Recent Advances in Adventitious Root Formation in Chestnut
Abstract
1. Introduction
2. Current Knowledge on Adventitious Root Formation
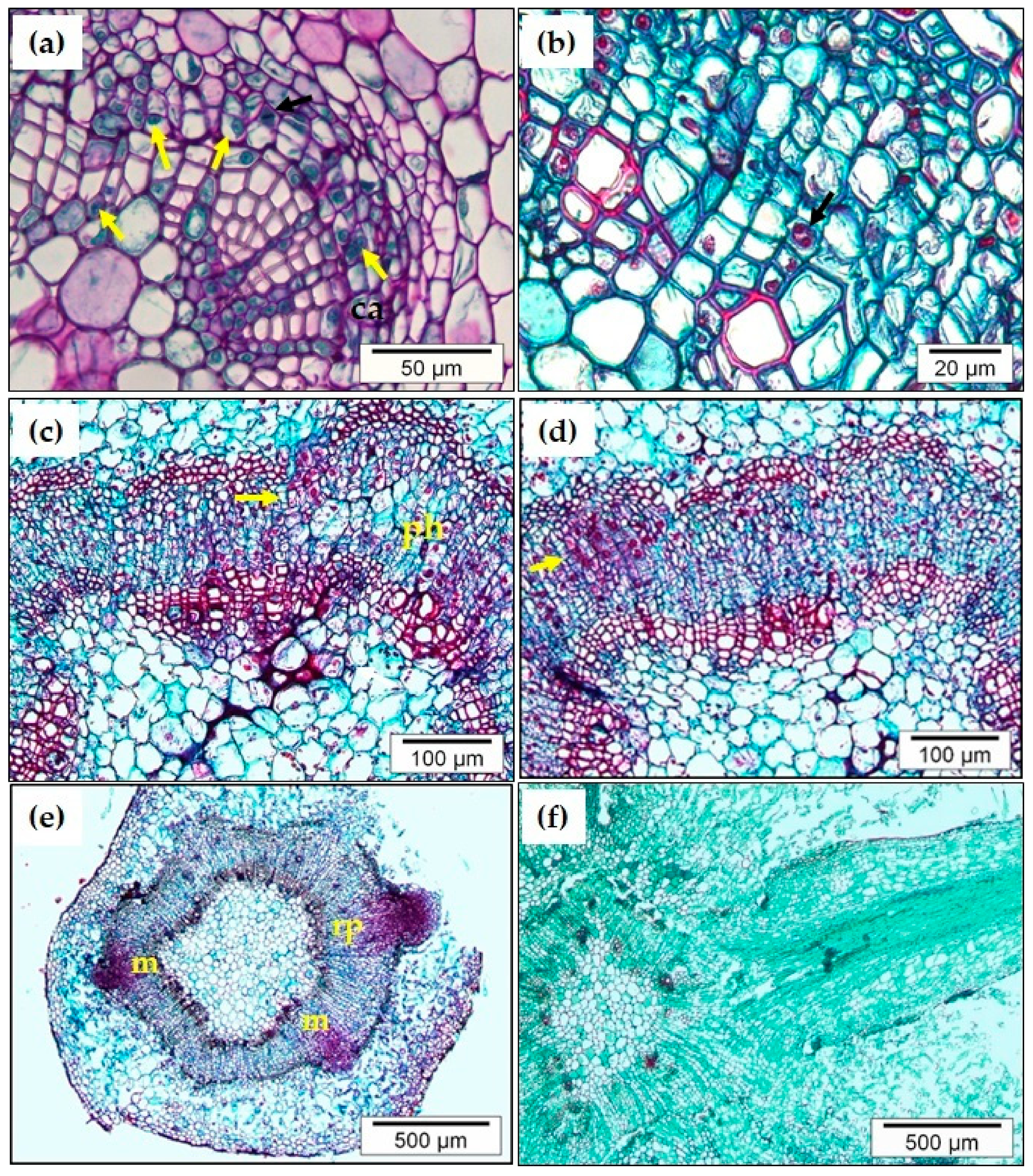
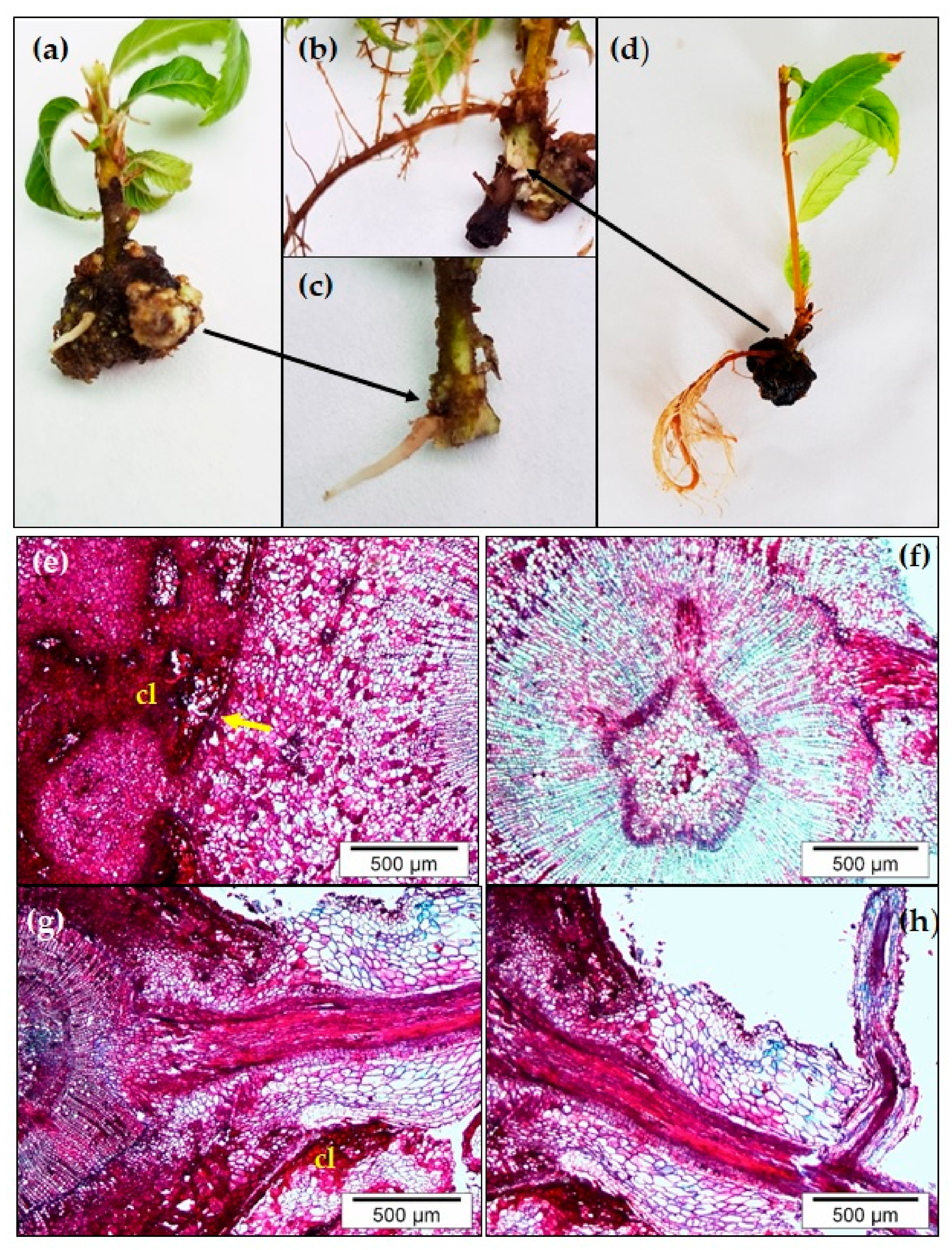
3. Developmental Phases of Adventitious Root Organogenesis in Chestnut: Histological Analysis
4. Biochemical Profiles during AR
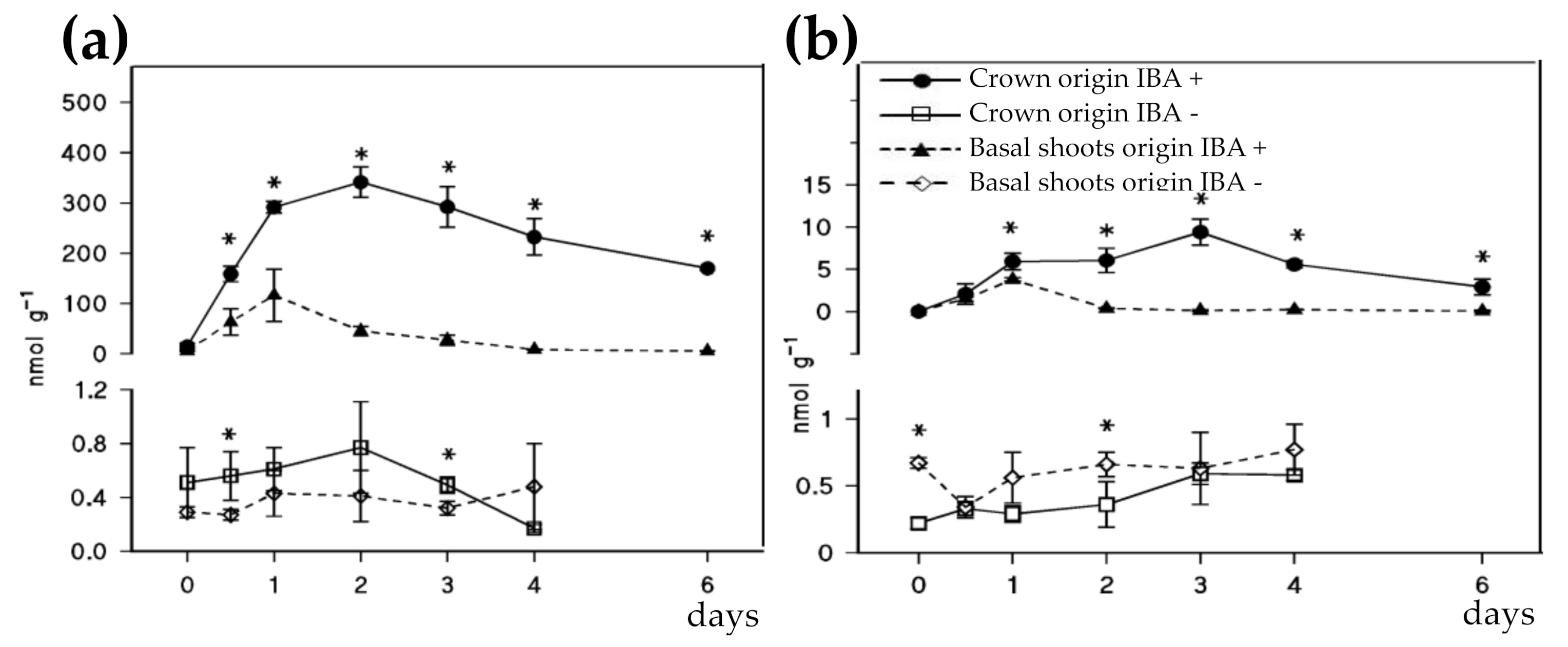
4.1. Auxin Content
4.2. Polyphenols and Polyamines
4.3. Auxin-Related Enzymatic Activity
5. Molecular Aspects of AR in Chestnut
5.1. Genetics
5.2. Epigenetics
6. Main Factors Influencing the Rooting Response
6.1. Genotype
6.2. Auxin Treatments
6.3. Non-Auxin Compounds
6.4. Ontogenetic Stage
6.5. Rooting Media
6.6. Light Conditions
7. Root System Architecture and Functionality: In Vitro vs. Ex Vitro Rooting
8. Acclimation of In Vitro Rooted Microshoots
9. Future Prospects of Biotechnological Approaches on AR
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vieitez, F.J.; Merkle, S.A. Castanea spp. Chestnut. In Biotechnology of Fruits and Nut Crops; Litz, R.E., Ed.; CAB International: Wallingford, UK, 2005; pp. 265–296. [Google Scholar]
- Maynard, C.A.; Powell, W.A.; Polin-McGuigan, L.D.; Vieitez, A.M.; Ballester, A.; Corredoira, E.; Merkle, S.A.; Andrade, G.M. Chestnut. In Compendium of Transgenic Crop Plants: Transgenic Forest Tree Species; Kole, C., Hall, T.C., Eds.; Blackwell Publishing: Chichester, UK, 2014; pp. 169–192. [Google Scholar]
- Conedera, M.; Tinner, W.; Krebs, P.; de Rigo, D.; Caudullo, G. Castanea Sativa in Europe: Distribution, Habitat, Usage and Threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Durrant, T.H., Mauri, A., Eds.; Publication Office of the European Union: Luxembourg, 2016; pp. 78–79. [Google Scholar]
- Nelson, C.D.; Powell, W.A.; Merkle, S.A.; Carlson, J.E.; Hebard, F.V.; Islam-Faridi, N.; Staton, M.E.; Georgi, L. Biotechnology of Trees: Chestnut. In Tree Biotechnology; Ramawat, K., Merillon, J.M., Ahuja, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 3–25. [Google Scholar]
- Corredoira, E.; Martínez, M.T.; Cernadas, M.J.; San José, M.C. Application of biotechnology in the conservation of the genus Castanea. Forests 2017, 8, 394. [Google Scholar] [CrossRef]
- Vollmeier, R.; Osterc, G.; Luthar, Z. Preservation of sweet chestnut genetic resources (Castanea sativa Mill.) against attack by chestnut gall wasp (Dryocosmus kuriphilus Yasumatsu, 1951). Acta Agric. Slov. 2018, 111, 209–217. [Google Scholar] [CrossRef]
- Huang, H.; Norton, J.D.; Boyhan, G.E.; Abrahams, B.R. Graft compatibility among chestnut (Castanea) species. J. Amer. Soc. Hort. Sci. 1994, 119, 1127–1132. [Google Scholar] [CrossRef]
- Wright, J.W. Introduction to Forest Genetics; Academic Press: New York, NY, USA, 1976; p. 463. [Google Scholar]
- Vieitez, E. The Lack of Rootablility of Chestnut Cuttings. In Proceedings of the International Chestnut Conference; Double, M.L., MacDonald, W.L., Eds.; West Virginia University Press: Morgantown, WV, USA, 1992; pp. 82–88. [Google Scholar]
- López-Villamor, A.; Míguez-Soto, B.; Fernández-López, J. Adventitious root formation in Castanea spp. semi-hard cuttings is under moderate genetic control caused mainly by non-additive genetic variance. Can. J. For. Res. 2017, 47, 946–956. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Vieitez, M.L.; Vieitez, E. Chestnut (Castanea spp.). In Biotechnology in Agriculture and Forestry 1. Trees, I; Bajaj, Y.P.S., Ed.; Springer: Berlin, Germany, 1986; pp. 393–413. [Google Scholar]
- Vieitez, A.M.; Sánchez, M.C.; García-Nimo, M.L.; Ballester, A. Protocol for micropropagation of Castanea sativa. In Protocols for Micropropagation of Woody Trees and Fruits; Jain, S.M., Hägmann, H., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 299–312. [Google Scholar]
- Lakehal, A.; Bellini, C. Control of adventitious root formation: Insights into synergistic and antagonistic hormonal interactions. Physiol. Plant. 2019, 165, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Steffens, B.; Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 2016, 170, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Murphy, A.; Peer, W.; Gan, L.; Li, Y.; Chen, Z.M. Physiological and molecular regulation of adventitious root formation. Crit. Rev. Plant Sci. 2015, 34, 506–521. [Google Scholar] [CrossRef]
- Gonin, M.; Bergougnoux, V.; Nguyen, T.D.; Gantet, P.; Champion, A. What Makes Adventitious Roots? Plants 2019, 8, 240. [Google Scholar] [CrossRef]
- Druege, U.; Hilo, A.; Perez-Perez, J.M.; Klopotek, Y.; Acosta, M.; Shahinnia, F.; Zerche, S.; Franken, P.; Hajirezaei, M.R. Molecular and physiological control of adventitious rooting in cuttings: Phytohormone action meets resource allocation. Ann. Bot. 2019, 123, 929–949. [Google Scholar] [CrossRef]
- Pizarro, A.; Diaz-Sala, C. Cellular dynamics during maturation-related decline of adventitious root formation in forest tree species. Physiol. Plant 2019, 165, 73–80. [Google Scholar] [CrossRef]
- Druege, U.; Franken, P.; Hajirezaei, M.R. Plant hormone homeostasis, signaling and function during adventitious root formation in cuttings. Front. Plant Sci. 2016, 7, 381. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Sala, C. Direct reprogramming of adult somatic cells toward adventitious root formation in forest tree species: The effect of the juvenile–adult transition. Front. Plant Sci. 2014, 5, 310. [Google Scholar] [CrossRef] [PubMed]
- Kevers, C.; Hausman, J.F.; Faivre-Rampant, O.; Evers, D.; Gaspar, T. Hormonal control of adventitious rooting: Progress and questions. J. Appl. Bot. 1997, 71, 71–79. [Google Scholar]
- De Klerk, G.J.; Van der Krieken, W.; de Jong, J.C. The formation of adventitious roots: New concepts, new possibilities. In Vitro Cell. Dev. Biol. Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Da Costa, C.T.; de Almeida, M.R.; Ruedell, C.M.; Schwambach, J.; Maraschin, F.S.; Fett-Neto, A.G. When stress and development go hand in hand: Main hormonal controls of adventitious rooting in cuttings. Front. Plant Sci. 2013, 4, 133. [Google Scholar] [CrossRef]
- Sugimoto, K.; Gordon, S.P.; Meyerowitz, E.M. Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 2011, 21, 212–218. [Google Scholar] [CrossRef]
- Jiang, F.; Feng, Z.; Liu, H.; Zhu, J. Involvement of plant stem cells or stem cell-like cells in dedifferentiation. Front. Plant Sci. 2015, 6, 1028. [Google Scholar] [CrossRef]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef]
- Sugimoto, K.; Temman, H.; Kadokura, S.; Matsunaga, S. To regenerate or not to regenerate: Factors that drive plant regeneration. Curr. Opin. Plant Biol. 2019, 47, 138–150. [Google Scholar] [CrossRef]
- Lup, S.D.; Tian, X.; Xu, J.; Pérez-Pérez, J.M. Wound signaling of regenerative cell reprogramming. Plant Sci. 2016, 250, 178–187. [Google Scholar] [CrossRef]
- Nag, S.; Saha, K.; Choudhuri, M.A. Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J. Plant Growth Regul. 2001, 20, 182–194. [Google Scholar] [CrossRef]
- San-José, M.C.; Vidal, N.; Ballester, A. Anatomical and biochemical changes during root formation in oak and apple shoots cultured in vitro. Agronomie 1992, 12, 767–774. [Google Scholar] [CrossRef]
- Geiss, G.; Gutierrez, L.; Bellini, C. Adventitious Root Formation: New Insights and Perspectives. In Annual Plant Reviews—Root Development; Beeckman, T., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; Volume 37, pp. 127–156. [Google Scholar]
- Ballester, A.; San-José, M.C.; Vidal, N.; Fernández-Lorenzo, J.L.; Vieitez, A.M. Anatomical and biochemical events during in vitro rooting of microcuttings from juvenile and mature phases of chestnut. Ann. Bot. 1999, 83, 619–629. [Google Scholar] [CrossRef]
- Gonçalves, J.C.; Diogo, G.; Amancio, S. In vitro propagation of chestnut (Castanea sativa × C. crenata): Effects of rooting treatments on plant survival, peroxidase activity and anatomical changes during adventitious root formation. Sci. Hortic. 1998, 72, 265–275. [Google Scholar] [CrossRef]
- Vidal, N.; Arellano, G.; San-José, M.C.; Vieitez, A.; Ballester, A. Developmental stages during the rooting of in-vitro-cultured Quercus robur shoots from material of juvenile and mature origin. Tree Physiol. 2003, 23, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, A.; Rinaldi, L. Setting a model system to study in vitro rooting of chestnut: Main insights on adventitious root development. In Adventitious Root Formation of Forest Trees and Horticultural Plants—From Genes to Applications; Niemi, K., Scagel, C., Eds.; Research Signpost: Kerala, India, 2009; pp. 259–275. [Google Scholar]
- De Klerk, G.J.; Ter Brugge, J.; Keppel, M. Successive phases during rooting of microcuttings of Malus. Acta Hortic. 1993, 336, 249–256. [Google Scholar] [CrossRef]
- Banda, J.; Bellande, K.; von Wangenheim, D.; Goh, T.; Guyomarc’h, S.; Laplaze, L.; Bennett, M.J. Lateral root formation in Arabidopsis: A well-ordered LRexit. Trends Plant Sci. 2019, 24, 826–839. [Google Scholar] [CrossRef]
- Vilches-Barro, A.; Maizel, A. Talking through walls: Mechanisms of lateral root emergence in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2015, 23, 31–38. [Google Scholar] [CrossRef]
- Giovannelli, A.; Giannini, R.; Bennici, A.; Mori, B. In vitro organogenesis of chestnut (Castanea sativa Mill.) cotyledon explants: Responses to growth regulators and developmental aspects. In Vitro Cell Dev. Biol. Plant. 2004, 40, 509–514. [Google Scholar] [CrossRef]
- Varas, E.; Covelo, P.; Vidal, N.; Sánchez, C. Analysis of the hormonal manipulation during in vitro induction of adventitious roots in Castanea sativa. Acta Hortic. 2015, 1083, 219–226. [Google Scholar] [CrossRef]
- Sugimoto, K.; Jiao, Y.; Meyerowitz, E.M. Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 2010, 18, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Hosseini, S.A.; Hajirezaei, M.R.; Druege, U.; Geelen, D. Adventitious rooting declines with the vegetative to reproductive switch and involves a changed auxin homeostasis. J. Exp. Bot. 2015, 66, 1437–1452. [Google Scholar] [CrossRef] [PubMed]
- Vielba, J.M.; Varas, E.; Rico, S.; Covelo, P.; Sánchez, C. Auxin-mediated expression of a GH3 gene in relation to ontogenic state in Chestnut. Trees Struct. Funct. 2016, 30, 2237–2252. [Google Scholar] [CrossRef]
- Pacurar, D.I.; Perrone, I.; Bellini, C. Auxin is a central player in the hormone cross-talks that control adventitious rooting. Physiol. Plant. 2014, 151, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sheng, L.; Xu, Y.; Li, J.; Yang, Z.; Huang, H.; Xu, L. Wox11 and 12 are involved in the first-step cell fate transition during de novo root organogenesis in Arabidopsis. Plant Cell 2014, 26, 1081–1093. [Google Scholar] [CrossRef] [PubMed]
- Eliyahu, A.; Duman, Z.; Sherf, S.; Genin, O.; Cinnamon, Y.; Abu-Abied, M.; Weinstain, R.; Dag, A.; Sadot, E. Vegetative propagation of elite Eucalyptus clones as food source for honeybees (Apis mellifera); adventitious roots versus callus formation. Isr. J. Plant Sci. 2020, 67, 83–97. [Google Scholar] [CrossRef]
- Perrine-Walker, F.; Doumas, P.; Lucas, M.; Vaissayre, V.; Beauchemin, N.J.; Band, L.R.; Chopard, J.; Crabos, A.; Conejero, G.; Peret, B.; et al. Auxin carriers localization drives auxin accumulation in plant cells infected by Frankia in Casuarina glauca actinorhizal nodules. Plant Physiol. 2010, 154, 1372–1380. [Google Scholar] [CrossRef]
- Imanishi, L.; Perrine-Walker, F.M.; Ndour, A.; Vayssières, A.; Conejero, G.; Lucas, M.; Champion, A.; Laplaze, L.; Wall, L.; Svistoonoff, S.; et al. Role of auxin during intercellular infection of Discaria trinervis by Frankia. Front. Plant Sci. 2014, 5, 399. [Google Scholar] [CrossRef]
- Damodaran, S.; Strader, L.C. Indole-3-butyric acid metabolism and transport in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 851. [Google Scholar] [CrossRef]
- Fattorini, L.; Della Rovere, F.; Andreini, E.; Ronzan, M.; Falasca, G.; Altamura, M.M. Indole-3-butyric acid induces ectopic formation of metaxylem in the hypocotyl of Arabidopsis thaliana without conversion into indole-3-acetic acid and with a positive interaction with ethylene. Int. J. Mol. Sci. 2017, 18, 2474. [Google Scholar] [CrossRef]
- Negishi, N.; Nakahama, K.; Urata, N.; Kojima, M.; Sakakibara, H.; Kawaoka, A. Hormone level analysis on adventitious root formation in Eucalyptus globulus. New Forest 2014, 45, 577–587. [Google Scholar] [CrossRef]
- Almeida, M.R.; Bastiani, D.; Gaeta, M.L.; Mariath, J.E.A.; Costa, F.; Retallick, J.; Nolan, L.; Tai, H.H.; Strömvik, M.V.; Fett-Neto, A.G. Comparative transcriptional analysis provides new insights into the molecular basis of adventitious rooting recalcitrance in Eucalyptus. Plant. Sci. 2015, 239, 155–165. [Google Scholar] [CrossRef]
- Abu-Abied, M.; Szwerdszarf, D.; Mordehaev, I.; Levy, A.; Stelmakh, O.R.; Belausov, E.; Yaniv, Y.; Uliel, S.; Katzenellenbogen, M.; Riov, J.; et al. Microarray analysis revealed upregulation of nitrate reductase in juvenile cuttings of Eucalyptus grandis, which correlated with increased nitric oxide production and adventitious root formation. Plant J. 2012, 71, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.C.; Diogo, G.; Coelho, M.T.; Vidal, N.; Amâncio, S. Quantitation of endogenous levels of IAA, IAAsp and IBA in micro-propagated shoots of hybrid chestnut pretreated with IBA. In Vitro Cell. Dev. Biol. Plant 2008, 44, 412–418. [Google Scholar] [CrossRef]
- Hou, J.W.; Guo, S.J.; Wang, G.Y. Effects of in vitro subculture on the physiological characteristics of adventitious root formation in microshoots of Castanea mollissima Cv. ‘Yanshanhong’. J. For. Res. 2010, 21, 155–160. [Google Scholar] [CrossRef]
- Couée, I.; Hummel, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of polyamines in root development. Plant Cell Tiss. Organ Cult. 2004, 76, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Tahir, M.; Nawaz, M.; Mao, J.; Li, K.; Wei, Y.; Ma, D.; Lu, X.; Zhao, C.; Zhang, D. Spermidine application affects the adventitious root formation and root morphology of apple rootstock by altering the hormonal profile and regulating the gene expression pattern. Sci. Hort. 2020, 266, 109310. [Google Scholar] [CrossRef]
- Tsafouros, A.; Roussos, P.A. The possible bottleneck effect of polyamines catabolic enzymes in efficient adventitious rooting of two stone fruit rootstocks. J. Plant Phys. 2020, 244, 152999. [Google Scholar] [CrossRef]
- De Klerk, G.J.; Guan, H.; Huisman, P.; Marinova, S. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul. 2011, 63, 175–185. [Google Scholar] [CrossRef]
- Méndez, J.; Mato, M. Methyl gallate and related polyphenols as auxin protectors. Phytochemistry 1997, 44, 41–43. [Google Scholar] [CrossRef]
- Fernández-Lorenzo, J.; Ballester, A.; Rigueiro, A. Phenolic content of microcuttings of adult chestnut along rooting induction. Plant Cell Tiss. Organ Cult. 2005, 83, 153–159. [Google Scholar] [CrossRef]
- Vieitez, J.; Kingston, D.; Ballester, A.; Vieitez, E. Identification of two compounds correlated with lack of rooting capacity of chestnut cuttings. Tree Physiol. 1987, 3, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lorenzo, J.L.; Rigueiro, A.; Ballester, A. Phenolic compounds as potential markers to differentiate juvenile and mature chestnut shoot cultures. Tree Physiol. 1999, 19, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Osterc, G.; Štampar, F. Initial cutting length modifies polyphenol profile in Castanea cuttings during the root formation process. Eur. J. Hortic. Sci. 2008, 73, 201–204. [Google Scholar]
- Osterc, G.; Štefančič, M.; Solar, A.; Štampar, F. Potential involvement of flavonoids in the rooting response of chestnut hybrid (Castanea crenata × Castanea sativa) clones. Aust. J. Expl. Agric. 2007, 47, 96–102. [Google Scholar] [CrossRef]
- Mellor, N.; Band, L.R.; Pěnčík, A.; Novák, O.; Rashed, A.; Holman, T.; Wilson, M.H.; Voß, U.; Bishopp, A.; King, J.R.; et al. Dynamic regulation of auxin oxidase and conjugating enzymes AtDAO1 and GH3 modulates auxin homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, 11022–11027. [Google Scholar] [CrossRef] [PubMed]
- Porco, S.; Pěnčík, A.; Rashed, A.; Voß, U.; Casanova-Sáez, R.; Bishopp, A.; Golebiowska, A.; Bhosale, R.; Swarup, R.; Swarup, K.; et al. Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 11016–11021. [Google Scholar] [CrossRef]
- Ribeiro, C.L.; Silva, C.M.; Drost, D.R.; Novaes, E.; Novaes, C.R.; Dervinis, C.; Kirst, M. Integration of genetic, genomic and transcriptomic information identifies putative regulators of adventitious root formation in Populus. BMC Plant Biol. 2016, 16, 66. [Google Scholar] [CrossRef]
- Gil, B.; Pastoriza, E.; Ballester, A.; Sánchez, C. Isolation and characterization of a cDNA from Quercus robur differentially expressed in juvenile-like and mature shoots. Tree Physiol. 2003, 23, 633–640. [Google Scholar] [CrossRef][Green Version]
- Vielba, J.M.; Valladares, S.; Corredoira, E.; Vieitez, A.M.; Sánchez, C. Cloning of CsCPE cDNA from chestnut somatic embryos: Comparative expression analysis with zygotic embryos. Acta Hortic. 2008, 784, 93–98. [Google Scholar] [CrossRef]
- Valladares, S.; Rico, S.; Vieitez, A.M.; Covelo, P.; Sánchez, C. Expression of the QrCPE gene is associated with the induction and development of oak somatic embryos. Tree Genet. Genomes. 2013, 9, 1383–1393. [Google Scholar] [CrossRef]
- Covelo, G.; Ferro, E.; Vielba, J.M.; Sánchez, C. Molecular aspects of adventitious root formation in Fagaceae species. In Adventitious Root Formation of Forest Trees and Horticultural Plants–From Genes to Applications; Niemi, K., Scagel, C., Eds.; Research Signpost: Kerala, India, 2009; pp. 105–122. [Google Scholar]
- Romano, J.M.; Dubos, C.; Prouse, M.B.; Wilkins, O.; Hong, H.; Poole, M.; Kang, K.Y.; Li, E.; Douglas, C.J.; Western, T.L.; et al. AtMYB61, an R2R3-MYB transcription factor, functions as a pleiotropic regulator via a small gene network. New Phytol. 2012, 195, 774–786. [Google Scholar] [CrossRef]
- Matías-Hernández, L.; Jiang, W.; Yang, K.; Tang, K.; Brodelius, P.E.; Pelaz, S. AaMYB1 and its orthologue AtMYB61 affect terpene metabolism and trichome development in Artemisia annua and Arabidopsis thaliana. Plant J. 2017, 90, 520–534. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, C.; Vielba, J.M.; Ferro, E.; Covelo, G.; Solé, A.; Abarca, D.; de Mier, B.S.; Díaz-Sala, C. Two Scarecrow-Like genes are induced in response to exogenous auxin in rooting-competent cuttings of distantly related forest species. Tree Physiol. 2007, 27, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Vielba, J.M.; Díaz-Sala, C.; Ferro, E.; Rico, S.; Lamprecht, M.; Abarca, D.; Ballester, A.; Sánchez, C. CsSCL1 is differentially regulated upon maturation in chestnut microshoots and is specifically expressed in rooting-competent cells. Tree Physiol. 2011, 31, 1152–1160. [Google Scholar] [CrossRef]
- Motte, H.; Parizot, B.; Fanf, T.; Beeckman, T. The evolutionary trajectory of root stem cells. Curr. Opin. Plant Biol. 2020, 53, 23–30. [Google Scholar] [CrossRef]
- Staswick, P.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.; Maldonado, M.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616–627. [Google Scholar] [CrossRef]
- Mishra, P.; Roggen, A.; Ljung, K.; Albani, M.C. Natural variation in adventitious rooting in the alpine perennial Arabis alpina. Plants 2020, 9, 184. [Google Scholar] [CrossRef]
- Valladares, S.; Varas, E.; Vielba, J.M.; Vidal, N.; Codesido, V.; Castro, R.; Sánchez, C. Expression of a Rap2-12 like-1 gene during adventitious rooting of chestnut and oak microshoots. Isr. J. Plant Sci. 2020, 67, 69–82. [Google Scholar] [CrossRef]
- Heyman, J.; Canher, B.; Bisht, A.; Christiaens, F.; de Veylder, L. Emerging role of the plant ERF transcription factors in coordinating wound defense responses and repair. J. Cell Sci. 2018, 131, jcs208215. [Google Scholar] [CrossRef]
- Poethig, R.S. Vegetative phase change and shoot maturation in plants. Cur. Topics Dev. Biol. 2013, 105, 125–152. [Google Scholar]
- Wang, J.W.; Park, M.Y.; Wang, L.J.; Koo, Y.; Chen, X.Y.; Weigel, D.; Poethig, R.S. MiRNA control of vegetative phase change in trees. PLoS Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Szwerdszarf, D.; Abu-Abied, M.; Mordehaev, I.; Yaniv, Y.; Riov, J.; Sadot, E. Profiling microRNAs in Eucalyptus grandis reveals no mutual relationship between alterations in miR156 and miR172 expression and adventitious root induction during development. BMC Genom. 2014, 15, 524. [Google Scholar] [CrossRef] [PubMed]
- Hasbún, R.; Valledor, L.; Santamaría, E.; Cañal, M.J.; Rodriguez, R. Dynamics of DNA methylation in chestnut trees development. Acta Hort. 2007, 760, 563–566. [Google Scholar] [CrossRef]
- Wendling, I.; Trueman, S.J.; Xavier, A. Maturation and related aspects in clonal forestry—Part I: Concepts, regulation and consequences of phase change. New For. 2014, 45, 449–471. [Google Scholar] [CrossRef]
- Serres, R.; Read, P.; Hackett, W.; Nissen, P. Rooting of American chestnut microcuttings. J. Environ. Hort. 1990, 8, 86–88. [Google Scholar]
- Dantas, Â.K.; Majada, J.; Dantas, F.K.; Delatorre, C.; Granda, V.; Vallejo, P.; Feito, I. Rooting of minicuttings of Castanea sativa Mill. hybrid clones. Revista Árvore 2016, 40, 465–475. [Google Scholar] [CrossRef]
- Galic, D.; Dale, A.; Alward, M. Vegetative propagation of American chestnut. Acta Hort. 2014, 1019, 99–103. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Vieitez, A.M. In Vitro morphogenetic competence of basal sprouts and crown branches of mature chestnut. Tree Physiol. 1991, 8, 59–70. [Google Scholar] [CrossRef]
- Vielba, J.M.; Vidal, N.; Ricci, A.; Castro, R.; Covelo, P.; San-José, M.C.; Sánchez, C. Effects of auxin and urea derivatives on adventitious rooting in chestnut and oak microshoots. Isr. J. Plant Sci. 2020, 67, 52–68. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Ballester, A.; Vieitez, A.M. Reinvigoration treatments for the micropropagation of mature chestnut. Ann. Sci. For. 1997, 54, 359–370. [Google Scholar] [CrossRef]
- Sánchez, M.C.; San-José, M.C.; Ferro, E.; Ballester, A.; Vieitez, A.M. Improving micropropagation conditions for adult-phase shoots of chestnut. J. Hortic. Sci. 1997, 72, 433–443. [Google Scholar] [CrossRef]
- Tchatchoua, D.; Aravanopoulos, F. Selection strategy for Chestnut (Castanea sativa Mill.) families originating from contrasting European populations. Open J. For. 2015, 5, 489–499. [Google Scholar]
- Giovanelli, A.; Gianini, R. Reinvigoration of mature chestnut (Castanea sativa) by repeated grafting and micropropagation. Tree Physiol. 2000, 20, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Satchwell, M.; Powell, W.A.; Maynard, C.A. Micropropagation of American chestnut: Increasing rooting rate and preventing shoot-tip necrosis. In Vitro Cell. Dev. Biol. Plant 1997, 33, 43–48. [Google Scholar] [CrossRef]
- Oakes, A.D.; Powell, W.A.; Maynard, C.A. Doubling acclimatization survival of micropropagated American chestnuts with darkness and shortened rooting induction time. J. Environ. Hort. 2013, 31, 77–83. [Google Scholar]
- Oakes, A.D.; Desmarais, T.; Powell, W.A.; Maynard, C.A. Improving rooting and shoot tip survival of micropropagated transgenic American chestnut shoots. Hort. Sci. 2016, 51, 171–176. [Google Scholar] [CrossRef]
- Tetsumura, T.; Yamashita, K. Micropropagation of Japanese chestnut (Castanea crenata Sieb. et Zucc.) seedlings. Hort. Sci. 2004, 39, 1684–1687. [Google Scholar] [CrossRef]
- Xiong, H.; Sun, H.; Zou, F.; Fan, X.; Niu, G.; Yuan, D. Micropropagation of Chinquapin (Castanea henryi) using axillary shoots and cotyledonary nodes. Hort. Sci. 2018, 53, 1482–1486. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Goulao, L.; Oliveira, C.; Gonçalves, J.C.; Amancio, S. RAPD assessment for identification of clonal identity and genetic stability of in vitro propagated chestnut hybrids. Plant Cell Tiss. Org. Cult. 2004, 77, 23–27. [Google Scholar] [CrossRef]
- Fernandes, P.; Tedesco, S.; Vieira da Silva, I.; Santos, C.; Machado, H.; Lourenço Costa, R. A new clonal propagation protocol develops quality root systems in Chestnut. Forests 2020, 11, 826. [Google Scholar] [CrossRef]
- Scaltsoyiannes, A.; Tsoulpha, P.; Panetsos, K.P.; Moulalis, D. Effect of genotype on micropropagation of walnut trees (Juglans regia). Silvae Genet. 1997, 46, 326–332. [Google Scholar]
- Vieitez, A.M.; Sánchez, M.C.; Amo-Marco, J.B.; Ballester, A. Forced flushing of branch segments as a method for obtaining reactive explants of mature Quercus robur trees for micropropagation. Plant Cell Tiss. Org. Cult. 1994, 37, 287–295. [Google Scholar]
- Sánchez, M.C.; San-José, M.C.; Ballester, A.; Vieitez, A.M. Requirements for in vitro rooting of Quercus robur and Q. rubra shoots derived from mature trees. Tree Physiol. 1996, 16, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.J.; Pijut, P.M.; Ostry, M.E.; Houston, D.R. Micropropagation of juvenile and mature American beech. Plant Cell Tiss. Org. Cult. 1997, 51, 209–213. [Google Scholar] [CrossRef]
- Martínez, M.T.; Vieitez, F.J.; Solla, A.; Tapias, R.; Ramírez-Martín, N.; Corredoira, E. Vegetative propagation of Phytophthora cinnamomi-tolerant holm oak genotypes by axillary budding and somatic embryogenesis. Forests 2020, 11, 841. [Google Scholar] [CrossRef]
- Pijut, P.M.; Woeste, K.E.; Michler, C.H. Promotion of adventitious root formation of difficult-to-root hardwood tree species. Hortic. Rev. 2011, 38, 213–251. [Google Scholar]
- Ilczuk, A.; Jacygrad, E. The effect of IBA on anatomical changes and antioxidant enzyme activity during the in vitro rooting of smoke tree (Cotinus coggygria Scop.). Sci. Hort. 2016, 210, 268–276. [Google Scholar] [CrossRef]
- Ljung, K. Auxin metabolism and homeostasis during plant development. Development 2013, 140, 943–950. [Google Scholar] [CrossRef]
- Strader, L.C.; Bartel, B. Transport and metabolism of the endogenous auxin precursor indole-3-butyric acid. Mol. Plant 2011, 4, 477–486. [Google Scholar] [CrossRef]
- Roussos, P.A.; Archimandriti, A.; Beldekou, I. Improving in vitro multiplication of juvenile European chestnut (Castanea sativa Mill) explants by the use of growth retardants. Sci. Hort. 2016, 198, 254–256. [Google Scholar] [CrossRef]
- Henrique, A.; Nogueira-Campinhos, E.; Orika-Ono, E.; Zambello de Pinho, S. Effect of plant growth regulators in the rooting of Pinus cuttings. Bra. Arch. Biol. Techn. 2006, 49, 189–196. [Google Scholar] [CrossRef]
- Copes, D.L.; Mandel, N.L. Effects of IBA and NAA treatments on rooting Douglas-fir stem cuttings. New For. 2000, 20, 249–257. [Google Scholar] [CrossRef]
- Pop, T.I.; Pamfil, D.; Bellini, C. Auxin control in the formation of adventitious roots. Not. Bot. Hortic. Agrobot. Cluj. 2011, 39, 307–316. [Google Scholar] [CrossRef]
- Ricci, A.; Bertoletti, C. Urea derivatives on the move: Cytokinin-like activity and adventitious rooting enhancement depend on chemical structure. Plant Biol. 2008, 11, 262–272. [Google Scholar] [CrossRef]
- Ricci, A.; Rolli, E. Some urea derivatives positively affect adventitious root formation: Old concepts and the state of the art. Plants 2020, 9, 321. [Google Scholar]
- Teale, W.; Palme, K. Naphthylphthalamic acid and the mechanism of polar auxin transport. J. Exp. Bot. 2018, 69, 303–312. [Google Scholar] [CrossRef]
- Zimmerman, R.H.; Hackett, W.; Pharis, R.P. Hormonal Aspects of Phase change and Precocious Flowering. In Hormonal Regulation of Development III. Role of Environmental Factors; Encyclopedia of Plant Physiology 11; Pharis, R.P., Reid, D.M., Eds.; Springer: Berlin, Germany, 1985; pp. 79–115. [Google Scholar]
- Fasoli, M.; Dal Santo, S.; Zenoni, S.; Tornielli, G.B.; Farina, L.; Zamboni, A.; Porceddu, A.; Venturini, L.; Bicego, M.; Murino, M.; et al. The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 2012, 24, 3489–3505. [Google Scholar] [CrossRef]
- Heide, O.M. Juvenility, maturation and rejuvenation in plants: Adventitious bud formation as a novel rejuvenation process. J. Hortic. Sci. Biotech. 2019, 94, 2–11. [Google Scholar] [CrossRef]
- Bonga, J.M. Vegetative Propagation in Relation to Juvenility, Maturity and Rejuvenation. In Tissue Culture in Forestry; Bonga, J.M., Durzan, D.J., Eds.; Martinus Nijhofl: Boston, MA, USA, 1982; pp. 78–90. [Google Scholar]
- Hackett, W.P. Juvenility, maturation and rejuvenation in woody plants. Hortic. Rev. 1985, 7, 109–155. [Google Scholar]
- Liu, H.; Gao, Y.; Song, X.B.; Ma, Q.G.; Zhang, J.P.; Pei, D. A novel rejuvenation approach to induce endohormones and improve rhizogenesis in mature Juglans tree. Plant Methods 2018, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lorenzo, J.L.; Fernández-López, M.J. Reinvigoration of mature Castanea sativa by serial micrografting onto a juvenile clone. Acta Hortic. 2005, 693, 293–298. [Google Scholar] [CrossRef]
- Fernández-Lorenzo, J.L.; Crecente-Campo, S. In vivo serial micrografting of Castanea sativa in short cycles. Acta Hortic. 2010, 866, 291–296. [Google Scholar] [CrossRef]
- Crecente-Campo, S.; Fernández-Lorenzo, J.L.; Mosquera-Losada, M.R.; Rigueiro-Rodríguez, A.; Ferreiro-Domínguez, N.; González, P.; Santiago-Freijanes, J.; Romero, R. Improvement of Micropropagation by Micrografting In Vitro Cultured Mature Scions of Castanea sativa Mill. on Physiologically Juvenile Rootstocks of the Same Genotype. In Proceedings of the 5th International Conference of the IUFRO Unit 2.09.02, Coimbra, Portugal, 10–15 September 2018; Bonga, J.M., Park, Y.S., Trontin, J.F., Eds.; Clonal Trees in the Bioeconomy Age: Opportunities and Challenges. pp. 34–42. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gresshoff, P.M.; Doy, C.H. Development and differentiation of haploid Lycopersicon esculentum. Planta 1972, 107, 161–170. [Google Scholar] [CrossRef]
- Lê, C.L. Factors influencing in vitro rooting of chestnut. For. Snow Landsc. Res. 2001, 76, 468–470. [Google Scholar]
- Nissen, S.; Sutter, E. Stability of IAA and IBA in nutrient medium to several tissue culture procedures. Hort. Sci. 1990, 25, 800–802. [Google Scholar] [CrossRef]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef]
- Yang, H.Y.; Klopotek, Y.; Hajirezaei, M.R.; Zerche, S.; Franken, P.; Druege, U. Role of auxin homeostasis and response in nitrogen limitation and dark stimulation of adventitious root formation in petunia cuttings. Ann. Bot. 2019, 124, 1053–1066. [Google Scholar] [CrossRef]
- Klopotek, Y.; Franken, P.; Klaering, H.P.; Fischer, K.; Hause, B.; Hajirezaei, M.R.; Druege, U. A higher sink competitiveness of the rooting zone and invertases are involved in dark stimulation of adventitious root formation in Petunia hybrida cuttings. Plant Sci. 2016, 243, 10–22. [Google Scholar] [CrossRef]
- Ballester, A.; Bourrain, L.; Corredoira, E.; Gonçalves, J.C.; Lê, C.L.; Miranda-Fontaíña, M.E.; San-José, M.C.; Sauer, U.; Vieitez, A.M.; Wilhelm, E. Improving chestnut micropropagation through axillary shoot development and somatic embryogenesis. For. Snow Landsc. Res. 2001, 76, 460–467. [Google Scholar]
- Atkinson, J.A.; Pound, M.P.; Bennett, M.J.; Wells, D.M. Uncovering the hidden half of plants using new advances in root phenotyping. Curr. Opin. Biotechnol. 2019, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Oakes, A.D.; Desmarais, T.R.; Powell, W.A.; Maynard, C.A. Ex vitro rooting of American chestnut improves acclimatization survival and plantlet quality. J. Environ. Hortic. 2016, 34, 75–79. [Google Scholar] [CrossRef]
- Badr, A.; Angers, P.; Desjardins, Y. Comprehensive analysis of in vitro to ex vitro transition of tissue cultured potato plantlets grown with or without sucrose using metabolic profiling technique. Plant Cell Tiss. Org. Cult. 2015, 122, 491–508. [Google Scholar] [CrossRef]
- Sáez, P.L.; Bravo, L.A.; Latsague, M.I.; Toneatti, M.J.; Coopman, R.E.; Álvarez, C.E.; Sánchez-Olate, M.; Ríos, D.G. Influence of in vitro growth conditions on the photosynthesis and survival of Castanea sativa plantlets during ex vitro transfer. Plant Growth Regul. 2015, 75, 625–639. [Google Scholar] [CrossRef]
- Pospisilova, J.; Synková, H.; Haisel, D.; Semoradova, S. Acclimation of plantlets to ex vitro conditions: Effects of air humidity, irradiance, CO2 concentration and abscisic acid (a Review). Acta Hortic. 2007, 748, 29–38. [Google Scholar] [CrossRef]
- De Klerk, G.J.; Massoumi, M. The roots of rooting. Proph. Ann. 2011, 30–33. [Google Scholar]
- Carvalho, L.; Amâncio, S. Effect of ex vitro conditions on growth and acquisition of autotrophic behaviour during the acclimatization of chestnut regenerated in vitro. Sci. Hort. 2002, 95, 151–164. [Google Scholar] [CrossRef]
- Mateja, S.; Dominik, V.; Franci, S.; Gregor, O. The effects of a fogging system on the physiological status and rooting capacity of leafy cuttings of woody species. Trees 2007, 21, 491–496. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, G.; Kozai, T. Development and application of photoautotrophic micropropagation plant system. Plant Cell Tiss. Org. Cult. 2011, 105, 149–158. [Google Scholar] [CrossRef]
- Vidal, N.; Blanco, B.; Cuenca, B. A temporary immersion system for micropropagation of axillary shoots of hybrid chestnut. Plant Cell Tiss. Org. Cult. 2015, 123, 229–243. [Google Scholar] [CrossRef]
- Cuenca, B.; Sánchez, C.; Aldrey, A.; Bogo, B.; Blanco, B.; Correa, B.; Vidal, N. Micropropagation of axillary shoots of hybrid chestnut (Castanea sativa × C. crenata) in liquid medium in a continuous immersion system. Plant Cell Tiss. Org. Cult. 2017, 131, 307–320. [Google Scholar] [CrossRef]
- Vidal, N.; Sánchez, C. Use of bioreactor systems in the propagation of forest trees. Eng. Life Sci. 2019, 19, 896–915. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Kubota, C. Developing a photoautotrophic micropropagation system for woody plants. J. Plant Res. 2001, 114, 525–537. [Google Scholar] [CrossRef]
- Maner, L.; Merkle, S. Polymerized peat plugs improve American chestnut somatic embryo germination in vitro. J. Am. Chestnut Found. 2010, 24, 16. [Google Scholar]
- Vidal, N.; Aldrey, A.; Blanco, B.; Correa, B.; Sánchez, C.; Cuenca, B. Proliferation and Rooting of Chestnut Under Photoautotrophic Conditions. In Proceedings of the 4th International Conference of the IUFRO Unit 2.09.02 on Development and Application of Vegetative Propagation Technologies in Plantation Forestry to Cope with a Changing Climate and Environment, La Plata, Argentina, 19–23 September 2016; published online. 2017; pp. 119–127. [Google Scholar]
- Beccaro, G.; Bonunous, G.; Cuenca, B.; Wardmund, M.; Xiong, H.; Zhang, L.; Zou, F.; Serdar, Ü.; Akyüz, B.; Mellano, M.G.; et al. Nursery Techniques. In The Chestnut Handbook Crop & Forest Management; Beccaro, G., Alma, A., Bounous, G., Gomes-Laranjo, J., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 115–150. [Google Scholar]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Boil. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, Y.; Zhang, Q.; Nie, X.; Sun, Y.; Zhang, Z.; Li, H.; Fang, K.; Wang, G.; Huang, H.; et al. Hybrid de novo genome assembly of Chinese chestnut (Castanea mollissima). GigaScience 2019, 8, 112. [Google Scholar] [CrossRef]
- Sun, Z.; Li, X.; Zhou, W.; Yan, J.; Gao, Y.; Li, X.; Sun, J.; Fang, K.; Zhang, Q.; Xing, Y.; et al. Agrobacterium-mediated genetic transformation of Chinese chestnut (Castanea mollissima Blume). Plant Cell Tiss. Org. Cult. 2020, 140, 95–103. [Google Scholar] [CrossRef]
- Gago, J.; Martínez-Núñez, L.; Landín, M.; Flexas, J.; Gallego, P.P. Modeling the effects of light and sucrose on in vitro propagated plants: A multiscale system analysis using artificial intelligence technology. PLoS ONE 2014, 9, e85989. [Google Scholar] [CrossRef]
- Nezami-Alanagh, E.; Garoosi, G.; Landín, M.; Gallego, P.P. Computer-based tools provide new insight into the key factors that cause physiological disorders of pistachio rootstocks cultured in vitro. Sci. Rep. 2019, 9, 9740. [Google Scholar] [CrossRef]

| Species | Donor Plant | Cutting Type | IBA (mM) | Time (seconds) | RS | RF (%) | Reference |
|---|---|---|---|---|---|---|---|
| C. sativa | 1-year-old | PS | 10 | 180–300 | Pl | 97.0 | [10] |
| C. dentata | Adult tress | Semihardwood SS | 49 | 5 | P/V | 65.0 | [89] |
| 1-yeard old rootstock | Softwood lateral branches | 49 | 5 | P/V | 59.0 | [89] | |
| C. sativa × C. crenata | 6-year-old tree | PS | 25 | 2 | P/Sa (3:1) | 22.7 | [64] |
| SB-derived plant | Minicutting | 12.3 | 10 | P/Pl (2:1) | 33.3 | [88] | |
| SB-derived plant | Minicutting | 49 | 10 | P/Pl (2:1) | 40.8 | [88] | |
| Ortet 7521 | SS | 10 | 180–300 | Pl | 50.0 | [10] | |
| Ortet 103 | SS | 10 | 180–300 | Pl | 87.0 | [10] |
| Species | Clone | Explant Source | Auxin | DK | RM | R (%) | Reference | |
|---|---|---|---|---|---|---|---|---|
| (mM) | Time | |||||||
| Castanea sativa (Cs) | P1 | C | 4.9 | 60 s | 0 | GD ⅓ | 8.6 | [90] |
| P1 | BS | 4.9 | 60 s | 0 | GD ⅓ | 86.1 | [90] | |
| P1 | BS | 0.05 | 24 h | 0 | GD ⅓ | 30.5 | [91] | |
| P1 | BS | 0.125 | 24 h | 0 | GD ⅓ | 94.4 | [91] | |
| P2 | C | 4.9 | 60 s | 0 | GD ⅓ | 11.4 | [90] | |
| P2 | BS | 4.9 | 60 s | 0 | GD ⅓ | 94.3 | [90] | |
| P2 | BS | 0.025 | 60 s | 0 | GD ⅓ | 81.4 | [91] | |
| P2 | BS | 25 | 5 d | 0 | GD ⅓ | 94.4 | [91] | |
| A2 | C | 4.9 | 120 s | 0 | GD ⅓ | 0.0 | [92] | |
| A2 | C+S | 4.9 | 120 s | 0 | GD ⅓ | 18.9 | [92] | |
| A2 | BS | 4.9 | 60 s | 0 | GD ⅓ | 43.2 | [90] | |
| A3 | C | 4.9 | 120 s | 0 | GD ⅓ | 8.9 | [92] | |
| A3 | C+S | 4.9 | 120 s | 0 | GD ⅓ | 22.8 | [92] | |
| L1 | BS | 0.125 | 24 h | 0 | GD ⅓ | 34.7 | [93] | |
| L1 | BS | 0.125 | 24 h | 0 | GD ⅓ + P/Pl/V | 91.7 | [93] | |
| L2 | BS | 0.125 | 24 h | 0 | GD ⅓ | 20.4 | [93] | |
| L2 | BS | 0.125 | 24 h | 0 | GD ⅓ + P/Pl/V | 54.7 | [93] | |
| L4 | C | 0.250 | 24 h | 0 | GD ⅓ + P/Pl/V | 13.6 | [93] | |
| Greek | BS | 2.7 * | 56 d | 0 | MS ½ + V | 56.3 | [94] | |
| Greek | BS | 5.4 * | 56 d | 0 | MS ½ + V | 50.0 | [94] | |
| Monte | SP | 0.015 | 5 d | 5 | WPM ½ | 90.0 | [95] | |
| Monte | C+G | 0.015 | 5 d | 5 | WPM ½ | 77.0 | [95] | |
| Pistol | CP | 0.015 | 5 d | 5 | WPM ½ | 75.0 | [39] | |
| Pistol | SP | 0.02 | 42 d | 0 | MS | 81.2 | [39] | |
| C. dentata (Cs) | B’ville | SS | 5 | 60 s | 0 | MS ½ + AC | 71.0 | [96] |
| Iow #2 | SP | 10 | 60 s | 0 | MS ½ + AC | 73.0 | [96] | |
| El#1W | SE | 10 | 120 s | 0 | MS ½ + AC | 67.0 | [97] | |
| El#1W | SE | 10 | 120 s | 8 | MS ½ + AC | 89.0 | [97] | |
| El#1 | SE | 10 | 30 s | 4 | WPM + HA | 33.3 | [98] | |
| El#1 | SE | 10 | 30 s | 4 | WPM + HA + AC | 83.0 | [98] | |
| C. crenata (Cc) | Tanza | SP | 0.015 | 5 d | 5 | ½BW + GG | 83.0 | [99] |
| C. mollisima (Cm) | Yansh | SP | 0.015 | 5 d | 5 | MS ½ (½NO3) | 73.3 | [55] |
| C. henryi (Ch) | Huali | Se | 0.025 | 28 d | 0 | MS + Pl | 23.3 | [100] |
| Huali | Se | 0.075 | 28 d | 0 | MS + Pl | 76.7 | [100] | |
| Huali | Se | 0.075 * | 28 d | 0 | MS + Pl | 0.0 | [100] | |
| Cs × Cc | HV | C | 4.9 | 60 s | 0 | GD ⅓ | 0.0 | [90] |
| HV | BS | 4.9 | 60 s | 0 | GD ⅓ | 19.4 | [90] | |
| HV-S3 | Se | 4.9 | 60 s | 0 | GD ⅓ | 33.3 | [90] | |
| HV-S1 | Se | 4.9 | 60 s | 0 | GD ⅓ | 100 | [90] | |
| 431 | C | 4.9 | 120 s | 0 | GD ⅓ | 16.9 | [92] | |
| 431 | C+S | 4.9 | 120 s | 0 | GD ⅓ | 30.4 | [92] | |
| 431 | C+S+R | 4.9 | 120 s | 0 | GD ⅓ | 51.0 | [90] | |
| 431 | BS | 4.9 | 60 s | 0 | GD ⅓ | 51.4 | [90] | |
| 431-S1 | Se | 4.9 | 30 s | 0 | GD ⅓ | 97.1 | [90] | |
| 110 | BS | 0.015 | 7 d | 5 | GD ⅓ | 83.0 | [93] | |
| 125 | BS | 0.015 | 7 d | 5 | GD ⅓ | 78.0 | [93] | |
| 90025 | BS | 0.015 | 7 d | 5 | GD ⅓ | 25.0 | [93] | |
| 90025 | BS | 0.25 | 24 h | 5 | GD ⅓ + AC | 26.0 | [93] | |
| Pr14 | BS | 0.125 | 24 h | 5 | GD ⅓ + P/Pl/V | 62.5 | [93] | |
| M3 | SS | 4.9 | 60 s | 0 | MS ½ + AC | 97.0 | [33] | |
| M3 | SS | 0.015 | 5 d | 0 | MS ½ + AC | 93.0 | [33] | |
| M3 | SS | 0.015 | 5 d | 0 | MS ½ | 93.0 | [101] | |
| Cs × Cm | SM904 | SS | 10 | 60 s | 0 | MMN | 90.0 | [102] |
| Species | Acclimation Period | Acclimation Conditions | Root Induction | Survival Rate (%) | Reference |
|---|---|---|---|---|---|
| C. crenata | 30 days | Vermiculite + BW ½ medium Continuous light | In Vitro | 85 | [100] |
| C. dentata | 8 weeks | Relative humidity (RH): 80% Fafard’s mix | In Vitro | 95 | [98] |
| 8 weeks | RH: 92%; Fafard’s mix | In Vitro/ ex vitro | 20/87 | [138] | |
| C. henryi | 90 days | RH: 80% → 65% Peat:perlite (2:1)+MN medium (30 days) Peat: perlite: loess (1:1:1) (60 days) | In Vitro | 80 | [101] |
| C. sativa × C. crenata | 4 weeks | RH: 95% → 50%; Peat:perlite (1:2) | In Vitro/ ex vitro | 50/100 | [33] |
| 42 days | RH: 98% → ambient; Peat:perlite (1:1) | In Vitro | 85 | [143] | |
| 8 weeks | RH: 90%; Rockwool cubes (4weeks) Peat:perlite (3:1)(4weeks) | In Vitro | 73 | [147] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vielba, J.M.; Vidal, N.; José, M.C.S.; Rico, S.; Sánchez, C. Recent Advances in Adventitious Root Formation in Chestnut. Plants 2020, 9, 1543. https://doi.org/10.3390/plants9111543
Vielba JM, Vidal N, José MCS, Rico S, Sánchez C. Recent Advances in Adventitious Root Formation in Chestnut. Plants. 2020; 9(11):1543. https://doi.org/10.3390/plants9111543
Chicago/Turabian StyleVielba, Jesús M., Nieves Vidal, M. Carmen San José, Saleta Rico, and Conchi Sánchez. 2020. "Recent Advances in Adventitious Root Formation in Chestnut" Plants 9, no. 11: 1543. https://doi.org/10.3390/plants9111543
APA StyleVielba, J. M., Vidal, N., José, M. C. S., Rico, S., & Sánchez, C. (2020). Recent Advances in Adventitious Root Formation in Chestnut. Plants, 9(11), 1543. https://doi.org/10.3390/plants9111543









