco Plantlets Derived from Mesophyll Protoplasts
3. Materials and Methods
3.1. Plant Materials and In-Vitro Growth Conditions
3.2. Isolation and Culture of Protoplasts from Leaf of Nicotiana benthamiana
3.3. Effect of Radiation Intensity on the Tobacco Plant and Protoplast Cultures
3.4. Effect of NaCl Concentrations on Tobacco Protoplast Cultures
3.5. Selection of Salt-Resistant Mutants Derived from Tobacco Protoplasts and Plant Regeneration
3.6. Salt Resistance Verification for the Tobacco Mutant Plants
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, L.; Li, W.; Yu, L.; Li, P.; Li, Q.; Ma, S.; Dong, X.; Zhou, G.; Leloup, C. Linear energy transfer dependence of the effects of carbon ion beams on adventitious shoot regeneration from in vitro leaf explants of Saintpaulia ionahta. Int. J. Radiat. Biol. 2006, 82, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Mei, M.; Deng, H.; Lu, Y.; Zhuang, C.; Liu, Z.; Qiu, Q.; Qiu, Y.; Yang, T. Mutagenic effects of heavy ion radiation in plants. Adv. Space Res. 1994, 14, 363–372. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Cheng, Z.; Sun, Y. Space environment induced mutations prefer to occur at polymorphic sites of rice genomes. Adv. Space Res. 2007, 40, 523–527. [Google Scholar] [CrossRef]
- Lee, I.-S.; Kim, D.-S.; Lee, S.-J.; Song, H.-S.; Lim, Y.-P.; Lee, Y.-I. Selection and agronomic traits of radiation-induced variants in rice. J. Plant Biotechnol. 2003, 30, 19–25. [Google Scholar]
- Eeckhaut, T.; Lakshmanan, P.S.; Deryckere, D.; Van Bockstaele, E.; Van Huylenbroeck, J. Progress in plant protoplast research. Planta 2013, 238, 991–1003. [Google Scholar] [CrossRef]
- Sheen, J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 2001, 127, 1466–1475. [Google Scholar] [CrossRef]
- Jia, N.; Zhu, Y.; Xie, F. An Efficient Protocol for Model Legume Root Protoplast Isolation and Transformation. Front. Plant Sci. 2018, 9, 670. [Google Scholar] [CrossRef]
- Pasternak, T.; Lystvan, K.; Betekhtin, A.; Hasterok, R. From Single Cell to Plants: Mesophyll Protoplasts as a Versatile System for Investigating Plant Cell Reprogramming. Int. J. Mol. Sci. 2020, 21, 4195. [Google Scholar] [CrossRef]
- Carlson, P.S. Methionine sulfoximine--resistant mutants of tobacco. Science 1973, 180, 1366–1368. [Google Scholar] [CrossRef]
- Kiełkowska, A.; Grzebelus, E.; Lis-Krzyścin, A.; Maćkowska, K. Application of the salt stress to the protoplast cultures of the carrot (Daucus carota L.) and evaluation of the response of regenerants to soil salinity. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 137, 379–395. [Google Scholar] [CrossRef]
- Woo, J.W.; Kim, J.; Kwon, S.I.; Corvalán, C.; Cho, S.W.; Kim, H.; Kim, S.-G.; Kim, S.-T.; Choe, S.; Kim, J.-S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Cocking, E.C. A method for the isolation of plant protoplasts and vacuoles. Nature 1960, 187, 962–963. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Jia, S.R.; Lin, Y. Plant regeneration from mesophyll protoplast culture of cabbage (Brassica oleracea var ‘capitata’). Theor. Appl. Genet. 1985, 71, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.-L.M.; Rietveld, E.M.; Van Marrewijk, G.A.; Kool, A.J. Regeneration of leaf mesophyll protoplasts of tomato cultivars (L. esculentum): Factors important for efficient protoplast culture and plant regeneration. Plant Cell Rep. 1987, 6, 172–175. [Google Scholar] [CrossRef]
- Kang, H.H.; Naing, A.H.; Kim, C.K. Protoplast Isolation and Shoot Regeneration from Protoplast-Derived Callus of Petunia hybrida Cv. Mirage Rose. Biology 2020, 9, 228. [Google Scholar] [CrossRef]
- Rahmani, M.-S.; Pijut, P.M.; Shabanian, N. Protoplast isolation and genetically true-to-type plant regeneration from leaf-and callus-derived protoplasts of Albizia julibrissin. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 127, 475–488. [Google Scholar] [CrossRef]
- Dovzhenko, A.; Dal Bosco, C.; Meurer, J.; Koop, H.U. Efficient regeneration from cotyledon protoplasts in Arabidopsis thaliana. Protoplasma 2003, 222, 107–111. [Google Scholar] [CrossRef]
- Sinha, A.; Wetten, A.C.; Caligari, P. Effect of biotic factors on the isolation of Lupinus albus protoplasts. Aust. J. Bot. 2003, 51, 103–109. [Google Scholar] [CrossRef]
- Lin, C.S.; Hsu, C.T.; Yang, L.H.; Lee, L.Y.; Fu, J.Y.; Cheng, Q.W.; Wu, F.H.; Hsiao, H.C.W.; Zhang, Y.; Zhang, R. Application of protoplast technology to CRISPR/Cas9 mutagenesis: From single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef]
- Cvejić, S.; Afza, R.; Jocić, S.; Prodanović, S.; Miklič, V.; Škorić, D.; Dragin, S. Radiosensitivity of sunflower inbred lines to mutagenesis. Helia 2011, 34, 99–106. [Google Scholar] [CrossRef]
- Eun, J.; Kim, J.; Lim, H.; Han, S.; Choi, S.; Jang, Y. Effect of proton ion and gamma-ray irradiation on radiosensitivity of M1 seedlings in Brassica napus. Korean J. Hortic. Sci. Technol. 2007, 25, 17–23. [Google Scholar]
- Ryu, J.; Im, S.; Kim, D.; Ahn, J.; Kim, J.; Kim, S.; Kang, S. Effects of Gamma-ray irradiation on growth characteristics and DNA damage in licorice (Glycyrrhiza uralensis). Radiat. Indust 2014, 8, 89–95. [Google Scholar]
- Yamaguchi, H.; Hase, Y.; Tanaka, A.; Shikazono, N.; Degi, K.; Shimizu, A.; Morishita, T. Mutagenic effects of ion beam irradiation on rice. Breed. Sci. 2009, 59, 169–177. [Google Scholar] [CrossRef]
- Nair, K.; Netrawali, M. Sensitivity of light-grown and dark-grown Euglena cells to gamma-irradiation. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1979, 36, 223–231. [Google Scholar] [CrossRef]
- Wada, H.; Koshiba, T.; Matsui, T.; Satô, M. Involvement of peroxidase in differential sensitivity to γ-radiation in seedlings of two Nicotiana species. Plant Sci. 1998, 132, 109–119. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ryu, T.H.; Lee, S.S.; Lee, S.; Chung, B.Y. Ionizing radiation manifesting DNA damage response in plants: An overview of DNA damage signaling and repair mechanisms in plants. Plant Sci. 2019, 278, 44–53. [Google Scholar] [CrossRef]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Um, M.; Kang, S.Y.; Lee, J.W.; Lee, O.R. Effect of Gamma Ray on Germination, Growth and Antioxidant Activity of Senna tora. Korean J. Med. Crop. Sci. 2017, 25, 290–295. [Google Scholar]
- Bertini, E.; Tornielli, G.B.; Pezzotti, M.; Zenoni, S. Regeneration of plants from embryogenic callus-derived protoplasts of Garganega and Sangiovese grapevine (Vitis vinifera L.) cultivars. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 138, 239–246. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Menczel, L.; Nagy, F.; Kiss, Z.R.; Maliga, P. Streptomycin resistant and sensitive somatic hybrids of Nicotiana tabacum + Nicotiana knightiana: Correlation of resistance to N. tabacum plastids. Theor. Appl. Genet. 1981, 59, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Gamborg, O.L.C.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, D.M.; Choi, S.H.; Lee, M.H.; Jie, E.Y.; Ahn, W.S.; Joo, S.J.; Ahn, J.-W.; Jo, Y.D.; Ahn, S.-J.; Kim, S.W. Development of a Rapid Selection System for Salt-Resistant Mutants of Nicotiana benthamiana through Protoplast Culture after Gamma Irradiation. Plants 2020, 9, 1720. https://doi.org/10.3390/plants9121720
Jin DM, Choi SH, Lee MH, Jie EY, Ahn WS, Joo SJ, Ahn J-W, Jo YD, Ahn S-J, Kim SW. Development of a Rapid Selection System for Salt-Resistant Mutants of Nicotiana benthamiana through Protoplast Culture after Gamma Irradiation. Plants. 2020; 9(12):1720. https://doi.org/10.3390/plants9121720
Chicago/Turabian StyleJin, Da Mon, Seung Hee Choi, Myoung Hui Lee, Eun Yee Jie, Woo Seok Ahn, Su Ji Joo, Joon-Woo Ahn, Yeong Deuk Jo, Sung-Ju Ahn, and Suk Weon Kim. 2020. "Development of a Rapid Selection System for Salt-Resistant Mutants of Nicotiana benthamiana through Protoplast Culture after Gamma Irradiation" Plants 9, no. 12: 1720. https://doi.org/10.3390/plants9121720
APA StyleJin, D. M., Choi, S. H., Lee, M. H., Jie, E. Y., Ahn, W. S., Joo, S. J., Ahn, J.-W., Jo, Y. D., Ahn, S.-J., & Kim, S. W. (2020). Development of a Rapid Selection System for Salt-Resistant Mutants of Nicotiana benthamiana through Protoplast Culture after Gamma Irradiation. Plants, 9(12), 1720. https://doi.org/10.3390/plants9121720






