Abstract
A new dihydroisocoumarin glucoside, vacillanoside (3), and two new anthrone C-glycosides microdantin derivatives; vacillantin A (10) and B (11), together with nine known compounds belonging to the anthraquinone, anthrone and isocoumarin groups were isolated from the leaves of Aloe vacillans. The structures were determined based on spectroscopic evidence including 1D and 2D nuclear magnetic resonance (NMR) spectroscopy and high resolution mass spectrometry (HRESIMS) data, along with comparisons to reported data. The leaves were used to extract compounds with different solvents. The extracts were tested for antioxidant activity with a variety of in vitro tests including 2,2-diphenyl-1-picrylhydrazyl (DPPH•), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate (ABTS•+), ferric reducing antioxidant power assay (FRAP), superoxide, and nitric oxide radical scavenging assays. The dichloromethane fraction was most active, displaying significant free radical scavenging activity. The n-butanol fraction also showed notable activity in all assays. Therefore, these findings support the potential use of A. vacillans leaves as an antioxidant medication due to the presence of polyphenolic compounds.
1. Introduction
Aloe spp. are members of the bitter or Asphodelaceae family (previously known as Liliaceae). This family is represented by more than 600 species endemic to tropical and southern Africa, Madagascar, Jordan, the Arabian Peninsula, East Asian countries, and various islands in the Indian Ocean [1]. Aloe plants are used as traditional medicines and dietary supplements in several countries including Egypt, China, and India [2,3].
In Arabic, Aloe is known as “Alloeh”, which means “shiny substance with bitter taste”, in reference to its exudate [4]. Aloe spp., with a waxy surface on succulent leaves, are well-adapted to harsh climatic conditions with infrequent precipitation [5]. Traditionally, Aloe was used as a purgative and bowel cleansing agent, a blood purifier, a gargle for a sore throat, and externally to treat burns, venereal ulcers, and shingles [6]. The Greek Herbal of Dioscorides (41–68 AD) recommended oral use of Aloe spp. for constipation, and external application for the treatment of wounds, hemorrhoids, ulcers, and hair loss [3,5].
A vast number of reports are available on the biological activities of different extracts from Aloe spp. and isolated secondary metabolites. For example, anti-inflammatory [7], antioxidant [8], anti-aging [9], anti-diabetic [4], anticancer [10], and immunomodulatory [11] effects have been observed. Aloe is also commonly used in the food supplement industry for the management of obesity and hyperlipidemia [12]. Furthermore, in the cosmetics industry, Aloe gel is incorporated into many pharmaceutical preparations that are used externally such as cleansers, moisturizers, shampoos, lotions, and sunscreen products [5]. The internal use of this gel is regulated as a dietary supplement in the USA [13] and Europe [14]. An edible coating material made from A. vera gel increases the shelf-life of grapes and reduces the total microbial counts of stored products [15]. Different plant parts including the leaves, roots, and gels from various Aloe species have been thoroughly investigated, affording several classes of secondary metabolites including alkaloids, pre-anthraquinones, anthraquinones, anthrones, chromones, flavones, coumarin derivatives, and pyrones [16].
With approximately 24 reported species, the Aloe genus is considered one of the largest groups of succulent plants growing in the Kingdom of Saudi Arabia [17]. Aloe vacillans, Forssk. (Syn. A. dhalensis Lavrans, and A. audhalica Lavrans and hardy) grows on rocky mountain slopes in Yemen and Saudi Arabia at an altitude of approximately 8000 ft [18]. The plant is stemless, forming small rosette-shaped succulent leaves that show brown tooth margins at the base of the plant. Bright yellow to orange-red flowers are grouped in inflorescences [17].
The reported biological, therapeutic, and economic importance of the genus Aloe encouraged us to explore the chemical composition and potential biological activities of the constituents from the endemic species A. vacillans Forssk. (Figure S1) The present study deals with the isolation and identification of some constituents of A. vacillans growing in Saudi Arabia and the evaluation of the antioxidant and free radical scavenging activities of the extract and its different fractions including 2,2-diphenyl-1-picrylhydrazyl (DPPH•), 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate) (ABTS•+), ferric reducing antioxidant power assay (FRAP), and superoxide and nitric oxide radical scavenging assays.
2. Results and Discussion
2.1. Structure Elucidation of New Compounds
A phytochemical study of the CH2Cl2 and EtOAc soluble fractions of the leaves of A. vacillans using diverse chromatographic methods, afforded twelve compounds. The known compounds were identified as aloe-emodin (1) [19], feralolide (2) [20,21], 10-hydroxyaloins A and B (4, 5) [22], aloin A and B (6, 7) [19], microdontin A and B (8, 9) [23], and elgonica-dimer A (12) [24,25] (Figure 1 and Figure S2) (Tables S1 and S2). The structures of the isolated compounds were identified based on a variety of spectroscopic techniques including 1D (1H, 13C, and DEPT-13C experiments) and 2D (1H-1H COSY, 1H-13C HSQC, 1H-13C HMBC, and 1H-1H NOESY) nuclear magnetic resonance (NMR) spectroscopy. Accurate mass measurements and comparisons with published data were also used (Figures S2–S24), and electronic circular dichroism (ECD) experiments were conducted to determine the absolute configurations.
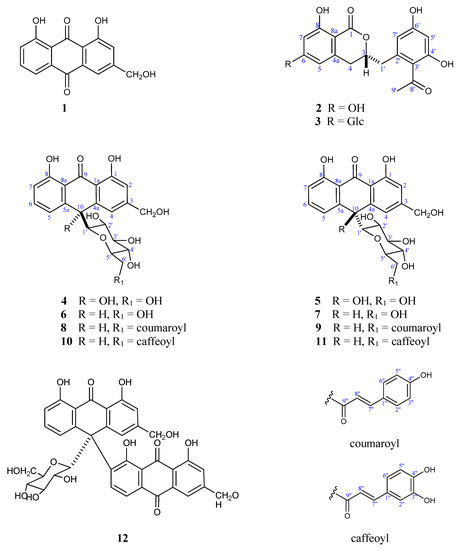
Figure 1.
Chemical structures of the isolated compounds (1–12) from A. vacillans (Glc = glucose).
Compound (3) was isolated as a white amorphous powder. The spectral data of (3) including IR, UV, and NMR were very similar to that of feralolide (2) [20], suggesting a similar dihydorisocoumarin skeleton. HRESIMS showed quasi-molecular ion peaks at m/z 507.1500 [M+H]+ (calcd 507.1503 for C24H27O12), m/z 529.1320 [M+Na]+ (calcd 529.1322 for C24H26O12Na), and m/z 545.1069 [M+K]+ (calcd 545.1061 for C24H26O12K) with 162 amu more than that of 2, suggesting the presence of a monosaccharide moiety. A significant fragment using the high-resolution electron impact mode (HR-EIMS) appeared at 327.0868 corresponding to C18H15O6 (M+-glucose). A positive Molisch’s test reflected the glycosidic nature [26]. Absorption bands at 3451, and 1645 cm−1 were observed in the IR spectrum assigned to OH and C=O, respectively.
The detailed NMR spectral analyses for 3 were also very similar to that of 2, particularly the aglycone that showed slight chemical shift differences due to the glycosylation site of the aglycone (i.e., left-hand side of the molecule). The main difference in the 1H NMR (Table 1) data between the two compounds was the downfield shift of H-5 and H-7 from δH 6.20 and δH 6.19 (2) to δH 6.51, representing two protons (3), respectively, accompanied by the downfield shift of H-4 from δH 2.87 in 2 to δH 2.92 in 3. As expected, C-5 and C-7 in 3 were also downfield shifted by + 0.4 and + 1.4 ppm compared to those in the aglycone (2). Furthermore, the monosaccharide part was identified as glucose by the appearance of a doublet signal H-1″ at δH 4.99 with a large J value (7.2 Hz), indicating its β-configuration. The remaining proton and carbon signals of glucose were assigned carefully by 13C, DEPT-13C, and 2D NMR, and were in good agreement with those reported for glucose [27]

Table 1.
1H and 13C NMR spectroscopic data for compounds 2 and 3.
The above data confirmed that 3 is the glycoside of 2. The glycosylation site was confirmed as C-6 by J2&3 bond correlations observed in the HMBC experiment from H-1″ to C-6; and H-5, H-7 to C-6. Other significant HMBC cross-peaks were observed from H-7 to C-1; H-3 to C-1 and C-4a; H-4 to C-3 and C8a; H-1′ to C-3′ and C-7′; H-7′ to C-3′, C-2′ and C-6′; and H-5′ to C-3′ and C-6′ (Figure 2). ECD spectral data of compound 3 were similar to those reported for the known dihydroisocoumarin derivative, feralolide (2) previously isolated from A. ferox [20]. The final structure of 3 was determined as 3,4 dihydro-6-glucopyranozyl-8-hydroxy-3-(2′-acetyl-3′,5′-dihydroxyphenyl)methyl-1H-[2]benzopyran-1-one, isolated for the first time from nature and given the name vacillanoside. Notably, a similar isocoumarin glycoside (feralolide 3′-O-glycosyl) from A. hildebrandtii [27] and A. arborescens [21] was previously reported, but with a different glycosylation position.
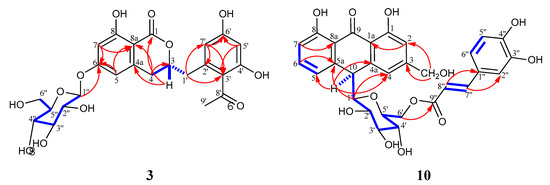
Figure 2.
Selected COSY ( ) and HMBC (H
) and HMBC (H C) correlations for compounds 3 and 10.
C) correlations for compounds 3 and 10.
 ) and HMBC (H
) and HMBC (H C) correlations for compounds 3 and 10.
C) correlations for compounds 3 and 10.
Compounds (10) and (11) were obtained as red amorphous powders. HR-ESI-MS of both compounds gave [M−H]− at m/z 579.1509 (calcd 579.1508 for C30H27O12−) with 17 degrees of unsaturation. Thirty carbon resonances were clear in the 13C-NMR and were sorted in a DEPT experiment into two oxymethylenes (δC 64.5, C-11 and δC 64.4, C-6′), 16 methines, and 12 quaternary carbons. The HR-EI-MS for both compounds showed strong peaks at 256.0735 with a relative abundance of 100% calculated for C15H12O4 and assigned to the aloe-emodin anthrone aglycone [19]. UV and IR spectral data of both compounds showed close similarity; each showed a λmax in the UV spectrum at 209, 244, 300, and 330 nm, and the IR spectrum showed bands at 3400, 1606, 1511, 1240, 1640, and 1720 cm−1, indicating the presence of hydroxyl groups, aromatic rings, and chelated carbonyl and conjugated ester carbonyl groups. Both compounds gave a positive Molisch’s test, reflecting their glycosidic nature [26].
The detailed NMR spectral analyses of 10 and 11 showed a resemblance to those of compounds 8 and 9, identified as microdontin A and B and originally isolated from A. microdonta [23]. The similarities of 10 and 11 in their NMR spectra can be summarized as follows: Both were C-glycosides of an aloe-emodin-9-anthrone derivative with a glucose unit esterified with caffeic acid at C-6′. This structure was confirmed by 1H-NMR signals for aloe-emodin-9-anthrone observed as a pair of meta-coupled aromatic protons resonating at δH 6.80 (brs, H-2) and 6.97 (brs, H-4) in (10) and 6.77 ppm (d, J = 2.2 Hz, H-2) and 6.97 (d, J = 2.2 Hz, H-4) in (11). Moreover, a monosubstituted ring A in both 10 and 11 was verified by three ABC coupled aromatic protons assigned to H-5, H-6, and H-7 that appeared at δH 6.90 (d, J = 8.0 Hz), δH 7.39 (t, J = 8.0 Hz), and δH 6.69 (d, J = 8.0 Hz) in 10, and at δH 7.02 (d, J = 7.9 Hz), δH 7.42 (br t, J = 7.9 Hz), and δH 6.81 (d, J = 7.9 Hz) in 11. The remaining protons for the aloe-emodin-9-anthrone moiety resonated at δH 4.46 (br s, H-10) in 10 and 4.54 ppm (br s) in 11, and the hydroxymethyl group at position 3 resonated at δH 4.62 (br s) and δH 4.60 (br s) and was interpreted for H2-11 in 10 and 11, respectively. The downfield carbons in the 13C-NMR at δC 195.3 ppm for 10 and 195.4 ppm for 11 were assigned to C-9.
The sugar moiety for both (10) and (11) proved to be β-D-glucopyranosyl connected to the aglycone via a C–C bond (C-glycoside), similar to microdontins A and B (8 and 9). This structure is indicated by the chemical shift of the anomeric proton of the sugar with β-configuration at δH 3.30 (d, J = 9.5 Hz) in (10) and δH 3.29 (d, J = 9.4 Hz) in (11) correlated to C-1′ carbons at δC 85.8 and 85.9 ppm, respectively, in the HSQC (Heteronuclear Single-Quantum Correlation) experiment. The remaining sugar signals (H2′–H6′) were in complete agreement with the signals reported for microdontins A and B [23]. The glycosidation site in 10 and 11 was confirmed at C-10 by significant cross-peaks in the HMBC experiment from H-1′ to C-4a and C-5a, and from H-10 to C-4, C-4a, C-5, C-5a and C-1′. Additionally, the methylene protons at C-6′ were downfield shifted to δH 3.83 (dd, J = 11.7, 6.9 Hz, H-6′a) and δH 4.23 (br d, J = 11.7 Hz, H-6′b) compared to aloins A (6) and B (7), indicating esterification at C-6′. The downfield shift of C-6′ from δC 63.1 ppm in aloin B to δC 64.4 and 64.5 ppm in 10 and 11, respectively, (around 1.5 ppm) further support C-6′ acylation. This result also agrees with HMBC correlations from H-6′ to C-9″ and from the trans-olefinic protons to C-1″, C-2″, and C-6″ (Figure 2).
A remarkable difference was observed in the aromatic region between microdontin A and B and 10 and 11. The phenolic acid in microdontins A and B was identified as p-hydroxycinnamic acid, but in 10 and 11 was identified as a caffeic acid moiety. This finding was confirmed by three coupled aromatic protons at δH 6.83 (d, J = 8.2 Hz), δH 6.97 (br d, J = 8.2 Hz), and δH 7.09 (br s), forming an ABX system and assigned for H-5″, H-6″, and H-2″. The system was accompanied with a trans-olefinic system H-7” and H-8” [δH 7.33 (d, J = 15.9 Hz), and δH 6.06 (d, J = 15.9 Hz)] in 10 and [δH 7.35 (d, J = 15.9 Hz), and δH 6.08 (d, J = 15.9 Hz)] in 11. These data matched data reported for caffeic acid [28]. The signal in 13C-NMR at δC 168.9 was assigned to C-9″ in the two compounds. HMBC correlations established further evidence for C-6′ where cross-peaks from H-6′ to C-9″ and from the trans-olefinic protons to C-1″, C-2″, and C-6″ were observed (Figure 2).
Overall, the above data prove that compounds 10 and 11 are diasteroisomers and derivatives of microdontins A and B (8 and 9). Isomer A or B was established in a NOESY experiment. Clear cross-peaks were observed from H-10 to H-4, H-5, and H-1′ in 11, but not in 10, confirming the α form in the latter. Furthermore, the α orientation of the glucose moiety at C-10 in 11 was confirmed by comparing its ECD spectrum with the related compounds, aloin B, microdontin B, and 10-hydroxy aloin B [29], proving that 11 is the β isomer and 10 the α isomer.
Trivial names, vacillantin A and B, were given to compounds 10 and 11. Notably, for all of the isolated compounds with α orientation at H-10 (6, 8, and 10), the chemical shift of H-4 was more downfield than H-5, while the opposite occurred in the β equivalents (7, 9, and 11) Table 2. Moreover, during RP C18 HPLC separation using MeOH/H2O, the polarity of the α isomer was less than the polarity of the β form.

Table 2.
1H (500 MHz) and 13C NMR (125 MHz) spectroscopic data in CD3OD for compounds 10 and 11.
2.2. Antioxidant Activities Results
Antioxidant activity was evaluated by five different spectrophotometric methods, namely DPPH (2,2-diphenyl-1-picrylhydrazyl), ABTS (2,20-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid), FRAP (Ferric reducing antioxidant power), superoxide, and nitric oxide radical-scavenging assays (Figures S25–S29). All of the extracts displayed dose-dependent reducing activity. Mean percent scavenging ± SD, was measured in DPPH, ABTS, and FRAP assays at four concentrations (10, 20, 50, and 100 µg/mL) (Table 3), while for the superoxide and nitric oxide scavenging assays, it was calculated at 20, 40, 60, 80, and 100 µg/mL. The IC50 ± SD was determined for each assay.

Table 3.
Antioxidant effects of A. vacillans extracts in DPPH, ABT, and FRAP assays.
The dichloromethane fraction demonstrated the highest radical scavenging activities at all concentrations (10, 20, 50, 100 µg/mL) (Table 3), with 81.90 ± 1.43% and 83.49 ± 3.09% scavenging at a concentration of 100 µg/mL, and IC50 of 22.14 ± 2.25 and 13.51 ± 2.33 in the DPPH and ABTS assays, respectively. These results are comparable to ascorbic acid (91.94 ± 0.92 and 90.51 ± 4.46% scavenging at 100 µg/mL concentration, and IC50 of 16.7 ± 1.96 and 10.56 ± 1.74 in the DPPH and ABTS assays, respectively).
The dichloromethane fraction also showed medium ferric reduction capability verified by a higher intensity of Perl’s Prussian blue color measured at 700 nm (1.44 ± 0.07% inhibition and IC50 30.64 ± 2.46). In comparison, ascorbic acid showed 1.84 ± 0.11 ferric reduction capability, at the same concentration (100 mg/mL) and IC50 of 16.3 ± 1.82. The n-butanol extract exhibited 0.86 ± 0.08% activity (IC50 30.64 ± 2.46), while the MeOH and the EtOAc extracts showed even weaker ferric reduction activities (IC50 of 94.83 ± 5.44 and 118.3 ± 5.8, respectively).
All tested samples produced concentration-dependent antioxidant effects in the superoxide scavenging assay (Table 4). Both CH2Cl2 and n-BuOH fractions displayed moderate free radical-scavenging, 87.90 ± 1.77% and 85.86 ± 2.26% (IC50 32.92 ± 2.99 and 46.86 ± 5.1, respectively), compared to ascorbic acid (90.92 ± 1.70% and IC50 7.09 ± 3.09).

Table 4.
Antioxidant effects of the A. vacillans extract using superoxide scavenging.
Similarly, the CH2Cl2 fraction produced the highest inhibition activity among the tested samples in the nitric oxide scavenging assay (Table 5) with 80.03 ± 3.43% inhibition at the concentration of 100 µg/mL (IC50 37.23 ± 3.72) compared to ascorbic acid (89.28 ± 2.02% inhibition, IC50 22.37 ± 3.82). Conversely, MeOH and EtOAc fractions showed moderate to weak inhibitory actions in all assays.

Table 5.
Antioxidant effect of thee A. vacillans extract using nitric oxide scavenging.
The higher activity of both CH2Cl2 and n-BuOH is mainly due to the presence of a higher content of polyphenolic compounds including anthraquinones, flavonides, tannins, etc. in these extracts [30]. Antioxidant activity of leaf gel from A. ferox, as estimated using the FRAP assay, was attributed to its polyphenolic and alkaloid contents [30]. Furthermore, extracts from several Aloe species showed potent antioxidant potential in various in vitro assays including A. barbadensis [31], A. arborescens [32], A. ferox [33], A. greatheadii var. davyana [34], A. harlana [35], A. saponaria [36], A. marlothii, and A. melanacantha [37]. The activity was attributed to several phytochemical constituents such as loesin, aloeresin A, and aloesone [38].
Aloe-emodin, one of the main anthraquinone compounds isolated from Aloe spp., displayed strong antioxidant activity [38], revealed by its powerful reducing properties and the ability to inhibit the oxidation of linolenic acid [39]. In contrast, aloin, found in most Aloe plants, exhibited very similar properties, inhibiting lipid peroxidation in the cerebral cortex by inactivation of Fe(II)-dependent ascorbate [40].
In addition, the anthrone C-glycoside microdentin A/B isolated from the leaf latex of A. schelpei displayed stronger antioxidant activity compared to aloinoside A/B and aloin A/B using vitamin C as a standard [41].
3. Materials and Methods
3.1. Apparatus and Chemicals
Silica gel (Merck 60 A, 230–400 mesh ASTM, Darmstadt, Germany) was used for column chromatography. Normal and reversed phase silica gel (Merck, Darmstadt, Germany) were used for thin-layer chromatography (TLC). Anthraquinones were detected using a 254/366 nm UV lamp, followed by exposure to concentrated ammonia vapors or by spraying with 10% alcoholic KOH or NaOH. Additionally, compounds were visualized spraying with 15% H2SO4/ethanol, followed by heating.
HPLC analysis was performed on a Prominence Shimadzu LC Solution (Shimadzu Corporation, Kyoto, Japan) system with an InertSustain® C18 analytical column (250 × 10 mm i.d.; 5 μm particle size) and a GL Sciences C18 preparative column (250 × 20 mm i.d.; 5 μm particle size) protected by a Waters Novapack RP C18 column guard. A binary LC-10AD pump, inline degasser, auto-sampler, and HP-1040A photodiode array detector coupled to an HP-85 personal computer were used for the analysis. UV–Vis spectra were recorded in the 200–700 nm range.
NMR spectroscopy was performed using deuterated solvents in an UltraShield Plus 500 (Bruker, Billerica, MA, USA) spectrometer operating at 500 MHz for 1H and 125 MHz for 13C. Some measurements used a Bruker AV-700 MHz NMR spectrometer (Bruker, Billerica, MA, USA) operating at 700 MHz for 1H and 175 MHz for 13C at the College of Pharmacy, King Saud University. Chemical shift values are reported in δ (ppm) relative to an internal standard (TMS) or residual solvent peak, and coupling constants (J) are reported in Hertz (Hz). The standard Bruker pulse program was used for the two-dimensional NMR analyses (COSY, HSQC, HMBC, and NOESY). HRMS was conducted by direct injection using a Thermo Scientific UPLC RS Ultimate 3000 Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer (company, city, country) (Mundelein, Illinois 60060 USA) combined with high-performance quadrupole precursor selection with high resolution, accurate-mass (HR/AM) Orbitrap™ detection. Direct infusion of isocratic elution was done using CH3CN/MeOH (7:3) as a solvent system with 0.1% formic acid. The experiment time was run for 1 min using nitrogen as the supplementary gas. A scan range from 160–1500 m/z was used. Detection was performed in both positive and negative modes, separately. The instruments were located at Prince Sattam Bin Abdulaziz University, College of Pharmacy. In addition, accurate mass determination was also achieved with a JEOL JMS-700 High-Resolution Mass Spectrophotometer (JEOL USA Inc., Peabody, MA, USA). The electron impact mode with an ionization energy of 70 eV was adopted. A direct probe was used with the following temperature ramp settings: Initial temperature of 50 °C; increasing by 32 °C/min, reaching a final temperature of 350 °C; resolution was adjusted to 10 k. IR spectrum was acquired using a JASCO 320-A spectrometer (JASCO International Co., Ltd., Easton, MD, USA). ECD analysis was performed on a J-815 CD spectrometer (JASCO INTERNATIONAL CO., LTD., Easton, MD, USA). A BioTek PowerWave 200 Microplate Spectrophotometer (BioTek, Winooski, VT, USA) and a PerkinElmer EnVision Multilabel Microplate Reader (PerkinElmer EnVision, Waltham, MA, USA) were used to monitor the antioxidant capacity of the tested samples. Reagents, chemicals, and solvents were analytical grade, purchased from Sigma-Aldrich (St. Louis, MO, USA), Loba Chemie Pvt. Ltd. (Mumbai, India), and SD Fine Chem. Ltd. (Mumbai, India).
3.2. Plant Material
The leaves of A. vacillans Forssk were collected in February 2018 in Mahayil Asir, in the southwestern region of Saudi Arabia (latitude: 18°13′0.4692″ N and longitude: 42°30′13.5540″ E). The specimens were kindly identified by Dr Raja Krishnan, Botany and Microbiology Department at the College of Science, King Saud University, Riyadh, Saudi Arabia. A voucher specimen (#11965) was submitted to the herbarium of the College of Science, King Saud University.
3.3. Extraction and Isolation
The succulent leaves of A. vacillans (4 kg) were chopped into small pieces and extracted in 70% hot methanol several times until exhaustion. The pooled extracts were then concentrated in a rotary evaporator to obtain a dark semi-solid residue (180 g). Total methanolic extract (MeOH) was dispersed in 300 mL of distilled water and successively partitioned with dichloromethane (CH2Cl2), ethyl acetate (EtOAc), and n-butanol (n-BuOH). Organic fractions were filtered over anhydrous sodium sulfate and evaporated to dryness to yield fractions A (DCM, 4.0 g), B (EtOAc, 8.5 g), C (n-BuOH, 70 g), and D (aqueous fraction, 90 g). The fractions were monitored on normal and RP C18 TLC using different solvent systems: n-hexane/EtOAc, CH2Cl2/MeOH, and CH2Cl2/MeOH/H2O at different ratios. The CH2Cl2 and EtOAc fractions were the richest in secondary metabolites including anthraquinones, triterpenes, and sterols; based on these results, these extracts were chosen for further chromatographic investigation.
Part of the CH2Cl2 fraction (3.5 g) was chromatographed on a silica gel column (100 × 4 cm, 358 g silica) using the n-hexane/EtOAc solvent system, followed by EtOAc/MeOH in gradient elution mode, yielding 251 fractions. Similar fractions, monitored on Kiesel gel 60 F254 TLC, were combined to give six main fractions (A–F). Direct crystallization of fraction C, eluted by 40% EtOAc/n-hexane, afforded compound 1, aloe-emodin, while compound 2, dihydroisocoumarin, was recovered from fraction E after sub-column treatment, on a silica gel column, using a CH2Cl2/MeOH solvent system in gradient elution mode.
The EtOAc extract (8 g) was applied on top of a silica gel column (100 × 4 cm, 500 g silica) and eluted with a CH2Cl2/MeOH mixture of increasing polarity. Eighty sub-fractions were collected and monitored on F254 TLC using n-hexane/EtOAc, and CHCl3/MeOH at different concentrations as well as on RP C18 TLC using MeOH/H2O at different ratios. Based on the results obtained from TLC, similar fractions were combined to yield seven main fractions (I–VII). Two fractions were chosen for further purification by reversed-phase C18-HPLC in a gradient elution mode starting with MeOH-H2O (60:40). Fraction II (130 mg) was eluted over 70 min (flow rate, 2 mL/min) to afford pure compounds 3 (1 mg, Rt = 17.88 min, 69.4% MeOH/H2O), 4 (15 mg, Rt = 21.25 min, 71.9% MeOH/H2O), 5 (12 mg, Rt = 23.12 min, 73.1% MeOH/H2O), 6 (12 mg, Rt = 30.97 min, 78.9% MeOH/H2O), 7 (9 mg, Rt = 32.42 min, 79.9% MeOH/H2O), 8 (7 mg, Rt = 38.95 min, 84.7% MeOH/H2O), and 9 (13 mg, Rt = 39.82 min, 85.3% MeOH/H2O). Similarly, fraction III (300 mg) was purified over 70 min to provide 10 (6 mg, Rt = 40.1 min, 85.5% MeOH/H2O), 11 (8 mg, Rt = 40.95 min, 86.1% MeOH/H2O), and 12 (3 mg, Rt = 47.86 min, 91.2% MeOH/H2O) (Scheme 1).
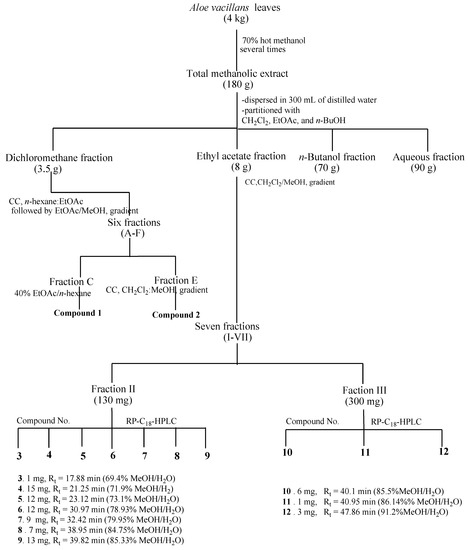
Scheme 1.
Extraction, purification, and isolation of Aloe vacillans leaves.
3.4. Antioxidant Activity
3.4.1. DPPH Radical Scavenging Assay
The antioxidant activity of the extracts and fractions was determined using DPPH (2,2-diphenyl-1-picrylhydrazyl) based on the method described by [42]. Absorbance was determined after 30 min at 520 nm, and percentage inhibition was obtained with the following the equation:
where At is the absorbance of the extract and A0 is the absorbance of the control.
Scavenging (%) = A0 − At/A0 × 100
3.4.2. ABTS Radical Cation Scavenging Assay
The assay was performed following the procedure described by [43]. The ability of samples to reduce the ABTS free radical (2,20-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) was also estimated using the above formula.
3.4.3. Reducing Power Assay
The reducing power of the extracts was determined using the method adapted by [44]. The antioxidant method (i.e., FRAP) is based on the capability of a test sample to reduce ferric ions (Fe3+) to ferrous ions (Fe2+) by electron donation.
3.4.4. Superoxide Radical Anion Scavenging Assay
Superoxide anion radical scavenging activity was assessed as previously described [45] with slight modification. Superoxide radicals were created by oxidation of NADH in a PMS-NADH system, and antioxidant activity was measured by the extent to which the extract and fractions of A. vacillans reduced nitro blue tetrazolium (NBT). The percentage of superoxide radical scavenging was also calculated using the above formula.
3.4.5. Nitric Oxide Radical Scavenging Assay
The assay was performed as previously described [46]. The free radical scavenging activity of the extract and fractions was determined by evaluating the % inhibition of the nitrite ions generated from the interaction of nitric oxide with oxygen using the same equation above-mentioned.
3.4.6. Spectral Data of the New Compounds
Vacillanoside (3)
White amorphous powder (1 mg); [α]23D − 56.2° (c 0.1, MeOH; UV λmax MeOH (log ε) nm: 211 (4.46), 274 (4.22), 306 (3.99); IR (KBr) vmax 3451, 1645, 1631, 1608, 1595, 1054, 1032, and 1016 cm−1; 1H and 13C NMR (500, 125 MHz, in CD3OD) HR-ESI-MS: m/z 505.1353 [M−H]+ (calcd 505.1346 for C24H25O12), m/z 507.1500 [M+H]+ (calcd 507.1503 for C24H26O12+H), m/z 529.1320 [M+Na]+ (calcd 529.1322 for C24H26O12Na), m/z 545.1069 [M+K]+ (calcd 545.1061 for C24H26O12K).
Vacillantin A (10)
Red amorphous powder (6 mg); [α]23D + 16.8° (c 0.05, MeOH); UV λmax MeOH (log ε) nm: 209 (4.63), 244 (4.42), 300 (3.92), 330 (3.61); IR (KBr) vmax 3400, 1720, 1640, 1606, 1511, 1240 cm−1; 1H and 13C NMR (see Table 2); HR-ESI-MS: m/z 579.1509 [M−H]+ (calcd 579.1503 for C30H27O12).
Vacillantin B (11)
Red amorphous powder (8 mg); [α]23D − 4.7° (c 0.05, MeOH); UV λmax MeOH (log ε) nm: 209 (4.63), 244 (4.42), 300 (3.92), 330 (3.61); IR (KBr) vmax 3400, 1720, 1640, 1606, 1511, 1240 cm−1; 1H and 13C NMR (see Table 2); HR-ESI-MS: m/z 579.1509 [M−H]+ (calcd 579.1503 for C30H27O12), m/z 581.1651 [M+H]+ (calcd 581.1659 for C30H28O12 + H), m/z 603.1469 [M+Na]+ (calcd 603.1478 for C30H28O12 + Na).
3.5. Statistical Analysis
Analysis of variance (ANOVA) was used to evaluate significance differences, followed by the Student’s t-test. Data were expressed as mean ± SD, and the difference was considered significant at p < 0.05 compared to the control. All statistical calculations used OriginLab software (version 8, Northampton, Massachusetts, USA) and Microsoft Excel.
4. Conclusions
In summary, a new dihydroisocoumarin derivative, vacillanoside (3), 3,4 dihydro-6 glucopyranozyl-8-hydroxy-3-(2′-acetyl-3′,5′-dihydroxyphenyl)-methyl-1H-[2]benzopyran-1-one (6), and two new anthraquinone derivatives, vacillantins A and B (10 and 11) were isolated from the leaves of A. vacillans (Asphodelaceae) together with nine known compounds (1, 2, 4–9, and 12). The structures of these compounds were elucidated through extensive spectroscopic analyses. The total alcohol extract and different fractions were tested for their antioxidant activities in five spectrophotometric assays. The dichloromethane fraction exhibited promising free radical scavenging activity in most of the assays. Our findings add new information to the literature on the structural diversity and pharmacological activities of Aloe species. Our results suggest A. vacillans as a potential source of secondary metabolites with pharmacological and industrial importance. Moreover, these results advocate further investigation of the remaining fractions with the aim of isolating bioactive compounds exhibiting interesting biological capacities.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/12/1632/s1, Figure S1: A photo of Aloe vacillans., Figures S2–S24 NMR spectral data (1D and 2D0 of compounds 3, 10 and 11: while Figures S9, S16 and S23 are HRESIMS spectra of compounds 3,10 and 11 and Figure S24 is the HRESIMS for the known compounds.
Author Contributions
M.A.-T., S.M.A.-M. and A.A.E.-G. chose the plants and designed the practical part. M.A.-T. achieved the practical part of the project. O.A.B. and M.S.A.-K. measured, interpreted, and assigned the NMR data and helped in preparing/revising the manuscript. W.M.A.-M. wrote, revised the manuscript, and prepared the supplementary material. S.M.A.-M. and A.A.E.-G. wrote the paper, interpreted the NMR data, and supervised. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at King Saud University through the Research Group Project No. RG 1437-021.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at King Saud University for funding the work through the Research Group Project No. RG 1437-021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Demmisew, S. Botanical aspects of Aloes of North East Africa. Bull. Chem. Soc. Ethiop. 1996, 10, 74–88. [Google Scholar]
- Gao, Y.; Kuok, K.I.; Jin, Y.; Wang, R. Biomedical applications of Aloe vera. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. S1), S244–S256. [Google Scholar] [CrossRef]
- Kojo, E.; Qian, H. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar]
- Dagne, E.; Bisrat, D.; Viljoen, A.; Van Wyk, B.E. Chemistry of Aloe Species. Curr. Org. Chem. 2000, 4, 1055–1078. [Google Scholar] [CrossRef]
- Steenkamp, V.; Stewart, M.J. Medicinal applications and toxicological activities of Aloe products. Pharmbiol. Biol. 2007, 45, 411–420. [Google Scholar] [CrossRef]
- Cock, I.E. The Genus Aloe: Phytochemistry and Therapeutic Uses Including Treatments for Gastrointestinal Conditions and Chronic Inflammation. Prog. Drug Res. 2015, 70, 179–235. [Google Scholar] [PubMed]
- Yagi, A.; Kabash, A.; Okamura, N.; Haraguchi, H.; Moustafa, S.M.; Khalifa, T.I. Antioxidant, free radical scavenging and anti-inflammatory effects of aloesin derivatives in Aloe vera. Planta Med. 2002, 68, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Yagi, A.; Kabash, A.; Mizuno, K.; Moustafa, S.M.; Khalifa, T.I.; Tsuji, H. Radical scavenging glycoprotein inhibiting cyclooxygenase-2 and thromboxane A2 synthase from Aloe vera gel. Planta Med. 2003, 69, 269–271. [Google Scholar] [CrossRef]
- Lim, B.O.; Seong, N.S.; Choue, R.W.; Kim, J.D.; Lee, H.Y.; Kim, S.Y.; Yu, B.P.; Jeon, T.I.; Park, D.K. Efficacy of dietary Aloe vera supplementation on hepatic cholesterol and oxidative status in aged rats. J. Nutr. Sci. Vitaminol. 2003, 49, 292–296. [Google Scholar] [CrossRef]
- Su, Y.T.; Chang, H.L.; Shyue, S.K.; Hsu, S.L. Emodin induces apoptosis in human lung adenocarcinoma cells through a reactive oxygen species-dependent mitochondrial signaling pathway. Biochem. Pharmacol. 2005, 70, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Oh, S.T.; Song, S.; Kim, M.R.; Kim, D.S.; Woo, S.S.; Jo, T.H.; Park, Y.I.; Lee, C.K. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int. Immunopharmacol. 2005, 5, 271–279. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wang, Y.; Huang, Z.S. The radio-protective effect of Aloe polysaccharides on irradiated mice. Chin. Trad. Herb. Drugs 2002, 33, 251–254. [Google Scholar]
- US Food and Drug Administration. Code of Federal Regulations (Food and Drugs), Title 21; US Government Printing Office: Washington, DC, USA, 1991; pp. 170–199.
- Council of Europe. Flavoring Substances and Natural Source of Flavorings, 3rd ed.; Maisonneuve: Moulins-les Metz, France, 1981; p. 376. [Google Scholar]
- Valverde, J.M.; Valero, D.; Martinez-Romera, D.; Guillén, A.; Castillo, S.; Serrano, M. Novel edible coating based on Aloe vera gel to maintain table grape quality and safety. J. Agric. Food Chem. 2005, 53, 7807–7813. [Google Scholar] [CrossRef]
- Abdalla, H.I.; Shaaban, M.; Shaaban, K.A.; Abu-Gabal, N.S.; Shalaby, N.M.; Laatsch, H. New bioactive compounds from Aloe hijazensis. Nat. Prod. Res. 2009, 23, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Collenette, S. Wild Flowers of Saudi Arabia. Riyadh; National Commission for Wild Life Conservation and Development (NCWCD): Riyadh, Saudi Arabia, 1999; p. 18. [Google Scholar]
- Wood, J.R.I.; Thomas, H.H. A Handbook of the Yemen Flora; Royal Botanic Gardens, Kew: Richmond, UK, 1997. [Google Scholar]
- Zhong, J.; Huang, Y.; Ding, W.; Wu, X.; Wan, J.; Luo, H. Chemical constituents of Aloe barbadensis Miller and their inhibitory effects on phosphodiesterase-4D. Fitoterapia 2013, 91, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Speranza, G.; Manitto, P.; Monti, D. Feralolide, a dihydroisocoumarin from Cape aloe. Phytochemistry 1993, 3, 175–178. [Google Scholar] [CrossRef]
- Kurizaki, A.; Watanabe, T.; Devkota, H.P. Chemical constituents from the flowers of Aloe arborescens. Nat. Prod. Commun. 2019, 14, 1–4. [Google Scholar] [CrossRef]
- Yasuda, K.; Uehara, S.; Takano, I.; Shindo, T.; Nishijima, M. Stability of barbaloin in aqueous solution. Food Preserv. Sci. 2000, 26, 85–90. [Google Scholar] [CrossRef]
- Farah, M.H.; Andersson, R.; Samuelsson, G. Microdontin A and B: Two new aloin derivatives from Aloe microdonta. Planta Med. 1992, 58, 88–93. [Google Scholar] [CrossRef]
- Shin, K.H.; Woo, W.S.; Lim, S.S.; Shim, C.S.; Chung, H.S.; Kennelly, E.J.; Kinghorn, D. Elgonica-Dimers A and B, two potent alcohol metabolism inhibitory constituents of Aloe arborescens. J. Nat. Prod. 1997, 60, 1180–1182. [Google Scholar] [CrossRef]
- Conner, J.M.; Alexander, I.; Gray, A.I.; Peter, G.; Waterman, P.G.; Reynolds, T. Novel anthrone-anthraquinone dimers from Aloe elgonica. J. Nat. Prod. 1990, 53, 1362–1364. [Google Scholar] [CrossRef]
- Abd-Alrahman, S.H.; Salem-Bekhit, M.M.; Elhalwagy, M.E.A.; Abdel-Mageed, W.M.; Radwan, A.A. Phytochemical Screening and Antimicrobial Activity of EthOH/Water Ziziphus jujuba Seeds Extracts. J. Pure Appl. Microbiol. 2013, 7, 823–828. [Google Scholar]
- Veitch, N.C.; Simmonds, M.S.J.; Blaney, W.M.; Reynolds, T. A dihydroisocoumarin glucoside from Aloe hildebrandtii. Phytochemistry 1994, 35, 1163–1166. [Google Scholar] [CrossRef]
- Dagne, E.; Bisrat, D.; Van Wyk, B.E.; Viljoen, A.; Hellwig, V.; Steglich, W. Anthrones from Aloe microstigma. Phytochemistry 1997, 44, 1271–1274. [Google Scholar] [CrossRef]
- Rauwald, H.W.; Lohse, K. Structure revision of 4-hydroxyaloin: 10-hydroxyaloins A and B as main In Vitro-oxidation products of the diastereomeric aloins. Planta Med. 1992, 58, 259–262. [Google Scholar] [CrossRef]
- Loots, D.T.; van der Westhuizen, F.H.; Botes, L. Aloe ferox leaf gel phytochemical content, antioxidant capacity, and possible health benefits. J. Agric. Food Chem. 2007, 55, 6891–6896. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Hu, Q. Evaluation of antioxidant potential of Aloe vera (Aloe barbadensis miller) extracts. J. Agric. Food Chem. 2003, 51, 7788–7791. [Google Scholar] [CrossRef]
- Beppu, H.; Koike, T.; Shimpo, K.; Chihara, T.; Hoshino, M.; Ida, C.; Kuzuya, H. Radical-scavenging effects of Aloe arborescens Miller on prevention of pancreatic islet B-cell destruction in rats. J. Ethnopharmacol. 2003, 89, 37–45. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. Phytochemical constituents and antioxidant activities of the whole leaf extract of Aloe ferox mill. Pharmacogn. Mag. 2011, 7, 325–333. [Google Scholar]
- Botes, L.; van der Westhuizen, F.H.; Loots, D.T. Phytochemical contents and antioxidant capacities of two Aloe Greatheadii Var. Davyana extracts. Molecules 2008, 13, 2169–2180. [Google Scholar] [CrossRef]
- Asamenew, G.; Bisrat, D.; Mazumder, A.; Asres, K. In vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of Aloe harlana reynolds. Phytother. Res. 2011, 25, 1756–1760. [Google Scholar] [CrossRef] [PubMed]
- Yoo, E.A.; Kim, S.D.; Lee, W.M.; Park, H.J.; Kim, S.K.; Cho, J.Y.; Min, W.; Rhee, M.H. Evaluation of Antioxidant, antinociceptive, and anti-Inflammatory activities of ethanol extracts from Aloe Saponaria Haw. Phytother. Res. 2008, 22, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Cardarellia, M.; Rouphaelb, Y.; Pellizzonic, M.; Collad, G.; Lucin, L. Profile of bioactive secondary metabolites and antioxidant capacity of leaf exudates from eighteen Aloe species. Ind. Crops Prod. 2017, 108, 44–51. [Google Scholar] [CrossRef]
- Salehi, B.; Albayrak, S.; Antolak, H.; Kręgiel, D.; Pawlikowska, E.; Sharifi-Rad, M.; Uprety, Y.; Tsouh Fokou, P.V.; Yousef, Z.; Amiruddin Zakaria, Z.; et al. Aloe genus plants: From farm to food applications and phytopharmacotherapy. Int. J. Mol. Sci. 2018, 19, 2843. [Google Scholar] [CrossRef] [PubMed]
- Abdul Qadir, M.; Shahzadi, S.K.; Bashir, A.; Munir, A.; Shahzad, S. Evaluation of phenolic compounds and antioxidant and antimicrobial activities of some common herbs. Int. J. Anal. Chem. 2017, 2017, 3475738. [Google Scholar] [CrossRef] [PubMed]
- Hęś, M.; Dziedzic, K.; Górecka, D.; Jędrusek-Golińska, A.; Gujska, E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants—A Review. Plant. Foods Hum. Nutr. 2019, 74, 255–265. [Google Scholar]
- Teka, T.; Kassahun, H. Characterization and Evaluation of Antioxidant Activity of Aloe schelpei Reynolds. Drug Des. Dev. Ther. 2020, 14, 1003. [Google Scholar] [CrossRef] [PubMed]
- Braca, A.; Tommasi, N.D.; Bari, L.D.; Pizza, C.; Politi, M.; Morelli, I. Antioxidant principles from Bauhinia terapotensis. J. Nat. Prod. 2001, 64, 892–895. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reactions. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Fontana, L.; Giagulli, C.; Minuz, P.; Lechi, A.; Laudanna, C. 8-Iso-PGF2 alpha induces beta 2-integrinmediated rapid adhesion of human polymorphonuclear neutrophils: A link between oxidative stress and ischemia/reperfusion injury. Arterioschler Thromb. Vasc. Biol. 2001, 21, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nagmoti, D.M.; Khatri, D.K.; Juvekar, P.R.; Juvekar, A.R. Antioxidant activity and free radical scavenging potential of Pithecellobium dulce Benth seed extracts. Free Rad. Antiox. 2011, 2, 37–43. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).