Dactylorhiza hatagirea (D. Don) Soo: A Critically Endangered Perennial Orchid from the North-West Himalayas
Abstract
1. Introduction
Methodology
2. Orchids in Medicine: Special Reference to D. hatagirea
3. Morphological and Anatomical Features of D. hatagirea
4. Distribution, Trade, and Consumption
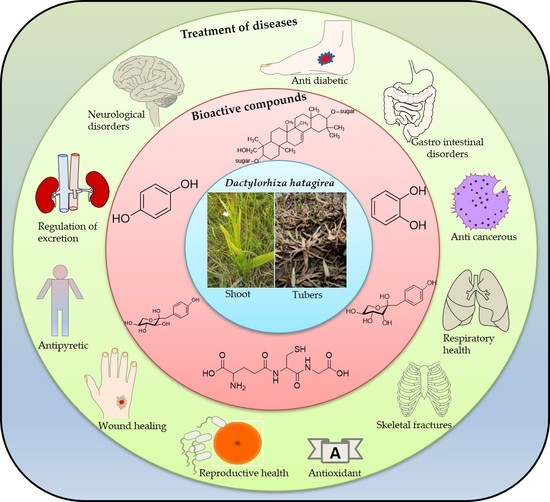
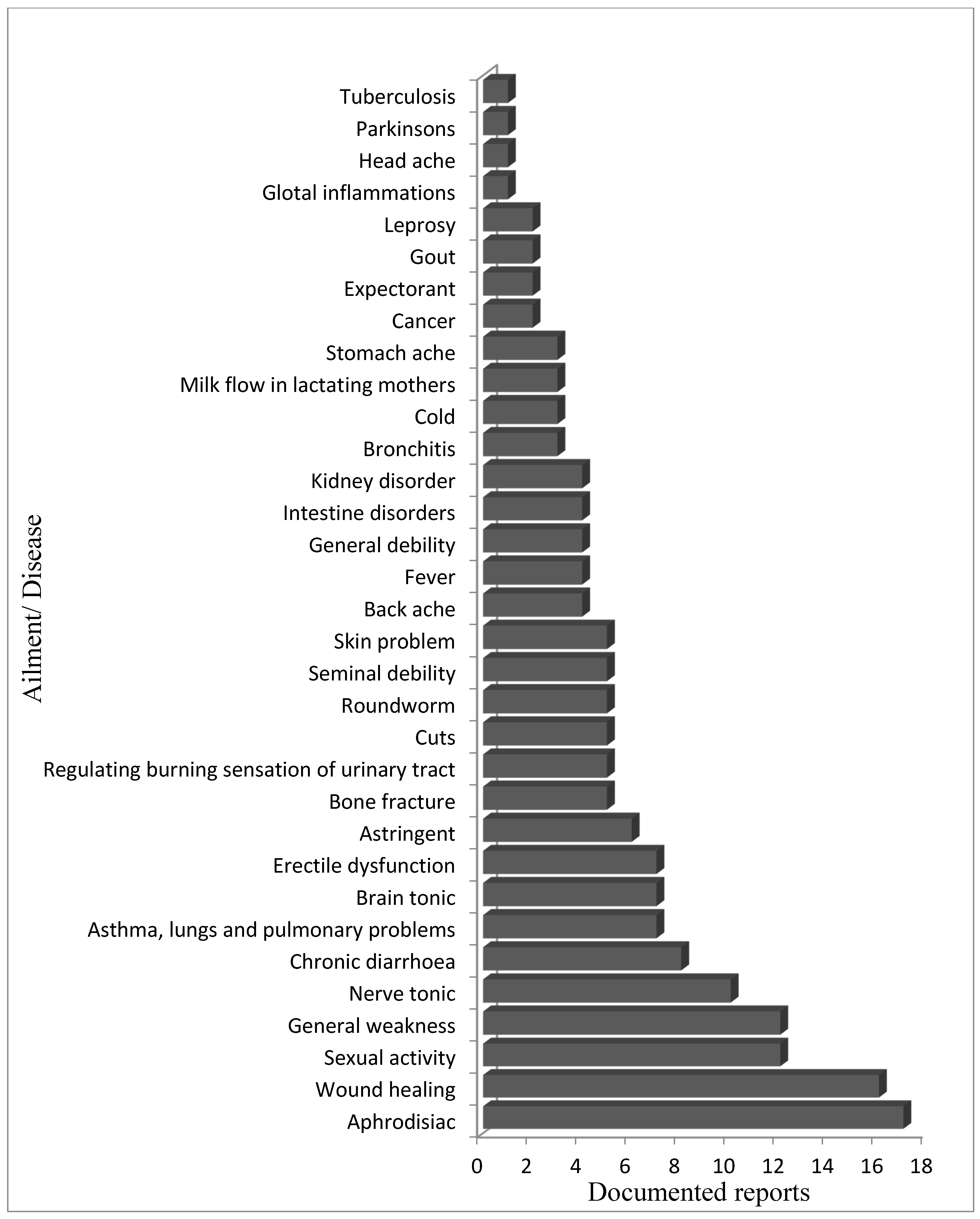
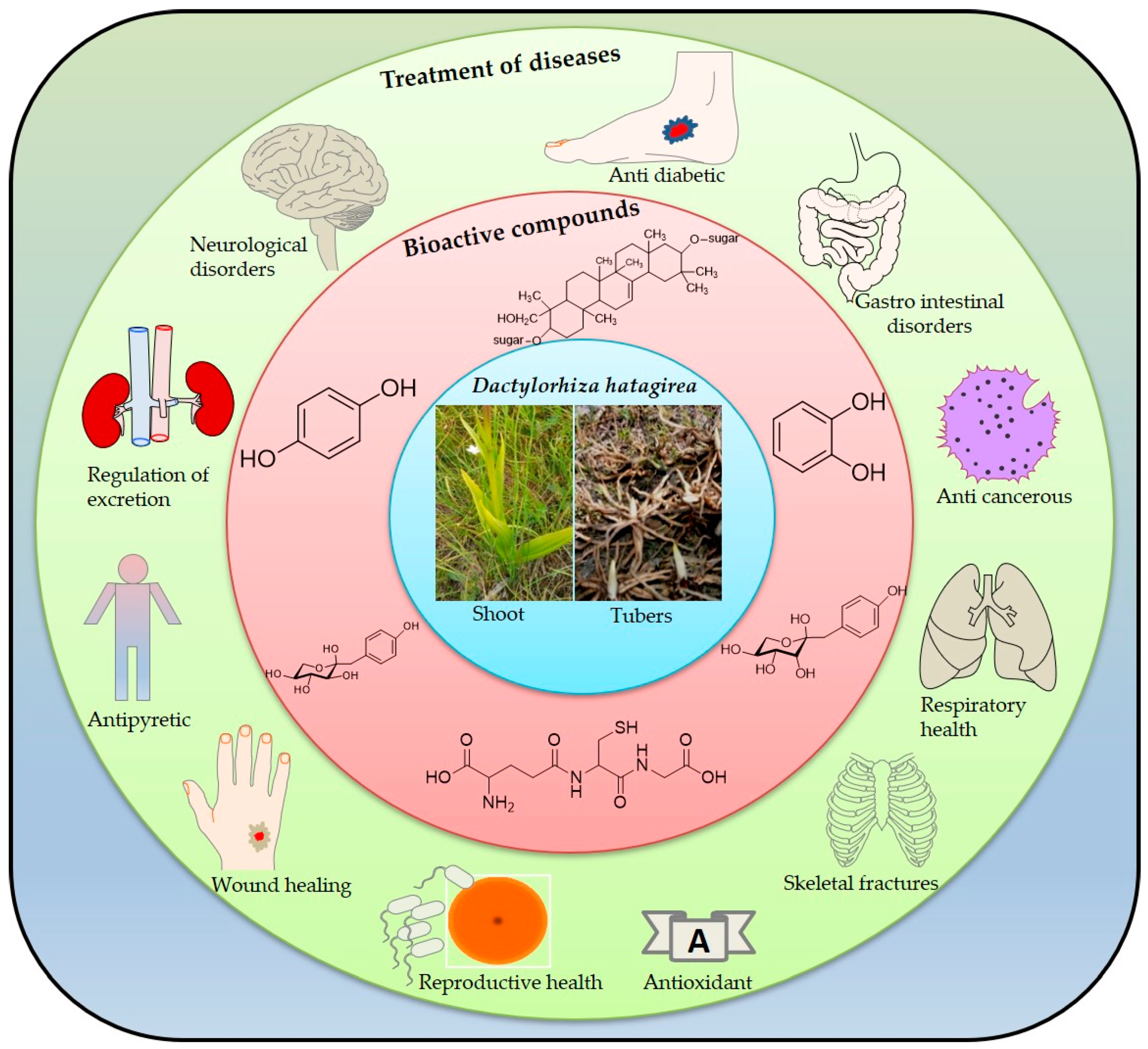
5. Ethnopharmacological Importance of D. hatagirea
6. Bioactive Compounds of D. hatagirea
7. Pharmacological Importance of D. hatagirea
7.1. Antibacterial Activity
7.2. Anti-Inflamatory Activity
7.3. Neuropharmacological Activity
7.4. Anti-Cancerous Activity
7.5. Anti-Diabetic Activity
7.6. Other Applications
8. Conservation Approaches
9. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Schipmann, U.; Leaman, D.J.; Cunningham, A.B. Impact of Cultivation and Gathering of Medicinal Plants on Biodiversity: Global Trends and Issues. In Biodiversity and the Ecosystem Approach in Agriculture; FAO: Rome, Italy, 2002; pp. 1–21. [Google Scholar]
- Prajapati, N.D.; Purohit, S.S.; Sharma, A.K.; Kumar, T. A Handbook of Medicinal Plants; Agrobios Publisher: Jodhpur, India, 2003. [Google Scholar]
- Malik, A.R.; Siddique, M.A.A.; Sofi, P.A.; Butola, J.S. Ethnomedicnal practices and conservation status of medicinal plants of North Kashmir Himalayas. Res. J. Med. Plant 2011, 5, 515–530. [Google Scholar]
- Joshi, K.; Chavan, P.; Warude, D. Molecular markers in herbal drug technology. Curr. Sci. 2009, 87, 159–165. [Google Scholar]
- Prota, A.E.; Bargsten, K.; Zurwerra, D.; Field, J.J.; Díaz, J.F.; Altmann, K.-H.; Steinmetz, M.O. Molecular mechanism of action of microtubule-stabilizing anticancer agents. Science 2013, 339, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Damale, M.G.; Shamkuwar, P.B.; Pawar, D. Traditional medicinal plants for anticancer activity. Int. J. Curr. Pharm. Res. 2013, 5, 4. [Google Scholar]
- Govind, P. Some important anticancer herbs: A review. Int. Res. J. Pharm. 2011, 2, 45–52. [Google Scholar]
- Bashir, A.; Singh, C.; Chauhan, N.; Rani, A. A review: Ethnobotanical studiy on medicinal plants of Kargil district, Ladakh, India. J. Emerg. Technol. Innov. Res. 2018, 5, 181–196. [Google Scholar]
- Dar, R.A.; Shahnawaz, M.; Qazi, P.H. General overview of medicinal plants: A review. J. Pharmacol. 2017, 6, 349–351. [Google Scholar]
- Sharma, S.; Jain, P.K.; Parkhe, G. Extraction, phytochemical screening and anti-inflammatory activity of hydro ethanolic extracts of roots of D. hatagirea. J. Drug. Discov. Ther. 2020, 19, 86–90. [Google Scholar] [CrossRef]
- Sirohi, B.; Sagar, R. Antipyretic activity of hydroalcholic extract of D. hatagirea roots and Lavandula stoechas flowers on Brewers yeast induced Pyrexia in Wistar rats. J. Drug. Discov. Ther. 2019, 9, 701–704. [Google Scholar]
- Popli, D.; Sood, H. Optimization of Liquid Media for Increasing The Biomass of Dactylorhiza Hatagirea; JUIT: Waknaghat, India, 2016. [Google Scholar]
- Sirohi, B.; Sagar, R. Effect of Hydroalcoholic Extract of D. hatagirea Roots & Lavandula Stoechas Flower on Thiopental Sodium Induced Hypnosis in Mice. J. Drug Deliv. Ther. 2019, 9, 414–417. [Google Scholar]
- Hossain, M.M. Therapeutic orchids: Traditional uses and recent advances-An overview. Fitoterapia 2011, 82, 102–140. [Google Scholar] [CrossRef]
- Reinikka, M.A. A History of the Orchid; Portland Timber Press: Portland, OR, USA, 1995; Volume 23. [Google Scholar]
- Jalal, J.S.; Kumar, P.; Pangtey, Y. Ethnomedicinal Orchids of Uttarakhand, Western Himalaya. Ethnobot. Leafl. 2008, 12, 1–5. [Google Scholar]
- Satish, M.N.; Abhay, P.S.; Chen-Yue, L.; Chao-Lin, K.; Hsin-Sheng, T. Studies on tissue culture of Chinese medicinal plant resources in Taiwan and their sustainable utilization. Bot. Bull. Acad. Sin. 2003, 44, 79–98. [Google Scholar]
- Lin, Y.L.; Chen, W.P.; Macabalang, A.D. Dihydrophenanthrenes from Bletilla formosana. Chem. Pharm. Bull. 2005, 53, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; He, S.; Pan, Y.J. New dihydrodibenzoxepins from Bulbophyllum kwangtungense. Planta Med. 2006, 72, 1244–1247. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Yut, H.; Qin, C.W.; Zhangt, Y.; Liu, Y.; Wang, J. Bulbophyllum Odoratissimum 3,7-Dihydroxy-2,4,6-trimethoxyphenanthrene. J. Korean Chem. Soc. 2007, 51, 352–355. [Google Scholar]
- Yoshikawa, M.; Murakami, T.; Kishi, A.; Sakurama, T.; Matsuda, H.; Nomura, M.; Matsuda, H.; Kubo, M. Novel indole S,O-bisdesmoside, calanthoside, the precursor glycoside of tryptanthrin, indirubin, and isatin, with increasing skin blood flow promoting effects, from two Calanthe species (Orchidaceae). Chem. Pharm. Bull. 1998, 46, 886–888. [Google Scholar] [CrossRef]
- Shimizu, M.; Shogawa, H.; Hayashi, T.; Arisawa, M.; Suzuki, S.; Yoshizaki, M.; Morita, N.; Ferro, E.; Basualdo, I.; Berganza, L.H. Anti-inflammatory constituents of topically applied crude drugs. III. Constituents and anti-inflammatory effect of Paraguayan crude drug “Tamandá cuná” (Catasetum barbatum LINDLE). Chem. Pharm. Bull. 1988, 36, 4447–4452. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Liu, G.; Zhang, J. Dactylorhin B reduces toxic effects of beta-amyloid fragment (25–35) on neuron cells and isolated rat brain mitochondria. Naunyn Schmiedebergs Arch. Pharmacol. 2006, 374, 117–125. [Google Scholar] [CrossRef]
- Shimura, H.; Matsuura, M.; Takada, N.; Koda, Y. An antifungal compound involved in symbiotic germination of Cypripedium macranthos var. rebunense (Orchidaceae). Phytochemistry 2007, 68, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.K.; Chen, C.C. Moscatilin from the orchid Dendrobrium loddigesii is a potential anticancer agent. Cancer Investig. 2003, 21, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Olof, T.C. Survival and flowering of some perennial herbs II. The behaviour of some orchids on permanent plots. Oikos 1972, 23, 23–28. [Google Scholar]
- Deciga-Campos, M.; Palacios-Espinosa, J.F.; Reyes-Ramirez, A.; Mata, R. Antinociceptive and anti-inflammatory effects of compounds isolated from Scaphyglottis livida and Maxillaria densa. J. Ethnopharmacol. 2007, 5, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Romero, Y.; Rojas, J.I.; Castillo, R.; Rojas, A.; Mata, R. Spasmolytic effects, mode of action, and structure-activity relationships of stilbenoids from Nidema boothii. J. Nat. Prod. 2004, 67, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.K.; Subramoniam, A.; Pushpangadan, P. Aphodisiac activity of Vanda tessellata. Indian J. Pharmacol. 2000, 32, 300–304. [Google Scholar]
- Chang, Y.Y.; Chiu, Y.F.; Wu, J.W.; Yang, C.H. Four orchid (Oncidium Gower Ramsey) AP1/AGL9-like MADS box genes show novel expression patterns and cause different effects on floral transition and formation in Arabidopsis thaliana. Plant Cell Physiol. 2009, 50, 1425–1438. [Google Scholar] [CrossRef]
- Kant, R.; Verma, J.; Thakur, K. Distribution pattern, survival threats and conservation of ‘Astavarga’ Orchids in Himachal Pradesh, North-West Himalaya. Plant Arch. 2012, 1, 165–168. [Google Scholar]
- Sut, S.; Maggi, F.; Acqua, S.D. Bioactive secondary metabolites from orchids (Orchidaceae). Chem. Biodivers. 2017, 14, e1700172. [Google Scholar] [CrossRef]
- Chauhan, N.S. Medicinal orchids of Himachal Pradesh. J. Orchid Soc. India 1990, 4, 99–105. [Google Scholar]
- Thakur, M.; Dixit, V.K. Evaluation of Antioxidant Activity and Ameliorative Effect Dactylorhiza Hatagirea on Sexual Dysfunction in Hyperglycemic Male Rats Department of Pharmaceutical Sciences; Dr. H.S. Gour University: Sagar, India, 2007. [Google Scholar]
- Polunin, O.; Stainton, A. Flowers of the Himalaya New Delhi; Oxford University Press: Oxford, UK, 1984; pp. 37–41. [Google Scholar]
- Bhatt, A.; Joshi, S.K.; Gairola, S. Dactylorhiza hatagirea (D.Don) Soo-a west Himalayan Orchid in peril. Curr. Sci. 2005, 89, 610–612. [Google Scholar]
- Bhakta Bahadur Raskoti. The Orchid of Nepal. In Bhakta Bahadur Raskoti and Rita Ale; Bhakta Bahadur Raskoti: Beijing, China, 2009. [Google Scholar]
- Gale, S.W.; Cribb, P.J. Dactylorhiza Necker ex Nevski, Fl. URSS 4: 697, 713. 1935, nom. Cons. Flora China 2009, 25, 114–117. [Google Scholar]
- Samant, S.S.; Dhar, U.; Palni, L.M.S. Medicinal Plants of Indian Himalaya. In Diversity Distribution Potential Values; Gyanodaya Prakashan: Nanital, India, 1998. [Google Scholar]
- Badola, H.K.; Pal, M. Endangered Medicinal plant in Himachal Pradesh. Curr. Sci. 2002, 7, 797–798. [Google Scholar]
- Dhar, U.; Kachroo, P. Alpine flora of Kashmir Himalaya; Scientific Publishers: Jodhpur, India, 1983. [Google Scholar]
- Aswal, B.S.; Mehrotra, B.N. Flora of Lahaul-Spiti; Bishan Singh Mahendra Pal Singh: Dehra Dun, India, 1994. [Google Scholar]
- Hajra, P.K.; Balodi, B. Plant Wealth of Nanda Devi Biosphere Reserve; Botanical Survey of India: Calcutta, India, 1995. [Google Scholar]
- Samant, S.S.; Dhar, U.; Rawal, R.S. Himalayan Medicinal Plants-Potential and Prospects; Palni, L.M.S., Samant, S.S., Eds.; Gyanodaya Prakashan: Nainital, India, 2001; pp. 166–184. [Google Scholar]
- Warghat, A.R.; Bajpai, P.K.; Sood, H.; Chaurasia, O.P.; Srivastava, R.B. Morphometric analysis of Dactylorhiza hatagirea (D. Don), a critically endangered orchid in cold desert Ladakh region of India. Afr. J. Biotechnol. 2012, 11, 11943–11951. [Google Scholar]
- Nautiyal, S.; Rajan, K.S.; Shibasaki, R. Interaction of Biodiversity and Economic Welfare—A Case Study from the Himalayas of India. J. Environ. Inform. 2005, 2, 111–119. [Google Scholar] [CrossRef]
- Selvam, A.B.D. Pharmacognosy of Negative Listed Plants; Botanical Survey: Kolkata, India, 2012; pp. 59–68. [Google Scholar]
- Tripathi, I.P. Chemistry, Biochemistry and Ayurveda of Indian Medicinal Plants; International E—Publication 427: Indore, India, 2013; Volume 34. [Google Scholar]
- Dhiman, N.; Sharma, N.K.; Thapa, P.; Sharma, I.; Swarnkar, M.K.; Chawla, A.; Shankar, R.; Bhattacharya, A. De novo transcriptome provides insights into the growth behavior and resveratrol and trans-stilbenes biosynthesis in Dactylorhiza hatagirea—An endangered alpine terrestrial orchid of Western Himalayas. Sci. Rep. 2019, 9, 13133. [Google Scholar] [CrossRef] [PubMed]
- Kala, C.P. Assessment of species rarity. Curr. Sci. 2004, 86, 1058–1059. [Google Scholar]
- Badola, H.K.; Aitken, S. The Himalayas of India: A treasury of Medicinal plant under siege. Biodiversity 2003, 4, 3–13. [Google Scholar] [CrossRef]
- Kunwar, R.M.; Nepal, B.K.; Kshherti, H.B.; Rai, S.K.; Bussmann, R.W. Ethnomedicine in Himalaya: A case study from Dolpa, Humla, Jumla and Mustang Districts of Nepal. J. Ethnobiol. Ethnomed. 2006, 2, 1–6. [Google Scholar] [CrossRef]
- Man, R.; Samant, S.S. Diversity, indigenous uses and conservation status of medicinal plants in Manali wildlife sanctuary, North West Himalaya. Indian J. Tradit. Knowl. 2011, 3, 439–459. [Google Scholar]
- Uniyal, S.K.; Awasthi, A.; Rawat, G.S. Current status and distribution of commercially exploited medicinal and aromatic plants in upper Gori Valley, Kumaon Himalaya, Uttaranchal. Curr. Sci. 2002, 82, 1246–1252. [Google Scholar]
- Popli, D. Elicitation of Dactylorhin-E and Studying Anti-Cancerous Potential of Dactylorhiza Hatagirea D. Don; Dissertation submitted to Jay Pee University of Information and Technology: Waknaghat, India, 2017; pp. 35–39. [Google Scholar]
- Giri, D.; Tamata, S. A general account on medicinal uses of D. hatagirea. N. Y. Sci. J. 2010, 2, 78–79. [Google Scholar]
- Rana, C.S.; Tiwari, J.K.; Dangwal, L.R.; Sundriyal, R.C. Herbal remedies for sexual capability. Indian J. Tradit. Knowl. 2012, 4, 646–651. [Google Scholar]
- Chauhan, N.S. Medicinal Wealth of Pabbar Valley in Himachal Pradesh. Master’s Thesis, HPKVV Palampur, Palampur, India, 1984; pp. 68–69. [Google Scholar]
- Bancroft, J. The endocrinology of sexual arousal. J. Endocrinol. 2005, 186, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.M.; Raj, X.J.; Kumar, G.P.; Sunil, G.; Singh, S.B. Phytofoods of Nubra valley, Ladakh—The cold desert. Indian J. Tradit. Knowl. 2010, 2, 303–308. [Google Scholar]
- Balkrishan, A.; Srivastava, A.; Mishra, R.K.; Patel, S.P.; Vashistha, R.K.; Singh, A. Astavarga plants—Threatened medicinal herbs of the North-West Himalaya. Int. J. Med. Aromat. Plants 2012, 2, 661–667. [Google Scholar]
- Khajuria, A.K.; Kumar, G.; Bisht, N.S. Diversity with ethnomedicinal notes on Orchids: A case study of Nagdev forest range, PauriGarhwal, Uttarakhand, India. J. Med. Plants Stud. 2017, 1, 171–174. [Google Scholar]
- Pant, S.; Rinchen, T. Dactylorhiza hatagirea: A high value medicinal orchid. J. Med. Plants Res. 2012, 6, 3522–3524. [Google Scholar]
- Uprety, Y.; Asselin, H.; Emmanuel, B.K.; Yadav, S.; Shrestha, K.K. Indigenous use and bio-efficacy of medicinal plants in the Rasuwa District, Central Nepal. J. Ethnobiol. Ethnomed. 2010, 3, 187–195. [Google Scholar] [CrossRef]
- Rawat, V.S.; Jalal, J.S. Sustainable Utilization of Medicinal Plants by Local Community of Uttarkashi District of Garhwal, Himalaya, India. Eur. J. Med. Plants 2011, 1, 18–25. [Google Scholar] [CrossRef]
- Arora, M.; Mahajan, A.; Sembi, J.K. A Review on Phytochemical and Pharmacological Potential of Family Orchidaceae. Int. Res. J. Pharm. 2017, 8, 9–24. [Google Scholar] [CrossRef]
- Tsering, J.; Tam, N.; Tag, H.; Gogoi, B.J.; Apang, O. Medicinal Orchids of Arunachal Pradesh: A review Bulletin of Arunachal. For. Res. 2017, 32, 1–16. [Google Scholar]
- Ballabh, B.; Chaurasia, O.; Ahmed, Z.; Singh, S.B. Traditional medicinal plants of cold desert Ladakh Used against kidney and urinary disorders. J. Ethnopharmacol. 2008, 2, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.S. Dactylorhiza hatagirea D Don Soo. In Medicinal and Aromatic Plants of Himachal Pradesh; Indus Publication Company: New Delhi, India, 1999; pp. 180–182. [Google Scholar]
- Watanabe, T.; Rajbhandari, K.R.; Malla, K.J.; Yahara, S. A Handbook of Medicinal Plants of Nepal; Kobfai Publishing Project Foundation for Democracy and Development Studies: Bangkok, Thailand, 2005. [Google Scholar]
- Bhatt, D.; Sharma, P.; Sharma, L.; Joshi, G.C. Folk herbal remedies for skin in Kamaun Himalaya. J. Non Timber For. Prod. 2012, 4, 309–312. [Google Scholar]
- Vij, S.P.; Srivastav, R.C.; Mainra, A.K. On the occurrence of Dactylorhiza hatagirea (D.Don) Soo in Sikkim. Orchard News 1992, 9, 14–15. [Google Scholar]
- Sharma, P.V.; Charaka, S. Chaukhambha orientalis. Varanasi India 2001, 2, 7–14. [Google Scholar]
- Hamilton, A.C.; Radford, E.A. Identification and Conservation of Important Plant Areas for Medicinal Plants in the Himalaya; Plant life International: Salisbury, UK; Ethnobotanical Society of Nepal: Kathmandu, Nepal, 2007; pp. 45–51. [Google Scholar]
- Arditti, J. Factors affecting the germination of orchid seeds. Bot. Rev. 1967, 33, 1–97. [Google Scholar] [CrossRef]
- Khan, S.W.; Khatoon, S. Ethnobotanical Studies on Some Useful Herbs of Haramosh and Bugrote Valleys in Gilgit, Northern Areas of Pakistan. Pak. J. Bot. 2008, 1, 43–58. [Google Scholar]
- Ballabh, B.; Chaurasia, O.P. Medicinal plants of cold desert Ladakh used in the treatment of stomach disorders. Indian J. Tradit. Knowl. 2009, 8, 185–190. [Google Scholar]
- Dorji, K.L. Ecological status of high-altitude medicinal plants and their sustainability: Lingshi, Bhutan. BMC Ecol. 2016, 16, 45. [Google Scholar]
- Kizu, H.; Kaneko, E.I.; Tomimore, T. Studies on Nepalese Crude Drugs. XXVI.1) Chemical Constituents of PanchAunle, the Roots of Dactylorhiza hatagirea D. DON. Chem. Pharm. Bull. 1999, 11, 1618–1625. [Google Scholar] [CrossRef]
- Ranapal, S. An Assessment of Status and Antibacterial Properties of Dactylorhiza hatagirea in Annapurna Conservation Area (A Case Study of Paplekharka, Lete VDC, Mustang). Bachelor’s Thesis, Institute of Forestry, Tribhuvan University, Pokhara, Nepal, 2009. [Google Scholar]
- Vij, S.P.; Pathak, P.; Mahant, K.C. Green pod culture of a therapeutically important species D. hatagirea (D.Don) Soo. J. Orchid Soc. India 1995, 9, 7–12. [Google Scholar]
- Charpinella, M.C.; Herrero, G.G.; Alonso, R.A.; Palacios, S.M. Antifungal Activity of Melia azedarch Fruit Extract. Fitterapia 1999, 70, 296–298. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Badere, R.; Singh, S.B. Antibacterial and antioxidant activities of ethanol extracts from trans Himalayan medicinal plants. Pharmacogn. J. 2010, 2, 62–69. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Warren, M.S.; Lee, A.; Galazzo, J.; Fronko, R.; Lee, M.; Blais, J.; Cho, D.; Chamberland, S.; Renau, T.; et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: Novel agents for combination therapy. Antimicrob. Agents Chemother. 2001, 1, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Stavri, M.; Piddock, L.J.; Gibbons, S. Bacterial efflux pump inhibitors from natural sources. J. Antimicrob. Chemother. 2007, 6, 1247–1260. [Google Scholar] [CrossRef] [PubMed]
- Neyfakh, A.A.; Bidnenko, V.E.; Chen, L.B. Efflux-mediated multidrug resistance in Bacillus subtilis: Similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA. 1991, 88, 4781–4785. [Google Scholar] [CrossRef]
- Urmila; Jandaik, S.; Mehta, J.; Mohan, M. Synergistic and efflux pump inhibitory activity of plant extracts and antibiotics on Staphylococcus aureus strains. Asian J. Pharm. Clin. Res. 2016, 2, 277–282. [Google Scholar]
- Farooqui, A.; Khan, A.; Borghetto, I.; Kazmi, S.U.; Rubino, S.; Paglietti, B. Synergistic antimicrobial activity of Camellia sinensis and Juglans regia against multidrug-resistant bacteria. PLoS ONE 2015, 10, 0118431. [Google Scholar] [CrossRef]
- Guiotti, A.M.; Cunha, B.G.; Paulini, M.B.; Goiato, M.C.; Dos Santos, D.M.; Duque, C.; Caiaffa, K.S.; Brandini, D.A.; De Oliveira, D.T.N.; Brizzotti, N.S.; et al. Antimicrobial activity of conventional and plant-extract disinfectant solutions on microbial biofilms on a maxillofacial polymer surface. J. Prosthet. Dent. 2016, 116, 136–143. [Google Scholar] [CrossRef]
- Widen, C.; Renvert, S.; Persson, G.R. Antibacterial activity of berry juices, an in vitro study. Acta Odontol. Scand. 2015, 73, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Z.; Zou, X.; Zhong, X.; Liang, X.; Zhou, J. The antibacterial effect of Urena lobata L. from Guangxi on mice with Staphylococcus aureus pneumonia. Afr. J. Tradit. Complementary Altern. Med. 2017, 14, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiology 2017, 6, e00459. [Google Scholar] [CrossRef] [PubMed]
- Flower, A.; Harman, K.; Lewith, G.; Moore, M.; Bishop, F.L.; Stuart, B.; Lampert, N. Standardised Chinese herbal treatment delivered by GPs compared with individualised treatment administered by practitioners of Chinese herbal medicine for women with recurrent urinary tract infections (RUTI): Study protocol for a randomised controlled trial. Trials 2016, 17, 358. [Google Scholar]
- Wojnicz, D.; Kucharska, A.Z.; Sokol-Lętowska, A.; Kicia, M.; Tichaczek-Goska, D. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol. Res. 2012, 40, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Grootveld, M.; Arroo, R.; Ruiz-Rodado, V.; Price, P.; Laird, K. A multifactorial comparison of ternary combinations of essential oils in topical preparations to current antibiotic prescription therapies for the control of acne vulgaris-associated bacteria. Phytother. Res. 2017, 31, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Hajimahmoodi, M.; Shams-Ardakani, M.; Saniee, P.; Siavoshi, F.; Mehrabani, M.; Hosseinzadeh, H.; Foroumadi, P.; Safavi, M.; Khanavi, M.; Akbarzadeh, T.; et al. In vitro antibacterial activity of some Iranian medicinal plant extracts against Helicobacter pylori. Nat. Prod. Res. 2011, 25, 1059–1066. [Google Scholar] [CrossRef]
- Sirohi, B.; Sagar, R.; Jain, P. Evaluation of the Anti-Inflammatory Activity of Hydroalcoholic Extract of Dactylorhiza hatagirea Roots and Lavandula stoechas Flower in Rats EC Pharmacol. Toxicology 2019, 7, 110–118. [Google Scholar]
- Kesari, A.N.; Kesari, S.; Singh, S.K.; Gupta, R.K.; Watal, G. Studies on the glycemic and lipidemic effect of Murraya koenigii in experimental animals. J. Ethnopharmacol. 2007, 112, 305–311. [Google Scholar] [CrossRef]
- Mohan, S.; Alsawalha, M.; Al-Subaei, A.; Al-Jindan, R.; Bolla, S.; Sen, D.; Balakrishna, J.; Ravi, P.; Gollapalli, S.R.; Veeraraghavan, V.; et al. Anti-diabetic activities of Dactylorhiza hatagirea leaf extract in 3T3-L1 cell line model. Pharmacogn. Mag. 2019, 15, 212. [Google Scholar] [CrossRef]
- Choukarya, R.; Choursia, A.; Rathi, J. In vivo and in vitro anti-diabetic activity of hydroalcholic extract of D. hatagirea roots: An evaluation of possible phytoconstituents. J. Drug. Discov. Ther. 2020, 9, 76–81. [Google Scholar]
- Anon. The Wealth of India; CSIR: New Delhi, India, 1976; Volume 10, pp. 77–81. [Google Scholar]
- Singh, B.; Gupta, V.; Bansal, P.; Singh, R.; Kumar, D. Pharmacological potential of plant used as aphrodisiacs. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 104–113. [Google Scholar]
- Bulpitt, C.J. The Uses and Misuses of Orchids in Medicine. QJM Int. J. Med. 2005, 98, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Warghat, A.R.; Bajpai, P.K.; Srivastava, B.R.; Chaurasia, O.P.; Chauhan, R.S.; Sood, H. In vitro protocorm development and mass multiplication of an endangered orchid, Dactylorhiza hatagirea. Turk. J. Bot. 2014, 38, 737–746. [Google Scholar] [CrossRef]
- Aggarwal, S.; Zettler, L.W. Reintroduction of an endangered terrestrial orchid, Dactylorhiza hatagirea (D. Don) Soo, assisted by symbiotic seed germination: First report from the Indian subcontinent. Nat. Sci. 2010, 8, 139–145. [Google Scholar]
- Novotna, K.W.; Vejsadova, H.; Kindlmann, P. Effects of sugars and growth regulators on in vitro growth of Dactylorhiza species. Biol. Plant. 2007, 51, 198–200. [Google Scholar] [CrossRef]
- Shrestha, N.; Shrestha, K.K. Vulnerability assessment of high-valued medicinal plants in Langtang National Park, Central Nepal. Biodiversity 2012, 1, 24–36. [Google Scholar] [CrossRef]
- Giri, D.; Tamta, S. Propagation and conservation of Dactylorhiza hatagirea (D.Don) Soo, an endangered alpine orchid. Afr. J. Biotechnol. 2012, 11, 12586–12594. [Google Scholar]
- Pillon, Y.; Fay, M.F.; Shipunov, A.B.; Chase, M.W. Species diversity versus phylogenetic diversity: A practical study in the taxonomically difficult genus Dactylorhiza (Orchidaceae). Biol. Conserv. 2006, 4, 129. [Google Scholar] [CrossRef]

| Status | Category | Reference(s) |
|---|---|---|
| Endangered | CAMP and CITES | [52] |
| Critically Endangered | CAMP and CITES | [53,54] |
| Vulnerable | CAMP | [53] |
| Listed among Appendix II | CITES | [55] |
| Critically rare | IUCN | [53,55] |
| Threatened | IUCN | [53] |
| NPSMHCC | MFSC, Kathmandu | [56] |
| S. No | Ailment/Use | Plant Part | Place/Country | Mode of Application | References |
|---|---|---|---|---|---|
| 1 | Respiratory (asthma, bronchitis, lungs, and other pulmonary problems) | leaves and tubers | India (Ladakh, Gharwal Himalaya) Nepal (Dolpa, Rasuwa, Humla, Jumla, and Mustang districts) |
| [35,61,62,63,64,65,66] |
| 2 | Neurological (brain tonic, nerve tonic) | leaves and tubers | India (Gharwal Himalaya), Nepal |
| [35,63,65,67] |
| 3 | Digestive (stomachache, chronic diarrhea, intestinal disorders) | tubers | India (Gharwal Himalaya, Arunachal Pradesh), Nepal (Dolpa, Rasuwa, Humla, Jumla, and Mustang districts) |
| [35,37,58,65,67,68] |
| 4 | Urinary (kidney disorders, burning sensation, and urine discharge) | tubers | India (Gharwal Himalaya) |
| [53,61,69] |
| 5 | Sexual (sexual activity, seminal debility, erectile dysfunction) | tubers | India (Gharwal Himalaya), Pakistan (Gilgit), Nepal (Dolpa, Rasuwa, Humla, Jumla, and Mustang districts) |
| [70,71] |
| 6 | External uses (headache, wound healing, skin problems) | tubers | India (Gharwal Himalaya, Kuman Himalayas, Arunachal Pradesh), Nepal (Dolpa, Rasuwa, Humla, Jumla, and Mustang districts) |
| [65,72,73,74,75] |
| 7 | Others (backache, bone fracture, fever, weakness, general debility, milk flow in lactating mothers) | tubers and leaves | India (Gharwal Himalaya, Western Himalaya, Manali), Pakistan (Gilgit and Bugrot valley), Nepal (Rasuwa district) |
| [13,54,57,58,62,65,66,76,77,78,79,80] |
| Name | Synonym | Structure | References |
|---|---|---|---|
| 1-deoxy-1-4 hydroxyphenyl-L-sorbose | Dactylose A |  | [80] |
| 1-deoxy-1-4 hydroxyphenyl-L-tagatose | Dactylose B | 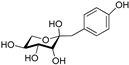 | [80] |
| (2R)-2-β-D-glucopyranosyloxy-2(2-methylpropyl) butanedioic acid bis (4- β-D-glucopuranosyloxybenzyl) ester | Dactylorhin A | 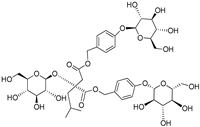 | [80] |
| (2R-3S)-2- β-D-glucopyranosyloxy-3-hydroxy-2(2-methylpropyl) butanedioic acid bis (4 β-D—glucopyranosyloxybenzyl) ester | Dactylorhin B | 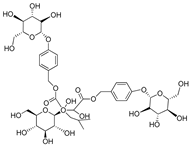 | [80] |
| (2R)-2-β-D-glucopyranosyloxy-2(2-methylpropyl) butanedioic acid | Dactylorhin C | 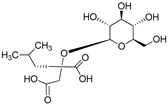 | [80] |
| (2R-3S)-2- β-D-glucopyranosyloxy-3-hydroxy-2(2-methylpropyl) butanedioic acid 1-(4- β-D- glucopyranosyloxybenzyl) ester | Dactylorhin D | 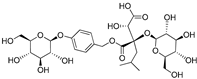 | [80] |
| (2R)-2-β-D-glucopyranosyloxy-2(2-methylpropyl) butanedioic acid 1-(4- β-D-glucopuranosyloxybenzyl) ester | Dactylorhin E | 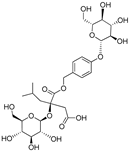 | [80] |
| (E)-5-(4-hydroxystyryl) benzene-1,3-diol | Resveratrol | 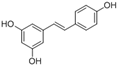 | [50] |
| 1H- Indole | Indole alkaloids | 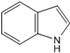 | [50] |
| Napthalane -1,4- dione | Napthoquinone | 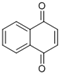 | [50] |
| (R)- 5- ((S)- 1,2- dihydroxyethyl)- 3,4 dihydroxy furan-2 (5H)-one | Ascorbic acid | 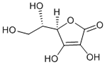 | [50] |
| 2- methyl- 3 ((7R, 11R,E), 3,7,11,15- tetra methylhexadec 2- en-1-yl) naphthalene-1,4-dione | Phylloquinone | 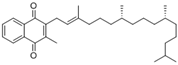 | [50] |
| Militarrin | 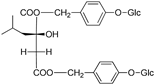 | [80] | |
| Lesoglossin | 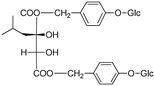 | [80] | |
| Pyrocatechol | 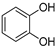 | [80] | |
| Glucomannan | 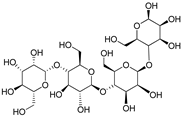 | [50] | |
| Saponin | 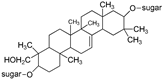 | [50] |
| Plant Extract/Antibiotics | Resistance Against | Dosage | Effect | Reference |
|---|---|---|---|---|
| Antibacterial | ||||
| Shoot extract | SA, EC, SF, PA, BS | 500 mg/mL | Best inhibition for EC, better for SA, and good for SF, SA, PA, and BS | [81] |
| Root extract | 500 mg/mL | Best inhibition for SF, better for SA, EC, BS, and good for PA | [81,84] | |
| Antioxidant | ||||
| Root extract | 3 μg/mL | Best antioxidant activity | [84] | |
| Plant extract | FRAP | 3% | Antioxidant activity | [102] |
| Root extract | NO, H2O2 | NA | Better antioxidant activity | [103] |
| Anti-inflammatory | ||||
| Root extract | Carrageenan-induced paw oedema | 100, 200, 300 mg/kg | Shows decrease in the volume of paw with increase in dosage | [11,98] |
| Root extract | Cotton pellet granuloma | 100, 200, 300 mg/kg | Reduced granuloma formation with increase in dosage | [98] |
| Neuropharmacological | ||||
| Root extract | Hypnosis | 100, 200, 300 mg/kg | Shows prolonged hypnosis with increase in dosage | [14] |
| Anti-pyretic | ||||
| Root extract | Brewer’s yeast induced pyrexia | 100, 200, 300 mg/kg | The influence of pathogenic fever was decreased with dose-dependent concentrations | [12] |
| Anti-diabetic Blood biochemical parameters and3T3-L1 diabetic cell line | ||||
| Root extract | Blood glucose | 100, 200 mg/kg | Shows dose-dependent decrease in blood glucose with increase in time | [101] |
| Root extract | TC, TG and TP | 100, 200 mg/kg | TC and TG show dose-dependent decrease while TP shows increase with increase in dosage concentration | |
| Leaf extract | α amylase activity | 31.25 and 500 μg/mL | α amylase activity decreased with increased dosage | [100] |
| Leaf extract | 3T3-L1 diabetic cell line | 25–400 μg/mL | 3T3-L1 cell line viability decreased with increase in dosage | |
| Leaf extract | GLUT 4 expression and NBDG uptake | 100 μg/mL | Increased expression | |
| Anti-cancerous potential | ||||
| Shoot extract | HEK- 239, MDA, MB- 231, MCF 7 cell lines | 250–1000 μg/mL | Cell viability decreases with increased dosage | [13] |
| Root extract | 250–1000 μg/mL | Cell viability decreases with increased dosage | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wani, I.A.; Kumar, V.; Verma, S.; Tasleem Jan, A.; Rather, I.A. Dactylorhiza hatagirea (D. Don) Soo: A Critically Endangered Perennial Orchid from the North-West Himalayas. Plants 2020, 9, 1644. https://doi.org/10.3390/plants9121644
Wani IA, Kumar V, Verma S, Tasleem Jan A, Rather IA. Dactylorhiza hatagirea (D. Don) Soo: A Critically Endangered Perennial Orchid from the North-West Himalayas. Plants. 2020; 9(12):1644. https://doi.org/10.3390/plants9121644
Chicago/Turabian StyleWani, Ishfaq Ahmad, Vijay Kumar, Susheel Verma, Arif Tasleem Jan, and Irfan A. Rather. 2020. "Dactylorhiza hatagirea (D. Don) Soo: A Critically Endangered Perennial Orchid from the North-West Himalayas" Plants 9, no. 12: 1644. https://doi.org/10.3390/plants9121644
APA StyleWani, I. A., Kumar, V., Verma, S., Tasleem Jan, A., & Rather, I. A. (2020). Dactylorhiza hatagirea (D. Don) Soo: A Critically Endangered Perennial Orchid from the North-West Himalayas. Plants, 9(12), 1644. https://doi.org/10.3390/plants9121644