Cracking in Sweet Cherry Cultivars Early Bigi and Lapins: Correlation with Quality Attributes
Abstract
1. Introduction
2. Results and Discussion
2.1. Total Yield and Yield Efficiency
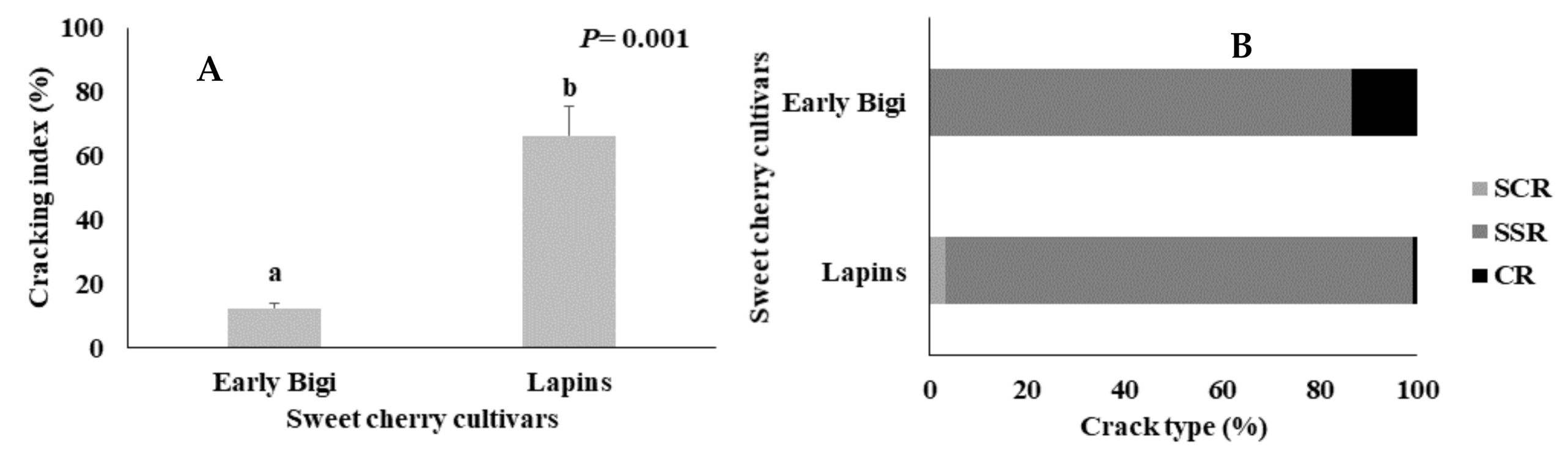
2.2. Cracking Index and Crack Type Incidence
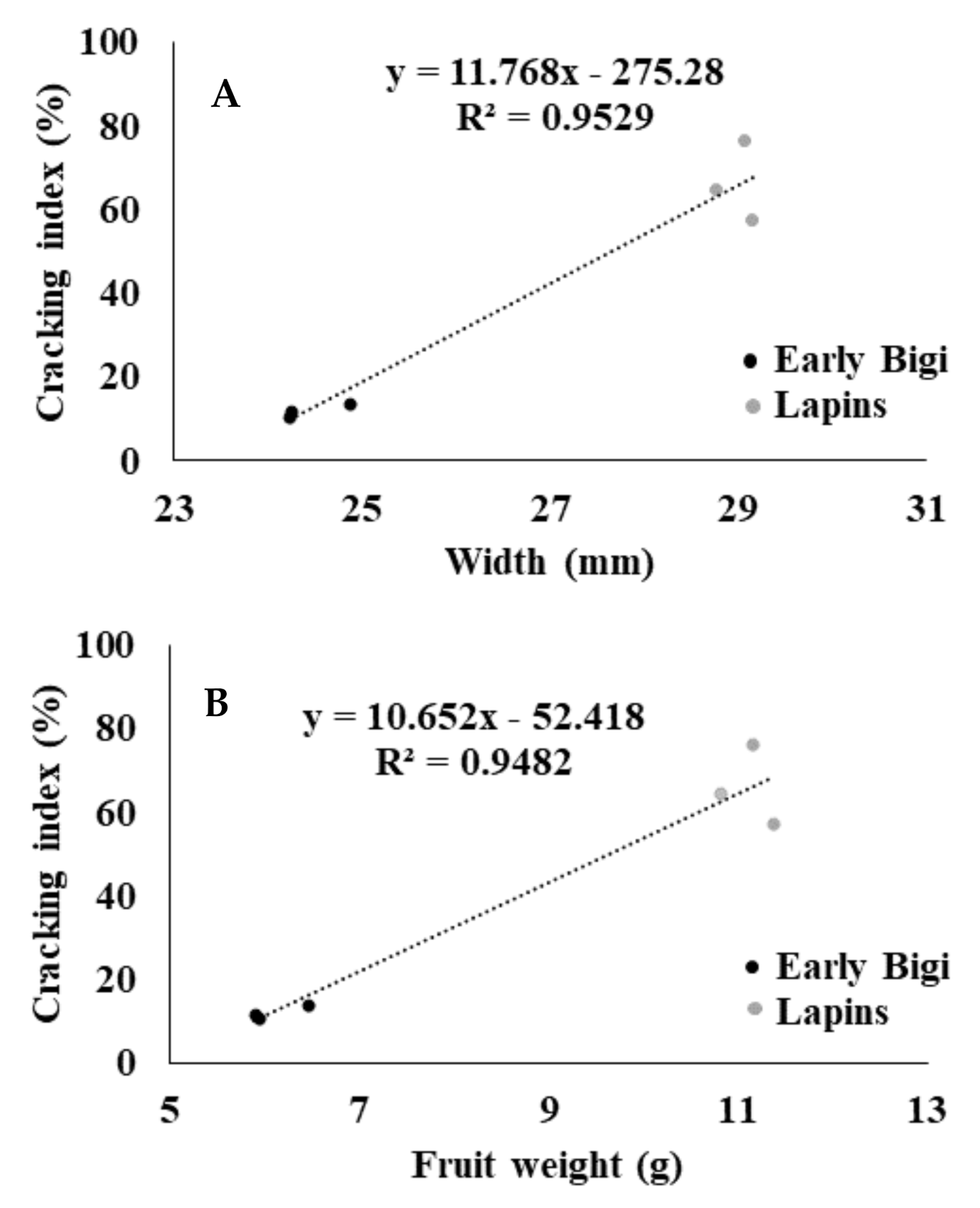
2.3. Cracking Index and Biometric Attributes
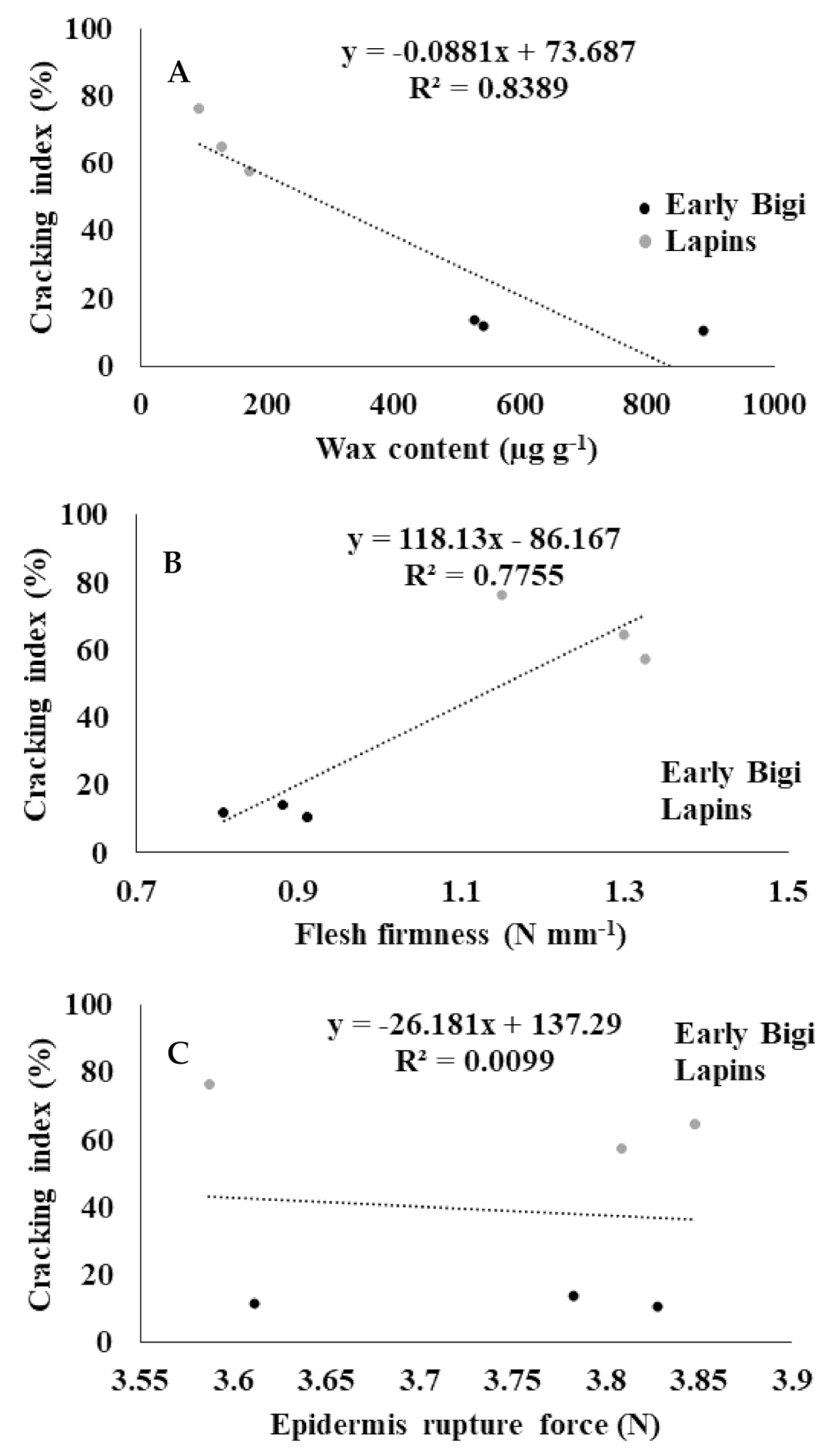
2.4. Cracking Index, Soluble Cuticular Wax Content, and Texture Parameters
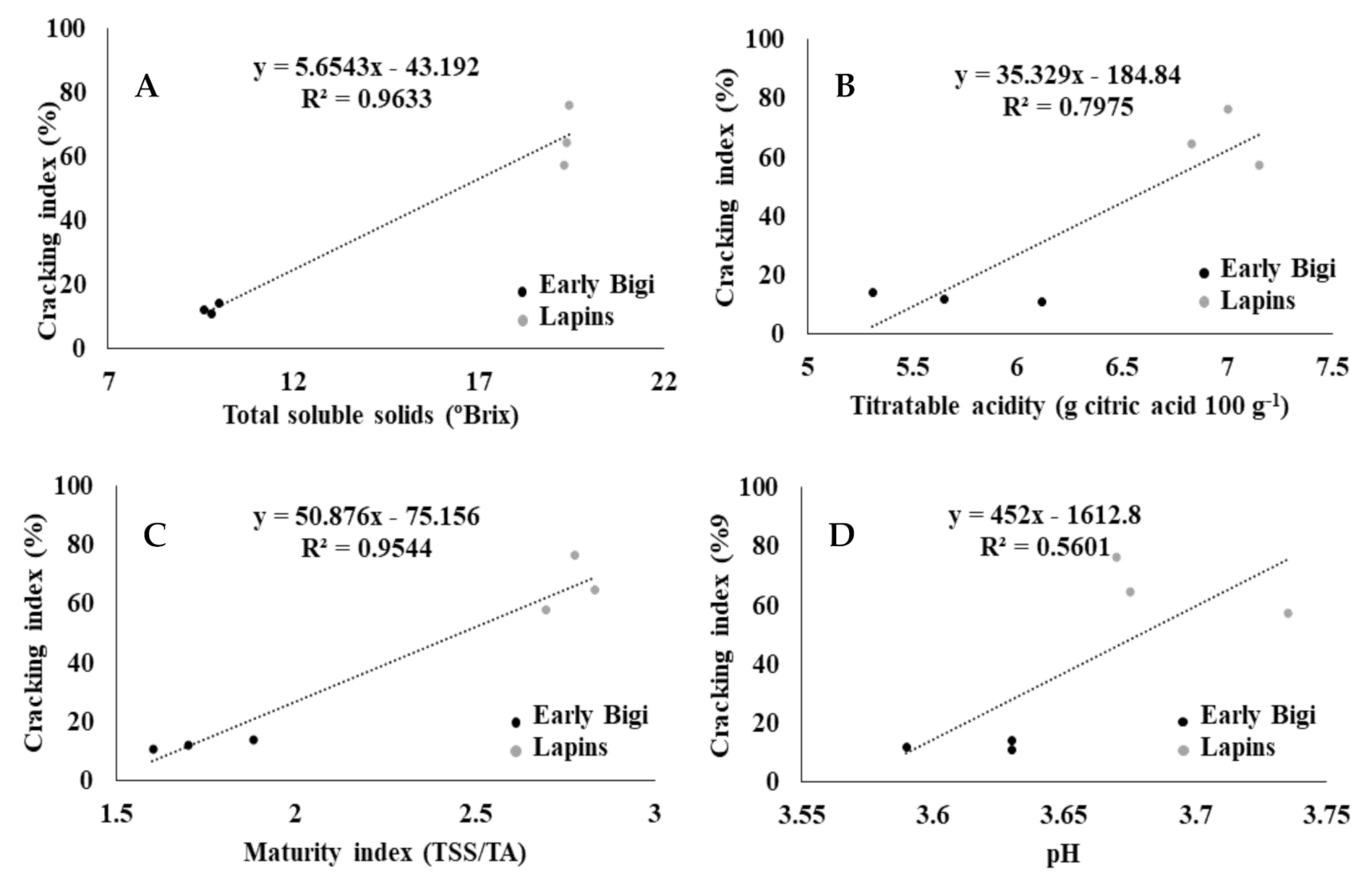
2.5. Cracking Index (CI) and Maturity Evaluation Parameters
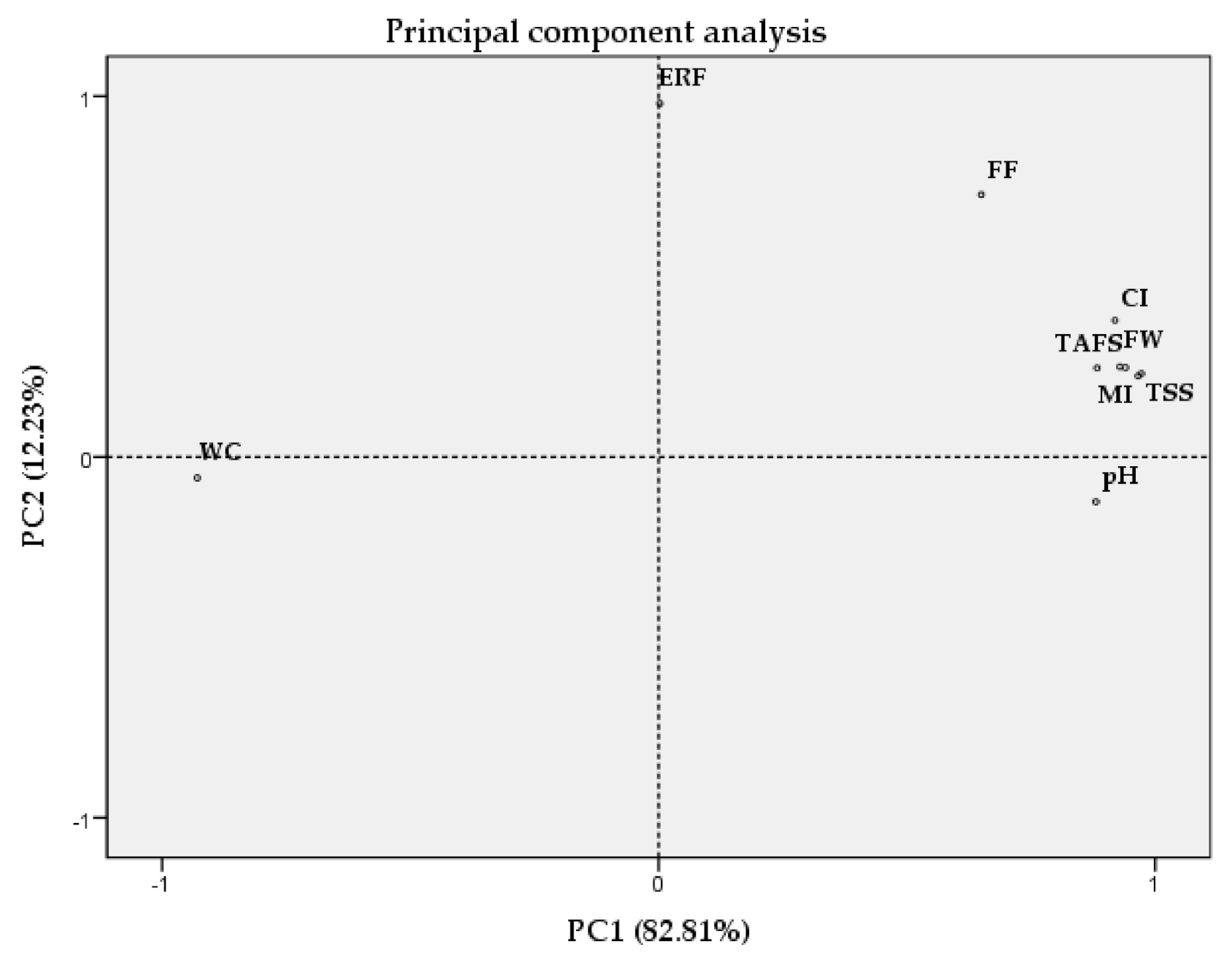
2.6. Principal Component Analysis (PCA)
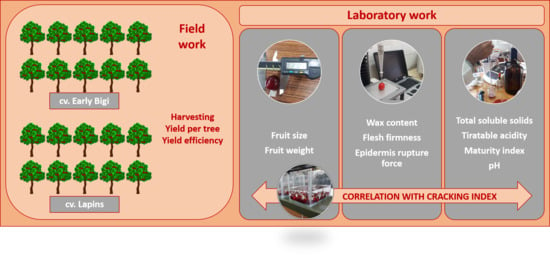
3. Material and Methods
3.1. Experimental Design and Sweet Cherry Raw Material
3.2. Production and Yield Efficiency
3.3. Cracking Index
3.4. Soluble Cuticular Waxes Content
3.5. Fruit Weight and Size
3.6. Epidermis Rupture Force and Flesh Firmness
3.7. Total Soluble Solids, Titratable Acidity, Maturity Index, and pH
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jakobek, L.; Seruga, M.; Voca, S.; Sindrak, Z.; Dobricevic, N. Flavonol and phenolic acid composition of sweet cherries (cv. Lapins) on six different vegetative rootstocks. Sci. Hortic. 2009, 123, 23–28. [Google Scholar] [CrossRef]
- FAOSTAT. Agricultural Production. Crops Primary–Cherries. Food and Agriculture Organization of the United Nations. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 10 July 2020).
- Lane, W.D.; Meheriuk, M.; McKenzie, D.-L. Fruit cracking of a susceptible, an intermediate, and a resistant sweet cherry cultivar. HortScience 2000, 35, 239–242. [Google Scholar] [CrossRef]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiol. Plant. 2015, 37, 1–14. [Google Scholar] [CrossRef]
- Knoche, M.; Peschel, S. Water on the surface aggravates microscopic cracking of the sweet cherry fruit cuticle. J. Am. Soc. Hortic. Sci. 2006, 131, 192–200. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Sweet cherry fruit cracking mechanisms and prevention strategies: A review. Sci. Hortic. 2018, 240, 369–377. [Google Scholar] [CrossRef]
- Koumanov, S. On the mechanisms of the sweet cherry (Prunus avium L.) fruit cracking: Swelling or shrinking? Sci. Hortic. 2015, 184, 169–170. [Google Scholar] [CrossRef]
- Knoche, M.; Winkler, A. Chapter 7: Rain-induced cracking of sweet cherries. In Cherries: Botany, Production and Uses; Quero-García, J., Iezzoni, A., Puławska, J., Lang, G., Eds.; CABI: Wallingford, UK, 2017; pp. 140–165. [Google Scholar]
- Christensen, J.V. Rain-induced cracking of sweet cherries: Its causes and prevention. In Cherries: Crop Physiology, Production and Uses; Webster, A.D., Looney, N.E., Eds.; CAB International: Wallingford, UK, 1996; pp. 297–327. [Google Scholar]
- Measham, P.F.; Bound, S.A.; Gracie, A.J.; Wilson, S.J. Crop load manipulation and fruit cracking in sweet cherry (Prunus avium L.). Adv. Hortic. Sci. 2012, 26, 25–31. [Google Scholar]
- Moing, A.; Renaud, C.; Christmann, H.; Fouilhaux, L.; Tauzin, Y.; Zanetto, A. Is there a relation between changes in osmolarity of cherry fruit flesh or skin and fruit cracking susceptibility? J. Am. Soc. Hortic. Sci. 2004, 129, 635–641. [Google Scholar] [CrossRef]
- Rozpara, E. Growth and yield of eleven sweet cherry cultivars in Central Poland. Acta Hortic. 2008, 795, 571–576. [Google Scholar] [CrossRef]
- Usenik, V.; Fajt, N. Sweet cherry cultivar testing in Slovenia. Acta Hortic. 2019, 1235, 265–270. [Google Scholar] [CrossRef]
- Wermund, U.; Fearne, A.; Hornibroo, S.A. Consumer purchasing behaviour with respect to cherries in the United Kingdom. Acta Hortic. 2005, 667, 539–544. [Google Scholar] [CrossRef]
- Christensen, J.V. Cracking in Cherries III. Determination of cracking susceptibility. Acta Agric. Scand. 1972, 22, 128–136. [Google Scholar] [CrossRef]
- Winkler, A.; Blumenberg, I.; Schurmann, L.; Knoche, M. Rain cracking in sweet cherries is caused by surface wetness, not by water uptake. Sci. Hortic. 2020, 269, 109400. [Google Scholar] [CrossRef]
- Gonçalves, B.; Silva, A.P.; Moutinho-Pereira, J.; Bacelar, E.; Rosa, E.; Meyer, A.S. Effect of ripeness and postharvest storage on the evolution of colour and anthocyanins in cherries (Prunus avium L.). Food Chem. 2007, 103, 976–984. [Google Scholar] [CrossRef]
- Usenik, V.; Fabcic, J.; Stampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Usenik, V.; Fajt, N.; Mikulic Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. Sweet cherry pomological and biochemical characteristics influenced by rootstock. J. Agric. Food Chem. 2010, 58, 4928–4933. [Google Scholar] [CrossRef]
- Christensen, J.V. Cracking in cherries VII. Cracking susceptibility in relation to fruit size and firmness. Acta Agric. Scand. 1975, 25, 301–312. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Sato, I.; Ishiguro, M. Influences of epidermal cell sizes and flesh firmness on cracking susceptibility in sweet cherry (Prunus avium L.) cultivars and selections. J. Jpn. Soc. Hortic. Sci. 2002, 71, 738–746. [Google Scholar] [CrossRef]
- Kunst, L.; Samuels, A.L. Plant cuticles shine: Advances in wax biosynthesis and export. Curr. Opin. Plant Biol. 2009, 12, 721–727. [Google Scholar] [CrossRef]
- Balbontín, C.; Ayala, H.; Bastías, R.M.; Tapia, G.; Ellena, M.; Torres, C.; Yuri, J.A.; Quero-García, J.; Ríos, J.C.; Silva, H. Cracking in sweet cherries: A comprehensive review from a physiological, molecular, and genomic perspective. Chil. J. Agric. Res. 2013, 73, 66–72. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Sato, I.; Watanabe, A.; Ishiguro, M. Cultivar differences in exocarp cell growth at apex, equator, stalk cavity and suture during fruit development in sweet cherry (Prunus avium L.). J. Jpn. Soc. Hortic. Sci. 2003, 72, 465–472. [Google Scholar] [CrossRef]
- Barret, D.M.; Gonzalez, C. Activity of softening enzymes during cherry maturation. J. Food Sci. 1994, 59, 574–577. [Google Scholar] [CrossRef]
- Wang, Y.; Long, L.E. Respiration and quality responses of sweet cherry to different atmospheres during cold storage and shipping. Postharvest Biol. Technol. 2014, 92, 62–69. [Google Scholar] [CrossRef]
- Li, M.; Zhi, H.H.; Dong, Y. Applications of glycine betaine on fruit quality attributes and storage disorders of ‘Lapins’ and ‘Regina’ cherries. HortScience 2019, 54, 1540–1545. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Dong, Y. Pre-harvest application of harpin β protein improves fruit on-tree and storage quality attributes of ‘Lapins’ and ‘Regina? Sweet cherry (Prunus avium L.). Sci. Hortic. 2020, 263, 109–115. [Google Scholar] [CrossRef]
- Martínez-Romero, D.; Alburquerque, N.; Valverde, J.M.; Guillén, F.; Castillo, S.; Valero, D.; Serrano, M. Postharvest sweet cherry quality and safety maintenance by Aloe vera treatment: A new edible coating. Postharvest Biol. Technol. 2006, 39, 93–100. [Google Scholar] [CrossRef]
- Romano, G.S.; Cittadini, E.D.; Pugh, B.; Schouten, R. Sweet cherry quality in the horticultural production chain. Stewart Postharvest Rev. 2006, 6, 1–8. [Google Scholar]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Simon, G.; Hrotkó, K.; Magyar, L. Fruit quality of sweet cherry cultivars grafted on four different rootstocks. Acta Hortic. 2004, 658, 365–370. [Google Scholar] [CrossRef]
- Christensen, J.V. Cracking in Cherries VI. Cracking susceptibility in relation to the growth rhythm of the fruit. Acta Agric. Scand. 1973, 23, 52–54. [Google Scholar] [CrossRef]
- Verner, L.; Blodgett, E.C. Physiological studies of the cracking of sweet cherries. University of Idaho. Agric. Exp. Stn. Bull. 1931, 184, 1–15. [Google Scholar]
- Hamilton, R.J. Waxes: Chemistry, Molecular Biology and Functions; Orly Press: Edinburgh, UK, 1995. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.; Silva, V.; Bacelar, E.; Guedes, F.; Silva, A.P.; Ribeiro, C.; Gonçalves, B. Cracking in Sweet Cherry Cultivars Early Bigi and Lapins: Correlation with Quality Attributes. Plants 2020, 9, 1557. https://doi.org/10.3390/plants9111557
Pereira S, Silva V, Bacelar E, Guedes F, Silva AP, Ribeiro C, Gonçalves B. Cracking in Sweet Cherry Cultivars Early Bigi and Lapins: Correlation with Quality Attributes. Plants. 2020; 9(11):1557. https://doi.org/10.3390/plants9111557
Chicago/Turabian StylePereira, Sandra, Vânia Silva, Eunice Bacelar, Francisco Guedes, Ana Paula Silva, Carlos Ribeiro, and Berta Gonçalves. 2020. "Cracking in Sweet Cherry Cultivars Early Bigi and Lapins: Correlation with Quality Attributes" Plants 9, no. 11: 1557. https://doi.org/10.3390/plants9111557
APA StylePereira, S., Silva, V., Bacelar, E., Guedes, F., Silva, A. P., Ribeiro, C., & Gonçalves, B. (2020). Cracking in Sweet Cherry Cultivars Early Bigi and Lapins: Correlation with Quality Attributes. Plants, 9(11), 1557. https://doi.org/10.3390/plants9111557