1. Introduction
The genus
Mertensia of the family Boraginaceae comprises 62 species of perennial herbs widely distributed in Europe, North and Central America, and Northern Asia [
1]. Several
Mertensia species are traditionally used to treat tuberculosis, venereal diseases, and whooping cough [
2]. Pyrrolizidine alkaloids such as lycopsamine and intermedine were found in
M. bakeri (Greene) and
M. ciliata (James) G. Don [
3].
M. maritima (L.) Gray, also recognized as an oyster plant, is largely found in the northern hemisphere. It has striking blue-green leaves and blooms from June to September with pink and blue flowers; thus, it has high ornamental value. The fresh leaves, taproots, and flowers of
M. maritima are eaten by the Iñupiat of Alaska [
2]. However, the presence of hepatotoxic pyrrolizidine alkaloids in the tissues of
M. maritima has not yet been disclosed. It contains several bioactive metabolites such as carotenoids, phenolic acid, terpenoids, tocopherol, fatty acids, and volatile compounds [
4,
5,
6], which are well-known to have many biological activities. In addition, the pharmacological activities of
Mertensia species have not yet been documented.
M. maritima is at risk of extinction owing to climate change and ocean warming [
7]. Although the cultivation of oyster plants in nurseries is often difficult [
7], it has been successfully grown in southwestern France and Northern Scotland [
4].
M. maritima is naturally propagated by seeds. However, the mass production of
M. maritima using conventional methods is hampered because of its poor seed germination. Therefore, an alternative mass production method for
M. maritima would be valuable. In vitro explant-culture methods are effectively used for ex situ germplasm maintenance and the massive production of plantlets and bioactive metabolites. Recently, the in vitro micropropagation of
M. maritima was reported [
6]; the authors used TDZ (Thidiazuron) for micropropagation. However, the mass production of healthy
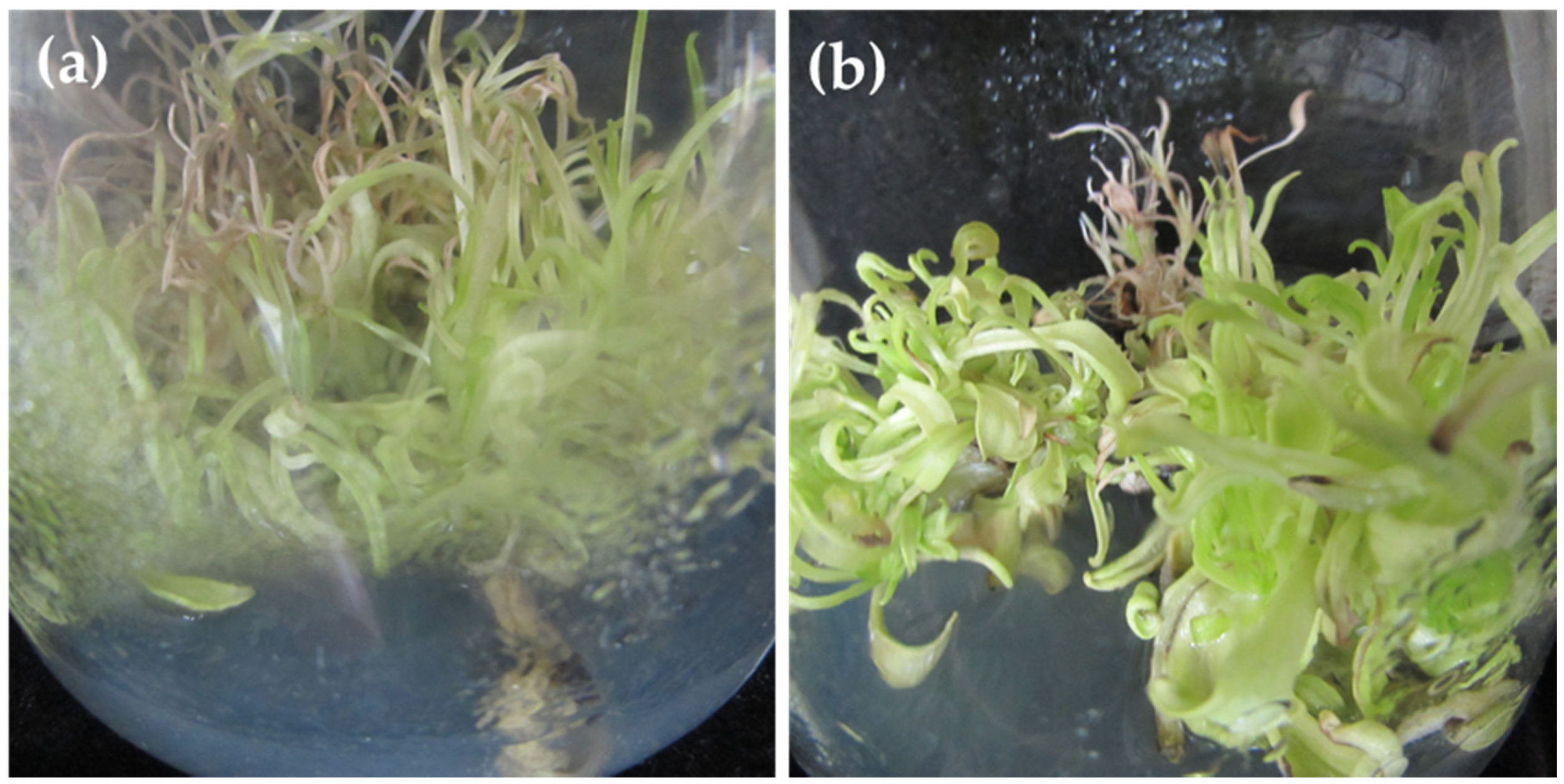
M. maritima shoots is affected by TDZ. Continuous TDZ exposure resulted in shoot tip necrosis and hyperhydricity (
Figure 1a,b). Hence, the identification of an efficient plant growth regulator (PGR) is necessary for the in vitro multiplication of
M. maritima shoots.
Several bioactive metabolites such as allantoin, rabdosiin, rosmarinic acid, all-
E-lutein, all-
E-β-carotene, all-
E-violaxanthin, 9-
Z-neoxanthin, (
Z)-β-carotene, all-
E-zeaxanthin, α-tocopherol, α-linolenic acid, palmitic acid, linoleic acid, γ-linolenic acid, stearidonic acid, stearic acid, lignoceric acid, behenic acid, and arachidic acid were obtained from
M. maritima callus and shoot cultures [
5,
6]. However, the screening of bioactive metabolites, including phenolics, in
M. maritima calli, shoots, and in vivo seedlings, has not yet been reported. Thus, the aims of this work were to (a) determine the effects of PGRs on callus and shoot proliferation, (b) assess the bioactive metabolite profile of in vitro-developed calli and shoots and in vivo seedlings, and (c) estimate the antioxidant capacity and enzyme inhibitory activity of
M. maritima.
3. Discussion
Callus induction and axillary shoot multiplication are significant in vitro technologies for the rapid commercial production of plantlets and bioactive metabolites. Leaf explants from
M. maritima initiated calli only on PGR (6-BA and NAA) supplemented MS medium. A combination of PGRs such as cytokinin and auxin are generally used to induce callus initiation in
M. maritima [
5] and several other Boraginaceae members such as
Alkanna orientalis (L.) Boiss. and
A. sieheana Rech. f. [
8],
Arnebia euchroma (Royle) Jonst. [
9],
Arnebia hispidissima (Lehm.) A. DC. [
10],
Echium italicum L. [
11],
Eritrichium sericeum (Lehm.) A. DC. [
12],
Onosma bulbotrichum [
13], and
Onosma sericeum Willd [
14]. In this study, the highest callus proliferation was attained after 5 weeks of cultivation under a 16-h photoperiod on MS medium augmented with 4 µM of each 6-BA and NAA. Fedoreyev et al. [
5] also obtained maximum callus growth in
M. maritima on W
B/A medium containing 2.2 µM 6-BA and 10.8 µM NAA after 30 days of cultivation in the dark. However, there was a considerable difference in the optimal levels of 6-BA and NAA. The differences in the optimal concentration of 6-BA and NAA required for the best callus growth may be due to culture conditions, callus type, and growth media.
Nodal explants of
M. maritima failed to regenerate in MS medium lacking PGR. This is consistent with an earlier study of
M. maritima [
6]. Similar results have been reported for Boraginaceae members such as
Hackelia venusta (Piper) H.St.John [
15] and
Trichodesma indicum (Linn) R. Br. [
16]. Axillary shoot multiplication can be achieved by supplementing the growth media with optimal levels of PGRs. Cytokinin is a class of PGR that promotes axillary shoot multiplication by antagonizing apical shoot dominance. Nodal segments of
M. maritima cultivated on medium with 6-BA and 6-KN produced axillary shoots (
Table 1). Cytokinins (for shoot production) and their concentrations were chosen based on our previous study [
6]. The explants cultivated on medium with 2 µM 6-BA and 4 µM 6-KN developed more shoots (13.4) than those on media containing the other five 6-BA and 6-KN combinations (
Table 1). The combinations of two cytokinins (6-BA and 6-KN) were found to be effective in the shoot regeneration of Boraginaceae members [
10,
17]. Kumar and Rao [
17] reported that
Heliotropium indicum L. axillary buds inoculated on medium with 4.7 µM 6-KN and 2.2 µM 6-BA developed 11.8 shoots.
Several studies have shown that media with cytokinin and auxin enhance shoot proliferation in numerous members of the Boraginaceae family, including
M. maritima [
6,
16,
17,
18,
19]. The ratio of cytokinin and auxin is crucial for the production of multiple shoots. In general, high cytokinin/auxin ratio promotes shoot proliferation in many plants [
20]. In this study, supplementing MS with a high level of 6-BA or 6-KN (8 µM) and low level of NAA (1 µM) yielded the maximum number of shoots. However, the incorporation of a high concentration of NAA (2 µM) in media containing 6-BA or 6-KN inhibited shoot proliferation. Similar results have been described for
A. hispidissima [
18],
H. indicum [
17], and
T. indicum [
16]. The MS added with 8 µM 6-KN and 1 µM NAA produced the maximum (8.4) number of shoots (
Table 2). This number was lower than that reported for oyster plant [
6]. Maximal shoot production was 17.7 shoots per node for
M. maritima and was stimulated by 4 µM TDZ and 1 µM NAA [
6].
The incorporation of IBA in ½ MS enhanced the rooting response of the explants (
Table 3). IBA was chosen for in vitro rooting because it has been reported to be beneficial for adventitious root induction in
M. maritima [
6] and other Boraginaceae members [
10,
16,
17,
18]. However, the rooting response of shoots under in vitro conditions varied with the IBA concentration. The optimal level of IBA required for the rooting of micro shoots depends on the rooting medium strength, the composition of shoot production medium, plant species, and genotype [
20]. In this study, the best rooting was attained with 2 µM IBA in
M. maritima. However, the rooting of
M. maritima was the most effective in half-strength medium with 4 µM IBA [
6]. MS with 9.8 µM IBA exhibited the best rooting in
A. hispidissima [
18], half-strength medium with 0.49 µM IBA showed the best rooting in
H. indicum [
17], and MS with 2.46 µM IBA displayed the best rooting in
T. indicum [
16].
Phenolics are unique compounds with significant biological properties, including antioxidant, anti-microbial, and anti-cancer activities [
21]. In this context, the determination of the TAP in plant extracts is the first step; for this purpose, a colorimetric method was developed by Folin and Ciocalteu [
22]. The TAP in the shoots was 216.4% and 369.5% higher than in seedlings and calli, respectively. The TAP in
M. maritima shoots extract was also larger (41.98 mg GAE/g of extract) than in calli (9.20 GAE/g of extract), in vivo leaves (16.06 GAE/g of extract), stems (14.55 GAE/g of extract), inflorescences (21.70 GAE/g of extract) and roots (3.17 GAE/g of extract) of
Heliotropium indicum [
23], flowers (18.43 GAE/g of extract) and roots (13.11 GAE/g of extract) of
Cynoglossum creticum [
24], and aerial parts (32.7 GAE/g of extract) [
25] and roots (11.45 GAE/g of extract) of
Symphytum anatolicum [
26]. The TAP in the shoots was 241.1% and 429.3% higher than in seedlings and calli, respectively. However, TAP in
M. maritima shoot tissues was lower (1.76 mg RE/g of extract) compared to in vivo leaves (4.39 RE/g of extract) and flowers (21.77 RE/g of extract) of
C. creticum [
24], aerial parts (20.9 RE/g of extract) of
Cynoglottis barrelieri [
25], leaves (3.32 QE/g of extract), stems (3.23 QE/g of extract) and inflorescences (4.90 QE/g of extract) of
H. indicum [
23], and aerial parts (13.3 RE/g of extract) [
25] and roots (2.74 RE/g of extract) of
S. anatolicum [
26]. Folin and Ciocalteu method, one of the most popular, is simple and requires inexpensive reagents. However, in recent times, there have been some concerns regarding the use of colorimetric methods, and some authors reported that these assays do not accurately reflect the levels of phytochemicals in extracts. This fact is related to the reduction of the Folin–Ciocalteu reagent by not only phenolics but also non-phenolic compounds [
27]. In this sense, at least one chromatographic technique (HPLC, LC-MS, or LC-MS/MS) is important for determining accurate levels of bioactive compounds. Hence, we determined the chemical profiles of
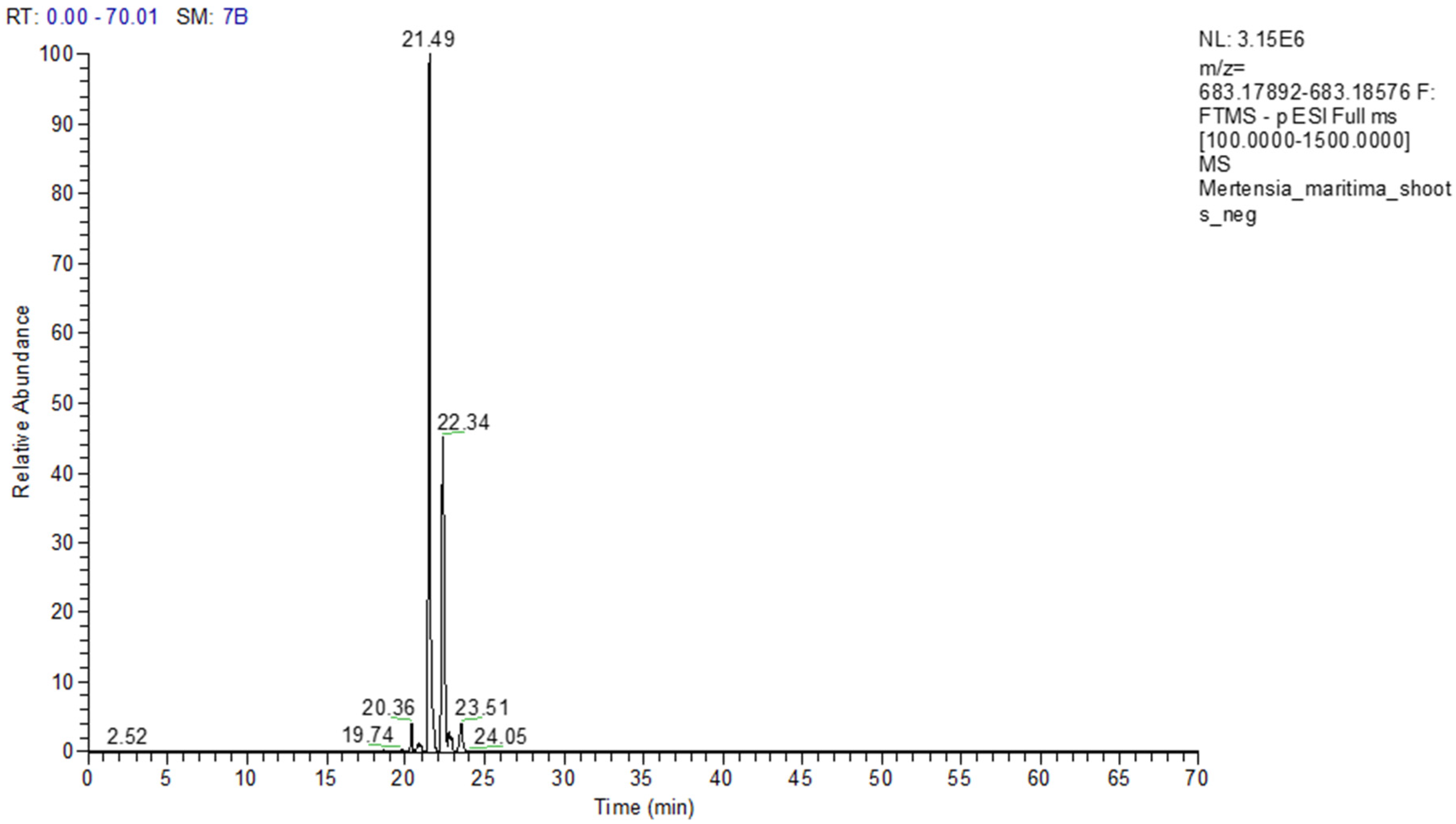
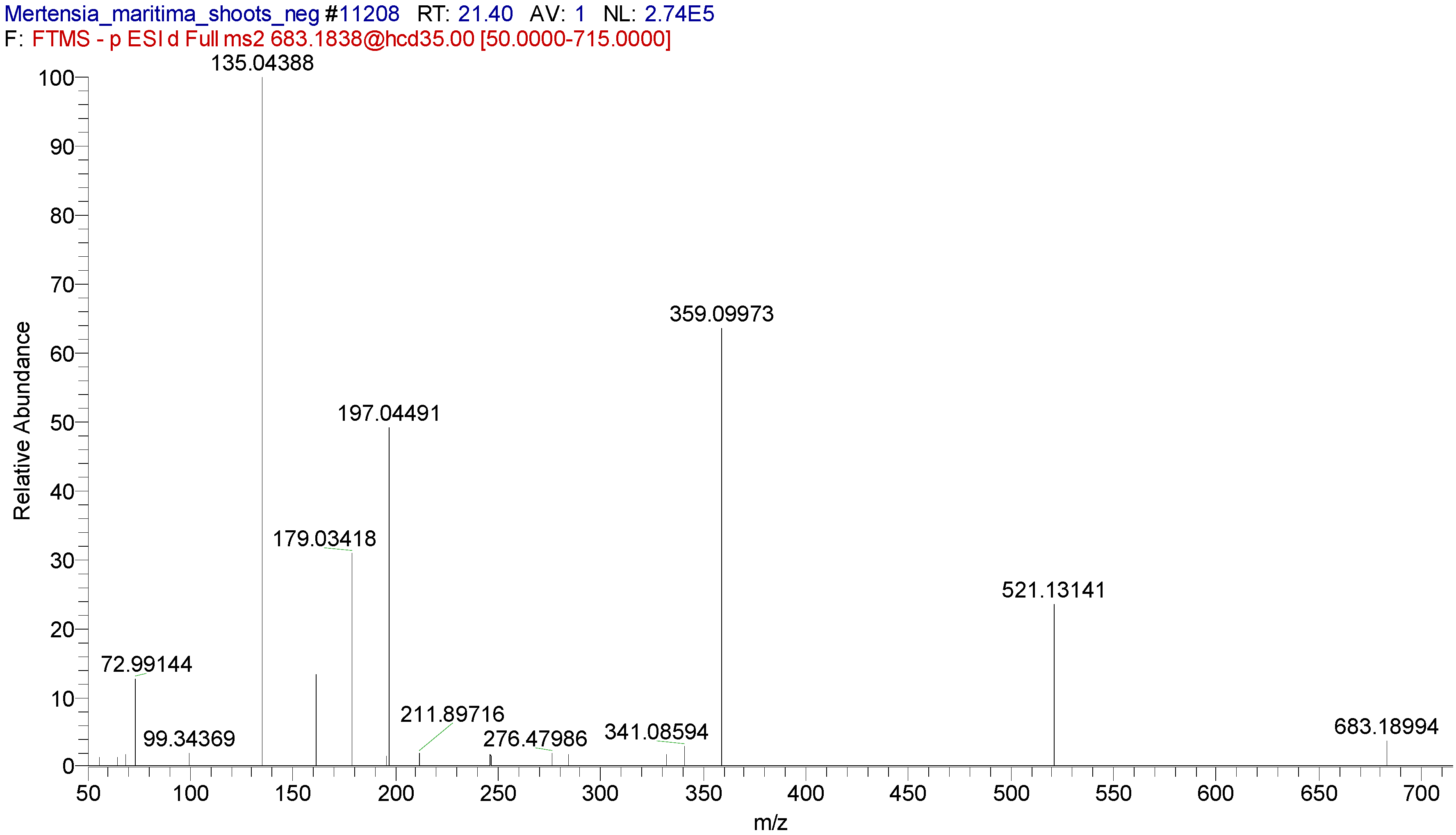
M. maritima extracts using the UHPLC-MS/MS technique.
The oyster plant is eaten by the Iñupiat of Alaska. It is cultivated as an edible plant in southwestern France and Northern Scotland [
4] for their fragrant leaves. Thus, knowledge of its chemical composition is necessary. Pyrrolizidine alkaloids are one of the main bioactive compounds of Boraginaceae members [
28]. For the first time, seven pyrrolizidine alkaloids, such as echimidine, heliosupine, heliotrine, intermedine or lycopsamine, and their N-oxides were identified in
M. maritima extracts by UHPLC-MS/MS (
Table 5,
Table 6 and
Table 7). The occurrence of these compounds was also disclosed in numerous members of the Boraginaceae family, including
Mertensia species [
3,
28,
29]. Four and six fatty acids were identified in callus and tissue extracts of
M. maritima by UHPLC-MS/MS (
Table 5–7). Among the fatty acids detected, α-linolenic acid and stearidonic acid were reported in
M. maritima [
6]. In this study, undecanedioic, dodecanedioic, tetradecanedioic, and hexadecanedioic acids were identified in
M. maritima, for the first time. Of the phenolic compounds detected, only rosmarinic acid was reported in the callus extract of
M. maritima [
5]. Whereas other phenolic compounds were found in members of the Boraginaceae [
30,
31,
32,
33,
34].
In the last few decades, the terms “antioxidant” and “oxidative stress” have become popular terms in the scientific community [
35]. This fact could be explained by the role of oxidative stress in the progression of chronic and degenerative diseases [
36]. In light of this information, we determined the antioxidant profiles of
M. maritima extracts using different chemical methods. Except for the metal chelation assay, the best antioxidant properties were obtained in the shoot, followed by the seedling and callus. The order is in line with the levels of TAP and TAF. These findings showed that phenolics were the main contributors of the antioxidant capacities of the
M. maritima extract. This fact also was confirmed by correlation analysis, and the results are given in
Figure 7. In addition, this approach was also confirmed by several authors who reported a linear relationship between total phenolics and antioxidant properties [
37,
38,
39]. In addition, individual compounds, including caffeic acid [
40], rosmarinic acid [
41], and rutin [
42], have been reported as significant antioxidants. The contents of these compounds are relatively high in members of the Boraginaceae family, including
M. maritima [
5,
30,
32,
33,
34]. Phenolic compounds with one or more hydroxyl groups are effective hydrogen or electron donors. Regarding the metal chelating ability, the contradictory results could be explained by the presence of non-phenolic chelators in
M. maritima shoot extracts, including polysaccharides, peptides, or vitamin C [
43].
According to WHO reports, diseases such as heart disease, stroke, chronic pulmonary disease, Alzheimer’s disease, and diabetes mellitus are the biggest killers worldwide [
44]. The inhibition of cholinesterase increases the level of acetylcholine, which could enhance the memory capacity in patients with Alzheimer’s [
45]. Similarly, the inhibition of carbohydrate-hydrolyzing enzymes can retard the increase in blood glucose levels in patients with diabetes [
46]. Hence, several compounds are synthetically produced as enzyme inhibitors in the pharmaceutical industry. However, several studies have reported that synthetic compounds have unpleasant side effects [
47,
48]. Thus, enzyme inhibitors from natural sources are needed to replace these synthetic ones. In recent investigations, several researchers reported enzyme inhibition properties of several Boraginaceae members such as,
Alkanna sfikasiana [
49],
Cynoglossum creticum [
24],
Cynoglottis barrelieri [
25],
Echium confusum [
50],
Onosma aucheriana,
Onosma sieheana,
Onosma frutescens,
Onosma stenoloba, and
Onosma sericea [
51,
52]
Symphytum anatolicum [
25,
26]. In the present study, the enzyme inhibitory effects of
M. maritima extracts were investigated using different enzymes. Among the three
M. martima tissue extracts studied, calli extracts exhibited ideal AChE (IC50: 0.74 mg/mL) inhibition, seedlings extracts exhibited ideal BChE (IC50: 0.74 mg/mL) inhibition and tyrosinase (IC50: 0.74 mg/mL) inhibition, while all three extracts exhibited similar amylase (IC50: 1.40–1.47 mg/mL) inhibition activity (
Table 9). The IC50 values attained from tyrosinase and amylase inhibition assays were lower compared to several other Boraginaceae members [
51,
52]. We observed different results for each enzyme inhibition ability. The observed enzyme inhibitory effects could be explained by the chemical profiles of the extracts. Some compounds in the chemical profiles, including caffeic acid [
53,
54], rosmarinic acid [
55,
56], and rutin [
57,
58] have been reported as significant enzyme inhibitor agents in previous studies. To our knowledge, the present study is the first report on the enzyme inhibitory effects of
M. maritima extracts, and these findings could provide valuable contributions to the scientific community.