Effects of Grafting on Morphophysiological and Yield Characteristic of Eggplant (Solanum melongena L.) Grafted onto Wild Relative Rootstocks
Abstract
1. Introduction
2. Results
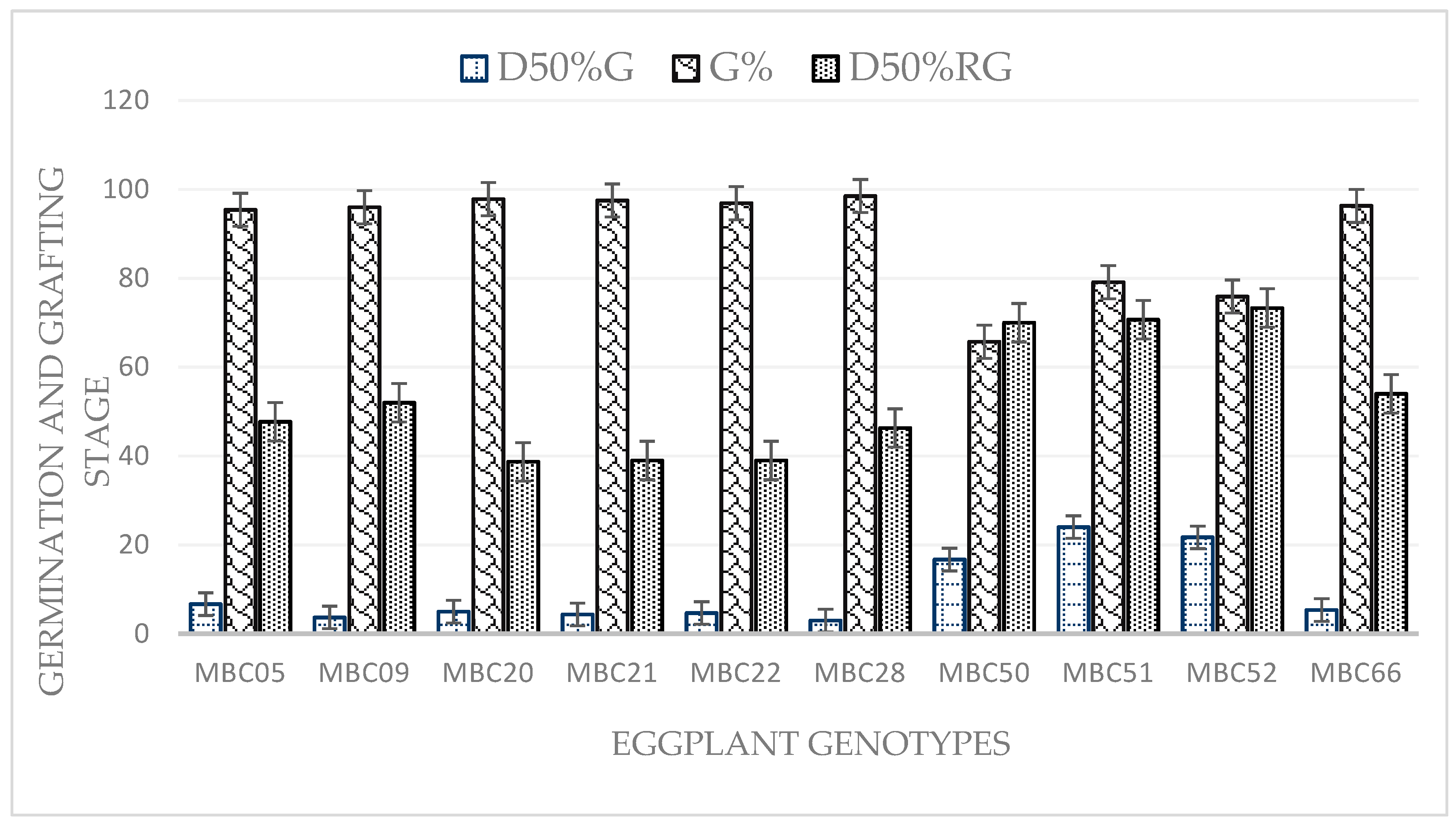
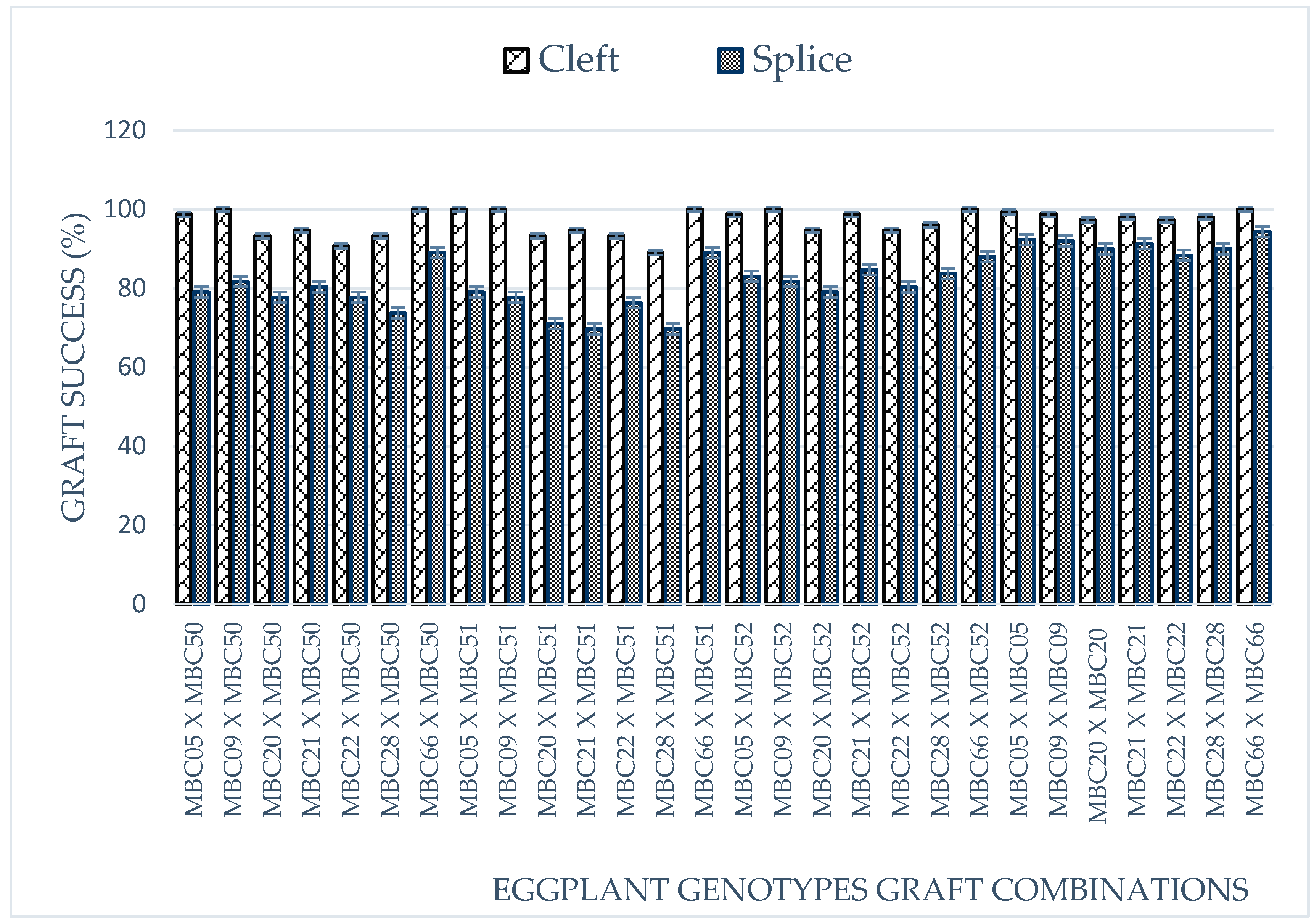
2.1. Observation of Seedlings at the Nursery Stage and Grafting Success (%)
2.2. Analysis of Variance for Vegetative, Yield and Yield Component Traits across the Cropping Systems
2.3. Analysis of Variance of Interaction between Rootstock and Scion for Vegetative, Yield and Yield Component Across the Cropping Systems
2.4. Effect of Grafting on Early Flowering and Harvesting across Two Cropping Systems
2.5. Effect of Grafting on Physiological, Yield and Yield Component Traits across Two Cropping System
3. Discussion
4. Materials and Methods
4.1. Planting Materials and Agronomic Practices
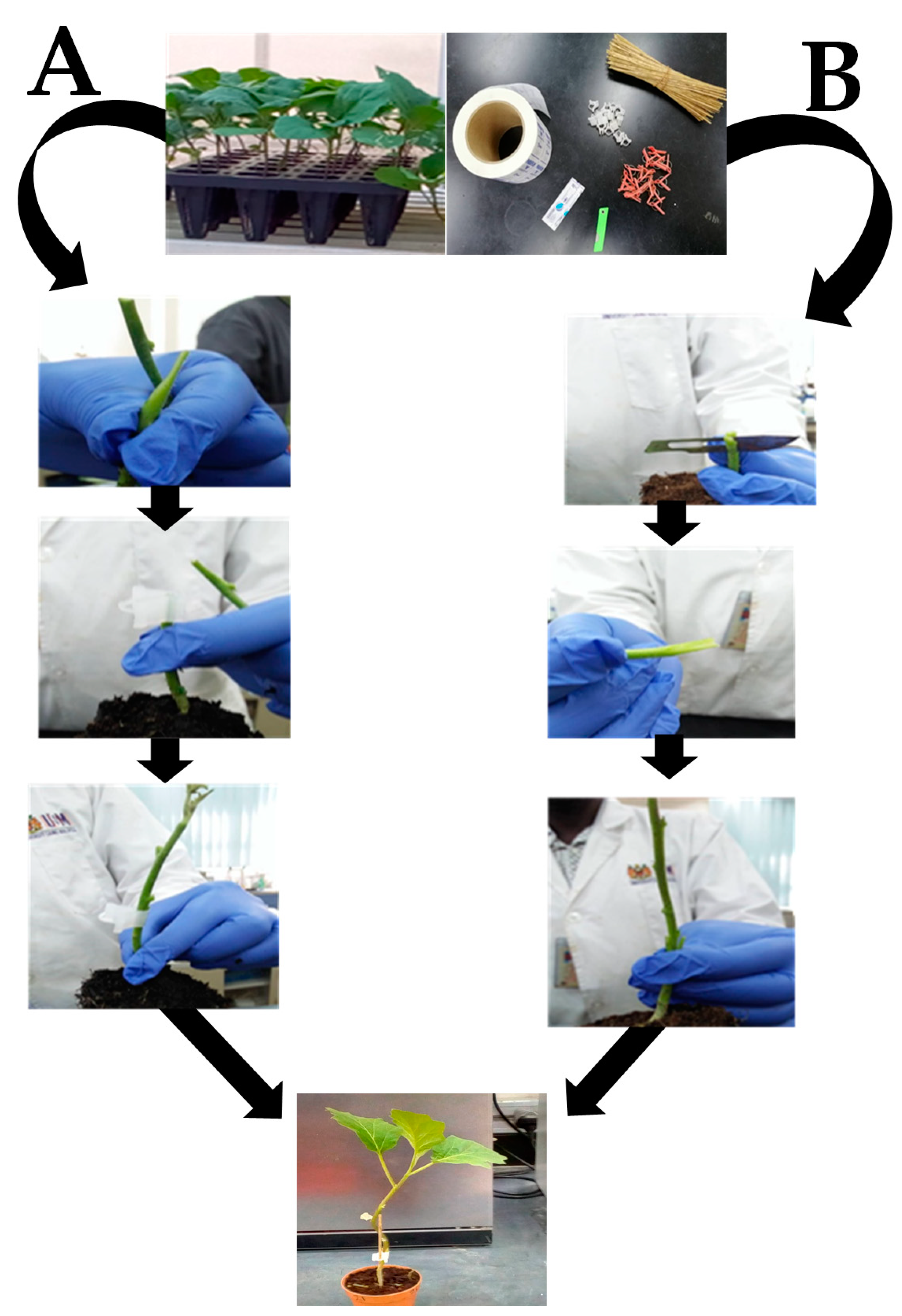
4.2. Seed Germination and Grafting Methods
4.3. Data Collection
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sulaiman, N.N.M.; Rafii, M.Y.; Duangjit, J.; Ramlee, S.I.; Phumichai, C.; Oladosu, Y.; Datta, D.R.; Musa, I. Genetic variability of eggplant germplasm evaluated under open field and glasshouse cropping conditions. Agronomy 2020, 10, 436. [Google Scholar] [CrossRef]
- Sabatino, L.; Iapichino, G.; D’Anna, F.; Palazzolo, E.; Mennella, G.; Rotino, G.L. Hybrids and allied species as potential rootstocks for eggplant: Effect of grafting on vigour, yield and overall fruit quality traits. Sci. Hortic. 2018, 228, 81–90. [Google Scholar] [CrossRef]
- Yang, R.Y. Application of Antioxidant Activity Analytical Methods for Studies on Antioxidant Activities of Vegetables. Ph.D. Thesis, Institute of Tropical Agriculture and International Cooperation of National Ping-Tung University of Science and Technology, Neipu, Taiwan, 2006. [Google Scholar]
- Miceli, A.; Sabatino, L.; Moncada, A.; Vetrano, F.; D’Anna, F. Nursery and field evaluation of eggplant grafted onto unrooted cuttings of Solanum torvum Sw. Sci. Hortic. 2014, 178, 203–210. [Google Scholar] [CrossRef]
- Louws, F.J.; Rivard, C.L.; Kubota, C. Grafting fruiting vegetables to manage soilborne pathogens, foliar pathogens, arthropods and weeds. Sci. Hortic. 2010, 127, 127–146. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Magaji, U.; Abdullah, N.; Miah, G.; Chukwu, S.C.; Hussin, G.; Ramli, A.; Kareem, I. Genotypic and phenotypic relationship among yield components in rice under tropical conditions. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Usman, M.G.; Rafii, M.Y.; Martini, M.Y.; Oladosu, Y.; Kashiani, P. Genotypic character relationship and phenotypic path coefficient analysis in chili pepper genotypes grown under tropical condition. J. Sci. Food Agric. 2017, 97, 1164–1171. [Google Scholar] [CrossRef]
- Kacjan Maršić, N.; Mikulič-Petkovšek, M.; Stampar, F. Grafting influences phenolic profile and carpometric traits of fruits of greenhouse-grown eggplant (Solanum melongena L.). J. Agric. Food Chem. 2014, 62, 10504–10514. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Ibrahim, M.; Munira, M.K.; Kabir, M.S.; Islam, A.K.M.S.; Miah, M.M.U. Seed germination and graft compatibility of wild solanum as rootstock of tomato. J. Biol. Sci. 2001, 1, 701–703. [Google Scholar]
- Gisbert, C.; Prohens, J.; Raigón, M.D.; Stommel, J.R.; Nuez, F. Eggplant relatives as sources of variation for developing new rootstocks: Effects of grafting on eggplant yield and fruit apparent quality and composition. Sci. Hortic. 2011, 128, 14–22. [Google Scholar] [CrossRef]
- Moncada, A.; Miceli, A.; Vetrano, F.; Mineo, V.; Planeta, D.; D’Anna, F. Effect of Grafting on yield and quality of eggplant (Solanum melongena L.). Sci. Hortic. 2013, 149, 108–114. [Google Scholar] [CrossRef]
- Khah, E.M.; Kakava, E.; Mavromatis, A.; Chachalis, D.; Goulas, C. Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J. Appl. Hortic. 2006, 8, 3–7. [Google Scholar] [CrossRef]
- Fita, A.; Picó, B.; Roig, C.; Nuez, F. Performance of Cucumis melo spp. agrestis as a rootstock for melon. J. Hortic. Sci. Biotechnol. 2004, 82, 184–190. [Google Scholar] [CrossRef]
- Khah, E.M. Effect of grafting on growth, performance and yield of aubergine (Solanum melongena L.) in greenhouse and open-field. Int. J. Plant. Prod. 2011, 5, 1735–8043. [Google Scholar]
- Passam, H.C.; Stylianoy, M.; Kotsiras, A. Performance of eggplant grafted on tomato and eggplant rootstocks. Eur. J. Hortic. Sci. 2005, 70, 130–134. [Google Scholar]
- Buller, S.; Inglis, D.; Miles, C. Plant growth, fruit yield and quality, and tolerance to verticillium wilt of grafted watermelon and tomato in field production in the Pacific Northwest. HortScience 2013, 48, 1003–1009. [Google Scholar] [CrossRef]
- Leonardi, C.; Giuffrida, F. Variation of plant growth and macronutrient uptake in grafted tomatoes and eggplants on three different rootstocks. Eur. J. Hortic. Sci. 2006, 71, 97–101. [Google Scholar]
- Trinchera, A.; Pandozy, G.; Rinaldi, S.; Crinò, P.; Temperini, O.; Rea, E. Graft union formation in artichoke grafting onto wild and cultivated cardoon: An anatomical study. J. Plant Physiol. 2013, 170, 1569–1578. [Google Scholar] [CrossRef]
- Kumar, P.; Edelstein, M.; Cardarelli, M.; Ferri, E.; Colla, G. Grafting affects growth, yield, nutrient uptake, and partitioning under cadmium stress in tomato. HortScience 2015, 50, 1654–1661. [Google Scholar] [CrossRef]
- Liu, Y.F.; Qi, H.Y.; Bai, C.M.; Qi, M.F.; Xu, C.Q.; Hao, J.H.; Li, Y.; Li, T.L. Grafting helps improve photosynthesis and carbohydrate metabolism in leaves of muskmelon. Int. J. Biol. Sci. 2011, 7, 1161–1170. [Google Scholar] [CrossRef][Green Version]
- Johnson, S.; Inglis, D.; Miles, C. Grafting effects on eggplant growth, yield and Verticillium wilt incidence. Int. J. Veg. Sci. 2014, 20, 3–20. [Google Scholar] [CrossRef]
- Bekhradi, F.; Kashi, A.; Delshad, M. Effect of three cucurbits rootstocks on vegetative and yield of ‘Charleston Gray’ watermelon. Int. J. Plant Prod. 2011, 5, 105–110. [Google Scholar]
- Bletsos, F.; Thanassoulpoulos, C.; Roupakias, D. Effect of grafting on growth, yield and Verticillium wilt of eggplant. HortScience 2003, 38, 183–186. [Google Scholar] [CrossRef]
- Yetisir, H.; Sari, N. Effect of different rootstock on plant growth, yield and quality of watermelon. Aust. J. Exp. Agric. 2003, 43, 1269–1274. [Google Scholar] [CrossRef]
- Schwarz, D.; Rouphael, Y.; Colla, G.; Vanema, J.K. Grafting as tool to improve tolerance of fruit vegetable to abiotic stresses: Thermal stress, water stress, and organic pollutants. Sci. Hort. 2010, 127, 162–171. [Google Scholar] [CrossRef]
| Source of Variation | df | D50%F | FH | NSB45DAT | NSB90DAT | PH45DAT | PH90DAT |
| Cropping systems (Cs) | 1 | 262.98 ** | 123.43 ** | 993.65 ** | 3554.70 ** | 30391 ** | 93773 ** |
| Blocks within Cs | 4 | 0.86 ns | 5.02 ns | 0.40 ** | 0.82 ns | 5.5487 ns | 9.5181 ns |
| Genotypes (G) | 34 | 546.39 ** | 530.70 ** | 9.72 ** | 42.72 ** | 158.50 ** | 1332.80 ** |
| G × Cs | 34 | 45.03 ** | 51.68 ** | 2.93 ** | 13.81 ** | 252.88 ** | 6555.00 ** |
| Error | 136 | 0.40 | 1.59 | 0.10 | 0.82 | 7.76 | 8.44 |
| Source of Variation | df | SD45DAT | SD90DAT | PR (μmol m−2 s−1) | SC (mmol m−2 s−1) | TR (mL cm d−1) | CC (ml/cm2) |
| Cropping systems (Cs) | 1 | 111.76 ** | 148.75 ** | 1000.60 ** | 0.20 ** | 34.49 ** | 44.44 ** |
| Blocks within Cs | 4 | 3.08 * | 1.60 ns | 0.80 * | 0.01 ns | 0.07 ** | 0.20 ** |
| Genotypes (G) | 34 | 10.37 ** | 28.27 ** | 49.57 ** | 0.06 ** | 4.66 ** | 3.01 ** |
| G × Cs | 34 | 2.26 ** | 1.81 ns | 1.93 ** | 0.01 ** | 0.32 ** | 0.13 ** |
| Error | 136 | 0.89 | 2.41 | 0.31 | 0.01 | 0.01 | 0.01 |
| Source of Variation | df | FL (cm) | FD (mm) | AFW (g) | NFPP | MFY (kg) | |
| Cropping systems (Cs) | 1 | 145.97 ** | 37.04 * | 1433.57 ** | 8510.96 ** | 53.56 ** | |
| Blocks within Cs | 4 | 1.29 ns | 16.28 ns | 67.06 ns | 58.51 ns | 0.01 ns | |
| Genotypes (G) | 34 | 257.95 ** | 2048.90 ** | 18,502.06 ** | 130,551.80 ** | 4.01 ** | |
| G × Cs | 34 | 4.95 ** | 97.61 ** | 2548.87 ** | 661.94 ** | 4.36 ** | |
| Error | 136 | 1.04 | 9.62 | 31.16 | 34.32 | 0.01 |
| Source of Variation | df | D50%F | FH | NSB45DAT | NSB90DAT | PH45DAT(cm) | PH90DAT(cm) |
| Cropping system (Cs) | 1 | 613.37 ** | 360.07 ** | 574.72 ** | 2407.96 ** | 18,089.32 ** | 46,712.82 ** |
| Blocks within Cs | 4 | 0.39 ns | 2.60 ns | 0.15 ns | 0.45 ns | 12.56 ns | 7.84 ns |
| Rootstocks (R) | 2 | 3045.10 ** | 3112.45 ** | 34.94 ** | 241.92 ** | 172.20 * | 3256.27 ** |
| Scions (S) | 6 | 152.20 ** | 169.61 ** | 7.33 ** | 15.05 ** | 196.37 ** | 727.81 ** |
| R × S | 12 | 297.37 ** | 280.65 ** | 9.59 ** | 34.42 ** | 161.28 ** | 1443.39 ** |
| Error | 100 | 5.33 | 6.69 | 0.71 | 3.73 | 49.61 | 133.70 |
| Source of Variation | df | SD45DAT (mm) | SD90DAT (mm) | PR (μmol m−2 s−1) | SC (mmol m−2 s−1) | TR (mL cm d−1) | CC (ml/cm2) |
| Cropping system (Cs) | 1 | 61.66 ** | 82.85 ** | 401.43 ** | 0.13 ** | 18.86 ** | 26.64 ** |
| Block within Cs | 4 | 1.32 ns | 2.03 ns | 0.46 ns | 0.01 ns | 0.03 ns | 0.13 ns |
| Rootstocks (R) | 2 | 25.46 ** | 126.54 ** | 48.89 ** | 0.01 ** | 0.15 ns | 13.62 ** |
| Scions (S) | 6 | 5.47 ** | 29.07 ** | 6.34 ** | 0.02 ** | 0.53 ** | 3.18 ** |
| R × S | 12 | 10.40 ** | 20.94 ** | 26.10 ** | 0.05 ** | 2.81 ** | 1.88 ** |
| Error | 100 | 1.02 | 2.45 | 0.32 | 0.01 | 0.11 | 0.05 |
| Source of Variation | df | FL (cm) | FD (mm) | AFW (g) | NFPP | MFY (kg) | |
| Cropping system (Cs) | 1 | 104.38 ** | 1.46ns | 5893.89* | 1922.71 ** | 35.51 ** | |
| Block within Cs | 4 | 1.29 ns | 2.80ns | 101.93ns | 25.89ns | 0.00 ns | |
| Rootstocks (R) | 2 | 2167.44 ** | 1195.31 ** | 99,079.51 ** | 109,510.59 ** | 8.87 ** | |
| Scions (S) | 6 | 59.67 ** | 1274.15 ** | 8753.32 ** | 4338.70 ** | 1.37 ns | |
| R × S | 12 | 44.21 ** | 3114.52 ** | 14,525.13 ** | 4645.01 ** | 3.44 ** | |
| Error | 100 | 1.82 | 22.24 | 618.25 | 131.35 | 1.18 |
| Scion/Rootstock | D50%F | FH | SB45 | SB90 | PH45 (cm) | PH90 (cm) | SD45 (mm) | SD90 (mm) |
|---|---|---|---|---|---|---|---|---|
| MCV1 | 73.83 i | 84.00 hi | 5.57 p–s | 15.83 cd | 56.03 k–p | 85.55 u | 9.57 lm | 13.85 h–0 |
| MCV2 | 75.50 h | 88.33 d–f | 5.65 o–r | 15.00 d–h | 58.85 i–l | 105.58 g–k | 10.97 f–i | 14.17 g–n |
| CCV1 | 52.33 t | 63.67 q | 4.58 xy | 9.88 op | 53.40 pq | 88.88 t | 9.02 m | 14.40 f–m |
| CCV2 | 57.17 rs | 68.83 no | 4.97 u–w | 11.17 l–n | 62.12 f–h | 96.38 n–p | 10.05 h–m | 13.05 m–o |
| CCV3 | 58.33 pq | 70.00 l–n | 4.45 y | 13.47 j | 55.93 l–p | 101.05 lm | 9.95 i–m | 13.43 j–o |
| NCV | 72.50 j | 83.17 i | 5.70 n–q | 15.30 d–h | 72.80 a | 123.95 d | 12.93 bc | 18.67 b |
| TCV | 72.33 j | 83.67 hi | 6.12 i–l | 17.00 b | 58.70 i–l | 89.18 st | 10.32 g–l | 13.45 i–o |
| MCV1/MCV1 | 78.33 ef | 89.67 cd | 6.22 hij | 15.00 d–h | 58.62 i–l | 89.97 rst | 11.17 e–g | 14.47 i–o |
| MCV2/MCV2 | 85.17 a | 95.33 a | 5.70 n–q | 13.47 j | 57.98 i–m | 107.58 f–i | 11.68 d–f | 14.03 g–o |
| CCV1/CCV1 | 63.67 k | 76.67 j | 5.27 stu | 14.13 h–j | 56.97 j–n | 94.98 o–p | 10.28 g–l | 13.27 k–o |
| CCV2/CCV2 | 60.50 n | 70.50 lm | 4.67 w–y | 9.58 p | 58.70 i–l | 108.08 f–h | 9.72 lm | 12.57 no |
| CCV3/CCV3 | 58.33 pq | 70.67 l | 4.75 v–y | 10.83 m–o | 52.17 q | 91.85 q–t | 9.88 i–m | 15.00 d–k |
| NCV/NCV | 76.33 g | 85.83 g | 5.57 p–s | 14.70 e–h | 60.12 gh | 128.52 c | 12.32 c–e | 16.37 cd |
| TCV/TCV | 78.17 f | 89.00 d–f | 8.22 d | 16.17 bc | 55.10 m–q | 92.45 q–t | 9.88 i–m | 13.55 i–o |
| MCV1/MWR | 76.67 g | 87.83 f | 5.77 l–o | 18.78 a | 52.13 q | 94.75 o–q | 12.63 cd | 15.23 c–i |
| MCV2/MWR | 77.83 f | 88.83 d–f | 6.05 i–n | 14.70 e–h | 70.10 ab | 118.00 e | 12.28 c–e | 16.93 g–o |
| CCV1/MWR | 56.50 s | 66.83 p | 5.88 k–p | 12.18 kl | 59.47 g–j | 104.97 h–k | 11.03 f–h | 13.13 l–o |
| CCV2/MWR | 57.67 o | 67.83 op | 6.08 i–m | 10.65 no | 57.78 i–n | 104.20 j–l | 10.82 f–k | 14.88 d–j |
| CCV3/MWR | 59.67 o | 71.00 l–n | 4.83 v–x | 9.37 p | 57.55 i–n | 102.82 kl | 9.58 lm | 12.28 o |
| NCV/MWR | 76.83 g | 88.17 ef | 5.37 q–t | 13.50 j | 59.27 h–j | 135.08 b | 13.32 a–c | 20.74 a |
| TCV/MWR | 76.83 g | 87.83 f | 8.58 c | 18.70 a | 53.71 o–q | 106.95 f–j | 10.37 g–l | 15.63 c–f |
| MCV1/BWR | 78.00 f | 90.83 bc | 5.67 o–q | 14.58 i–e | 58.72 i–l | 93.20 p–r | 11.38 e–g | 16.17 c–e |
| MCV2/BWR | 80.33 b | 91.50 b | 6.58 f–h | 15.55 c–e | 66.82 cd | 122.00 d | 12.35 c–e | 15.98 c–g |
| CCV1/BWR | 61.33 m | 71.33 l | 5.95 j–o | 13.58 ij | 62.60 e–g | 108.80 fg | 12.32 c–e | 16.03 c–f |
| CCV2/BWR | 59.83 no | 70.67 l | 5.75 m–p | 9.87 op | 59.60 g–j | 93.95 o–q | 10.90 i–m | 14.45 c–h |
| CCV3/BWR | 62.50 l | 73.50 k | 4.97 u–w | 11.53 k–n | 53.47 pq | 94.17 o–q | 10.85 f–j | 12.88 m–o |
| NCV/BWR | 74.50 i | 84.83 gh | 6.62 f–h | 14.20 g–j | 65.38 de | 135.93 b | 13.82 a | 18.35 b |
| TCV/BWR | 76.67 g | 87.83 f | 9.03 b | 15.22 c–g | 53.57 o–q | 96.97 o | 11.02 f–h | 14.97 d–k |
| MCV1/TWR | 79.50 cd | 89.66 cd | 6.98 e | 16.25 bc | 56.70 j–o | 91.85 q–t | 9.88 i–m | 13.93 g–o |
| MCV2/TWR | 79.00 de | 88.33 d–f | 6.77 ef | 14.42 f–j | 65.15 d–f | 115.33 e | 12.43 c–e | 16.35 cd |
| CCV1/TWR | 58.33 pq | 69.17 m–o | 5.30 r–u | 10.87 m–o | 54.73 n–q | 104.60 i–k | 10.37 g–l | 12.75 m–o |
| CCV2/TWR | 62.17 l | 72.83 k | 5.05 t–v | 11.70 k–m | 55.00 m–q | 104.28 i–l | 9.73 k–m | 15.18 c–j |
| CCV3/TWR | 58.83 p | 70.83 l | 4.83 v–x | 11.33 k–n | 55.35 m–p | 99.10 mn | 10.57 g–l | 15.58 c–g |
| NCV/TWR | 77.83 f | 89.00 d–f | 6.25 h–j | 14.62 e–i | 69.52 bc | 147.00 a | 14.22 a | 21.80 a |
| TCV/TWR | 79.83 bc | 89.17 d–f | 10.0 a | 19.28 a | 59.12 k | 109.52 f | 11.82 d–f | 15.48 c–h |
| Mean | 69.52 | 80.48 | 6.01 | 13.75 | 58.96 | 105.35 | 11.13 | 15.11 |
| SEM | 0.68 | 0.68 | 0.18 | 0.36 | 1.02 | 1.92 | 0.12 | 0.19 |
| LSD (p = 0.05) | 0.72 | 1.44 | 0.35 | 1.04 | 3.18 | 3.32 | 1.08 | 1.77 |
| Scion/Rootstock | CC | P R | S C | T R | FL (cm) | FD (mm) | AFW (g) | NFPP | MFY (kg) |
|---|---|---|---|---|---|---|---|---|---|
| MCV1 | 4.72 i | 22.10 l | 0.20 k | 3.43 no | 18.9 j | 32.6 pq | 90.2 ij | 54.8 l–n | 2.5 tu |
| MCV2 | 6.13 a | 18.63 m | 0.43 ef | 3.37 no | 20.1 h–j | 34.1 n–g | 142.6 e | 53.8 k–m | 4.0 fg |
| CCV1 | 4.02 p | 23.32 j | 0.30 j | 3.02 q | 15.9 kl | 33.3 o–q | 95.7 i | 51.3 m–o | 2.6 st |
| CCV2 | 3.98 p | 25.35 h | 0.33 i | 3.37 | 21.2 g | 36.4 m–o | 90.5 ij | 54.1 l–n | 2.3 v |
| CCV3 | 4.45 k | 25.32 h | 0.35 hi | 3.30 op | 15.7 l | 72.2 c | 154.1 d | 46.0 op | 2.9 r |
| NCV | 3.93 pq | 25.37 h | 0.28 j | 3.32 n–p | 11.3 op | 27.3 r | 33.9 k–m | 128.2 f | 3.2 n |
| TCV | 4.37 kl | 24.02 i | 0.43 ef | 3.48 m | 6.53 r | 19.7 s | 27.8 m–p | 115.3 g | 2.1 w |
| MCV1/MCV1 | 4.37 l | 23.32 j | 0.35 hi | 2.75 r | 22.6 f | 39.4 j–m | 114.4 g | 50.2 no | 2.8 r |
| MCV2/MCV2 | 5.57 d | 24.40 j | 0.45 de | 3.63 l | 25.0 de | 38.1 k–m | 116.1 g | 62.7 h–j | 4.3 e |
| CCV1/CCV1 | 4.82 h | 22.12 l | 0.30 j | 3.22 p | 10.5 pq | 37.0 l–n | 95.0 i | 66.6 h | 3.8 ij |
| CCV2/CCV2 | 4.55 j | 22.53 kl | 0.35 hi | 3.73 kl | 25.5 de | 30.8 q | 85.5 j | 55.0 l–n | 2.2 v |
| CCV3/CCV3 | 4.82 h | 22.82 jk | 0.42 fg | 3.80 k | 17.0 k | 66.4 d | 178.2 c | 41.9 p | 2.9 q |
| NCV/NCV | 3.85 rs | 26.80 g | 0.45 de | 4.17 ij | 13.8 m | 52.6 f | 25.7 op | 177.3 b | 3.5 lm |
| TCV/TCV | 4.45 k | 25.25 h | 0.40 g | 3.80 k | 8.68 q | 27.4 r | 28.6 m–p | 135.6 e | 2.5 tu |
| MCV1/MWR | 6.05b | 29.35 c | 0.50 c | 6.03 a | 29.4b | 43.4 hi | 134.2 f | 54.4 l–n | 3.8 i |
| MCV2/MWR | 5.73 c | 32.18 a | 0.55b | 5.03 e | 31.4 a | 47.9 g | 195.3 a | 52.2 m–o | 5.3 a |
| CCV1/MWR | 5.23 g | 25.68 h | 0.55b | 4.90 f | 25.5 de | 38.4 k–m | 103.2 h | 65.6 hi | 4.0 fg |
| CCV2/MWR | 4.18 mn | 27.97 de | 0.50 c | 5.20 d | 27.3 c | 36.0 m–p | 116.1 g | 55.1 k–n | 3.0 pq |
| CCV3/MWR | 4.22 m | 28.43 d | 0.75 a | 5.80 b | 20.4 g–i | 90.3b | 186.7b | 46.0 op | 3.5 lm |
| NCV/MWR | 5.58 J | 31.37 b | 0.55 b | 5.47 c | 13.9 m | 45.8 gh | 38.9 k | 130.8 ef | 3.8 hi |
| TCV/MWR | 4.57 j | 29.41 c | 0.55b | 5.02 e | 11.3 op | 39.4 j–m | 25.2 p | 157.6 c | 2.6 s |
| MCV1/BWR | 5.37 f | 27.95 de | 0.43 ef | 5.40 c | 25.8 d | 45.9 gh | 136.5 ef | 52.2 m–o | 3.6 jkl |
| MCV2/BWR | 5.15 g | 31.02 b | 0.50 c | 4.55 h | 30.0 b | 40.7 i–k | 139.9 ef | 59.9 i–l | 4.9 c |
| CCV1/BWR | 5.23 m | 25.78 h | 0.55b | 4.60 h | 23.0 f | 42.9 h–j | 115.9 h | 58.9 i–l | 3.8 hi |
| CCV2/BWR | 5.17 g | 26.80 g | 0.50 c | 5.07 e | 25.8 d | 40.1 i–l | 103.3 h | 61.8 h–k | 3.2 n |
| CCV3/BWR | 4.07o | 28.10 de | 0.55b | 5.47 c | 21.1 gh | 33.2 o–q | 192.3 ab | 49.3 no | 3.7 ij |
| NCV/BWR | 3.80 h | 27.80 d–f | 0.55b | 5.35 c | 13.8 m | 58.7 e | 31.9 l–o | 181.4 b | 4.6 d |
| TCV/BWR | 4.33 l | 27.62 ef | 0.50 c | 4.57 h | 10.9 p | 37.1 l–n | 33.4 k–n | 148.5 d | 3.2 n |
| MCV1/TWR | 6.07b | 27.53 ef | 0.45 de | 4.63 h | 24.7 de | 40.7 i–k | 136.8 ef | 48.9 no | 3.1 op |
| MCV2/TWR | 5.50 e | 27.58 ef | 0.40 g | 4.27i | 27.2 c | 42.5 h–j | 172.6 c | 54.7 l–n | 5.1b |
| CCV1/TWR | 4.82 h | 24.55 i | 0.37 h | 4.08 k | 24.5 e | 39.4 j–m | 117.6 g | 57.5 j–m | 3.7 ij |
| CCV2/TWR | 4.20 m | 25.30 h | 0.40 g | 5.83b | 22.5 f | 28.7 r | 86.5 j | 57.5 j–m | 2.4 u |
| CCV3/TWR | 4.13 no | 27.53 ef | 0.50 c | 4.80 fg | 21.2 g | 99.5 a | 192.8 ab | 49.2 no | 4.1 f |
| NCV/TWR | 4.13 no | 27.17 fg | 0.47 d | 4.67 gh | 12.9 mn | 57.6 e | 36.2 kl | 144.8 d | 3.9 gh |
| TCV/TWR | 3.88 qr | 27.12 fg | 0.45 de | 4.27 i | 12.2 op | 34.4 n–p | 27.4 n–p | 193.8 a | 3.7 ij |
| Mean | 4.68 | 26.22 | 0.44 | 4.35 | 19.91 | 45.14 | 102.89 | 82.09 | 3.44 |
| SEM | 0.06 | 0.25 | 0.01 | 0.07 | 0.46 | 1.30 | 4.06 | 3.31 | 0.09 |
| LSD (p = 0.05) | 0.08 | 0.64 | 0.02 | 0.13 | 1.17 | 3.54 | 6.37 | 6.69 | 0.11 |
| No | Code | Original Code | Type of Material | Fruit Type | Origin |
|---|---|---|---|---|---|
| Scion | |||||
| 1 | MCV1 | Green world white eggplant 330 | Commercial variety | Long | Malaysia |
| 2 | MCV2 | Green world purple dream eggplant 302 | Commercial variety | Long | Malaysia |
| 3 | CCV1 | China vegetable seed technology 62146129 | Commercial variety | Long | China |
| 4 | CCV2 | China vegetable seed technology 62119631 | Commercial variety | Long | China |
| 5 | CCV3 | China vegetable seed technology 62119631 | Commercial variety | Round | China |
| 6 | NCV | Yalo garden egg eggplant | Commercial variety | Round | Nigeria |
| 7 | TCV | Round eggplant (Chao-phaya) | Commercial variety | Round | Thailand |
| Rootstock | |||||
| 8 | MWR | MFS eggplant | Wild relatives | Round | Malaysia |
| 9 | BWR | Plate brush eggplant | Wild relatives | Round | Bangladesh |
| 10 | TWR | Pea eggplant | Wild relatives | Round | Thailand |
| Parameters | Denotation | Data Collection |
|---|---|---|
| Days for 50% seeds germination | D50%SG | Daily visual observations were recorded until the reaching of 50% of emerged seedlings |
| Germination % | G% | The observation was recorded at every day by visual observation until all germinated |
| Days taken to 50% of grafting stage plants | G50%RGS | Daily visual observations were recorded until 50% of plants reached grafting stage |
| Graft success (%) | GS | A graft success was recorded at 15 days after grafting based on wilting of the grafts at healing region. |
| Days taken for 50% flowering | D50%F | Number of days from sowing to 50% flowering. |
| First harvest | FH | Days to first fruit picking was measured by counting the number of days after transplanting to the day of the first picking at the marketable stage. |
| Plant height (cm) | PH | Plant height 45 and 90 days after transplanting. |
| Number of secondary branches | NSB | Numbers of secondary branches 45 and 90 days after transplanting. |
| Stem diameter | SD | The diameter of the stem was measured 5 cm above the ground level at 45 and 90 days after transplanting with an electronic digital caliper. |
| Fruit length (cm) | FL | The length of one matured fruit per plant from calyx to the apex of the fruit |
| Fruit diameter (cm) | FD | The diameter of one matured fruit (0.3 cm below the calyx) using venire calipers |
| Fruit weight (g) | FW | The weight of one matured fruit per plant. |
| Number of fruits per plant | NFPP | Total number of fruits from the first harvest until 90 days after transplanting |
| Average fruit weight (g) | AFW | By weighing of individual fruits. |
| Marketable fruit yield (Kg) | MFY | Total weight of fruit from the first harvest until 90 days after transplanting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musa, I.; Rafii, M.Y.; Ahmad, K.; Ramlee, S.I.; Md Hatta, M.A.; Oladosu, Y.; Muhammad, I.; Chukwu, S.C.; Mat Sulaiman, N.N.; Ayanda, A.F.; et al. Effects of Grafting on Morphophysiological and Yield Characteristic of Eggplant (Solanum melongena L.) Grafted onto Wild Relative Rootstocks. Plants 2020, 9, 1583. https://doi.org/10.3390/plants9111583
Musa I, Rafii MY, Ahmad K, Ramlee SI, Md Hatta MA, Oladosu Y, Muhammad I, Chukwu SC, Mat Sulaiman NN, Ayanda AF, et al. Effects of Grafting on Morphophysiological and Yield Characteristic of Eggplant (Solanum melongena L.) Grafted onto Wild Relative Rootstocks. Plants. 2020; 9(11):1583. https://doi.org/10.3390/plants9111583
Chicago/Turabian StyleMusa, Ibrahim, Mohd Y. Rafii, Khairulmazmi Ahmad, Shairul Izan Ramlee, Muhammad Asyraf Md Hatta, Yusuff Oladosu, Isma’ila Muhammad, Samuel Chibuike Chukwu, Nur Nadzirah Mat Sulaiman, Arolu Fatai Ayanda, and et al. 2020. "Effects of Grafting on Morphophysiological and Yield Characteristic of Eggplant (Solanum melongena L.) Grafted onto Wild Relative Rootstocks" Plants 9, no. 11: 1583. https://doi.org/10.3390/plants9111583
APA StyleMusa, I., Rafii, M. Y., Ahmad, K., Ramlee, S. I., Md Hatta, M. A., Oladosu, Y., Muhammad, I., Chukwu, S. C., Mat Sulaiman, N. N., Ayanda, A. F., & Halidu, J. (2020). Effects of Grafting on Morphophysiological and Yield Characteristic of Eggplant (Solanum melongena L.) Grafted onto Wild Relative Rootstocks. Plants, 9(11), 1583. https://doi.org/10.3390/plants9111583







