Transformation and Characterization of Δ12-Fatty Acid Acetylenase and Δ12-Oleate Desaturase Potentially Involved in the Polyacetylene Biosynthetic Pathway from Bidens pilosa
Abstract
1. Introduction
2. Results
2.1. Cloning and Sequence Analysis of Putative Δ12-Oleate Desaturase and Δ12-Fatty Acid Acetylenase Genes from Bidens pilosa var. radiata
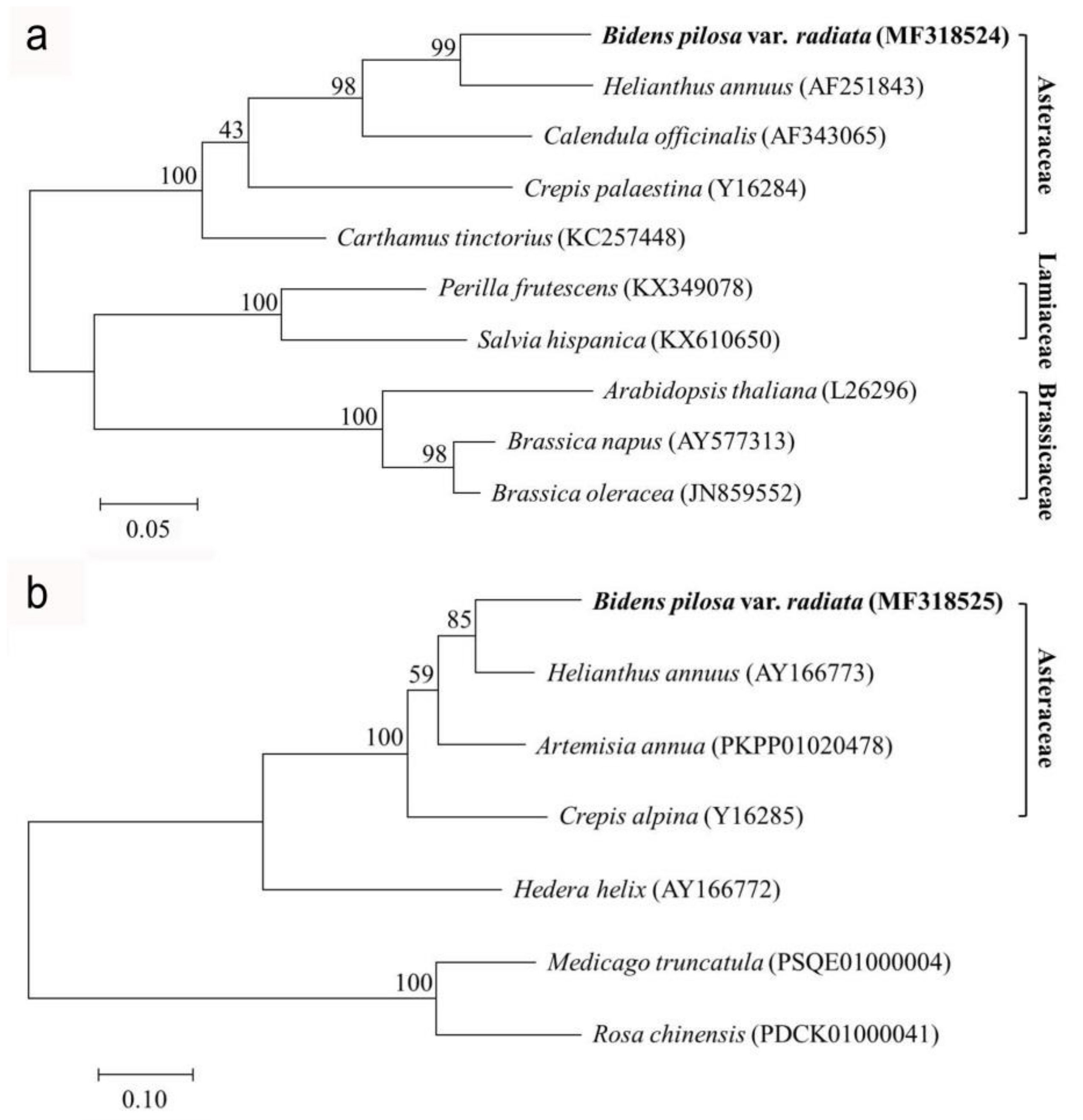
2.2. Phylogenetic Analysis of Δ12-Oleate Desaturase and Δ12-Fatty Acid Acetylenase Genes from Bidens pilosa var. radiata
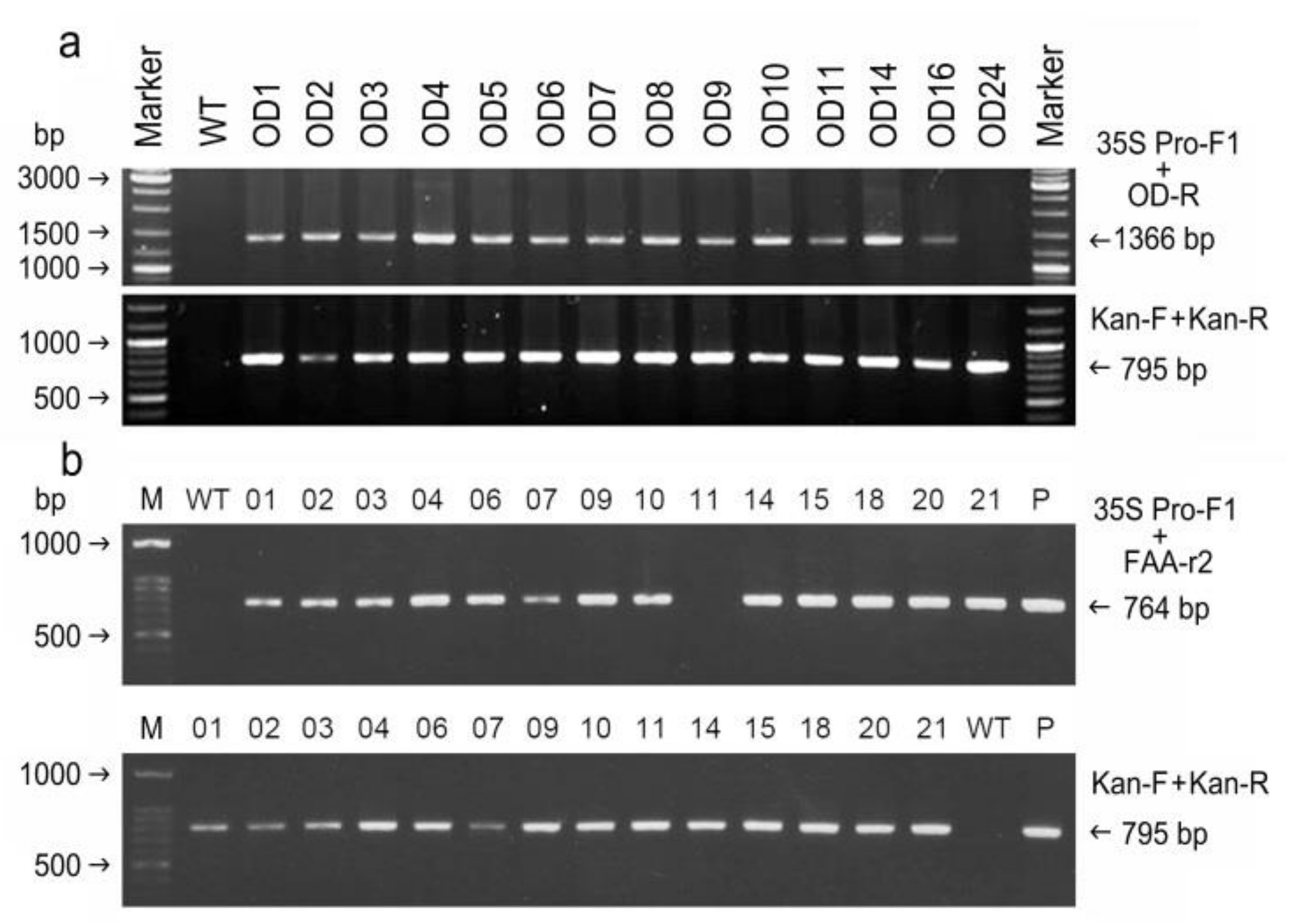
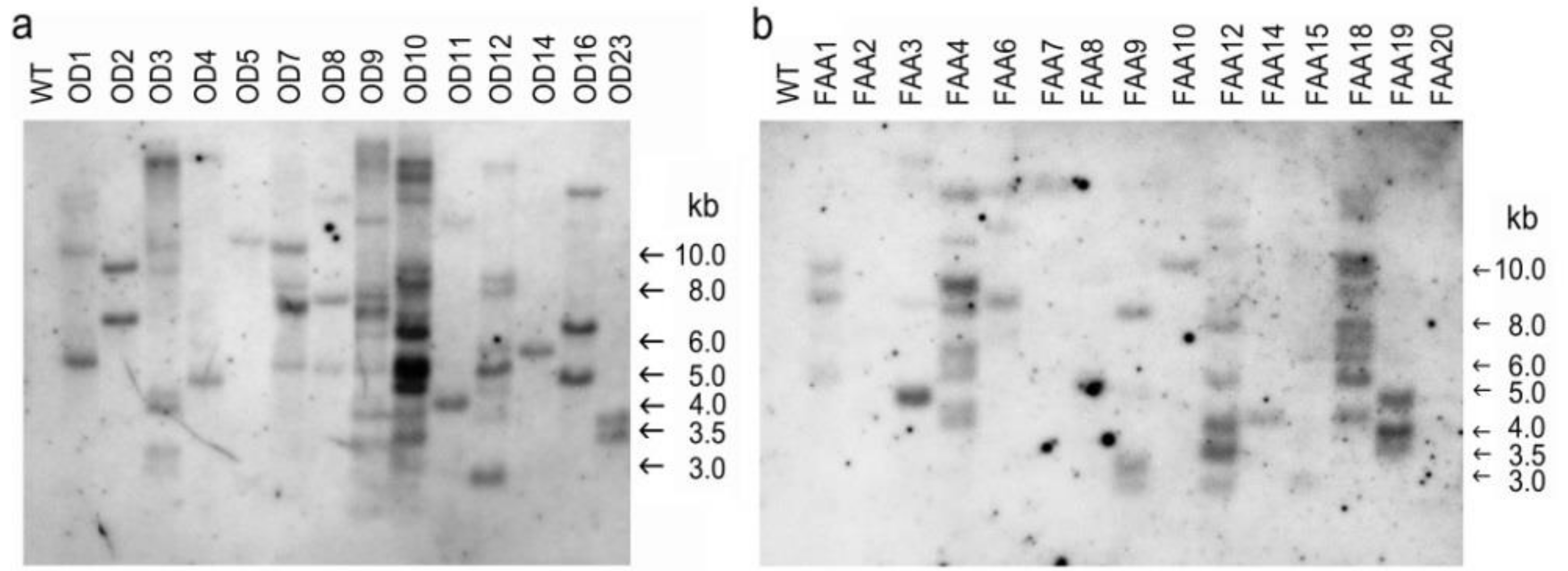
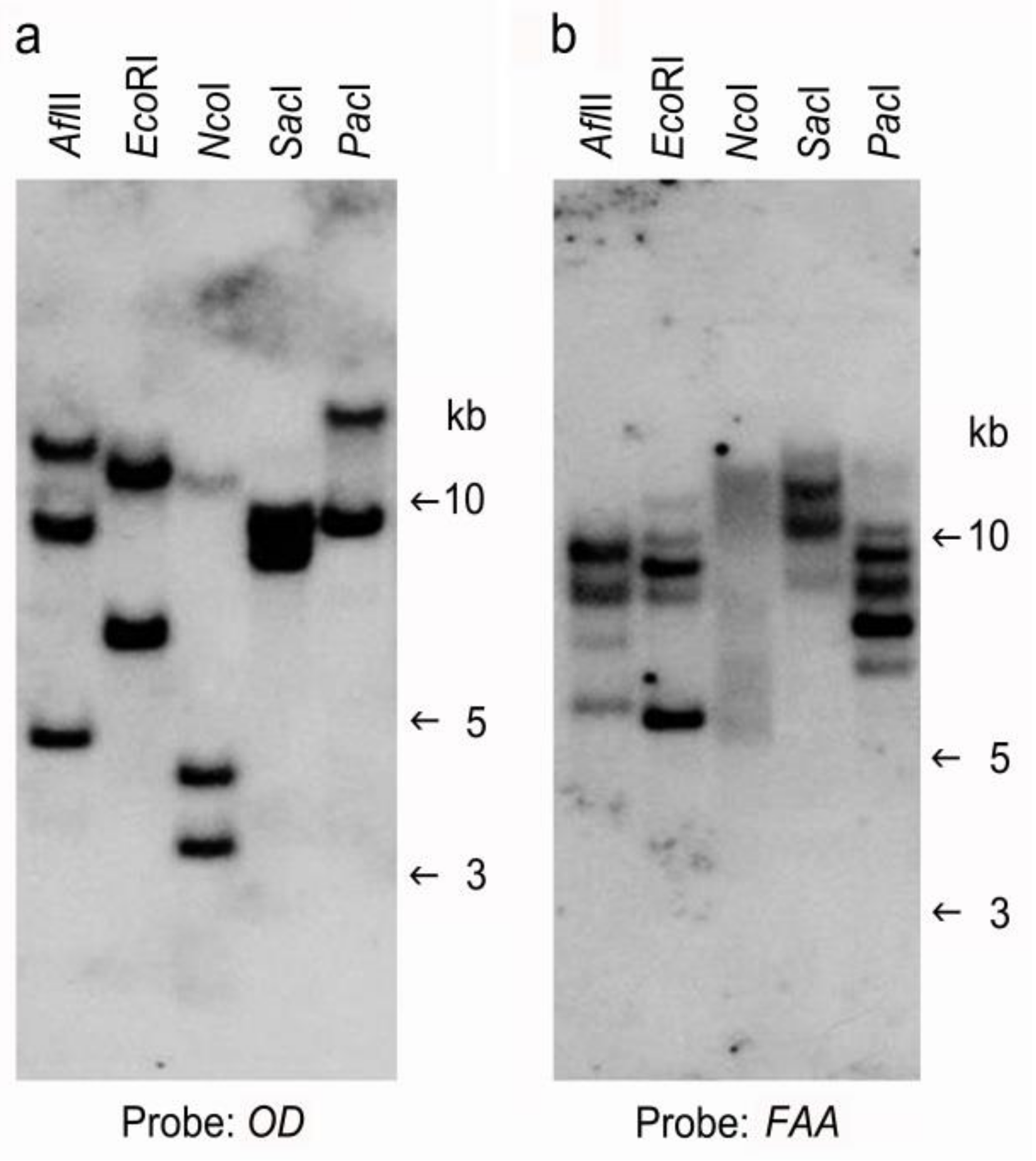
2.3. Confirmation of Putative Transformants
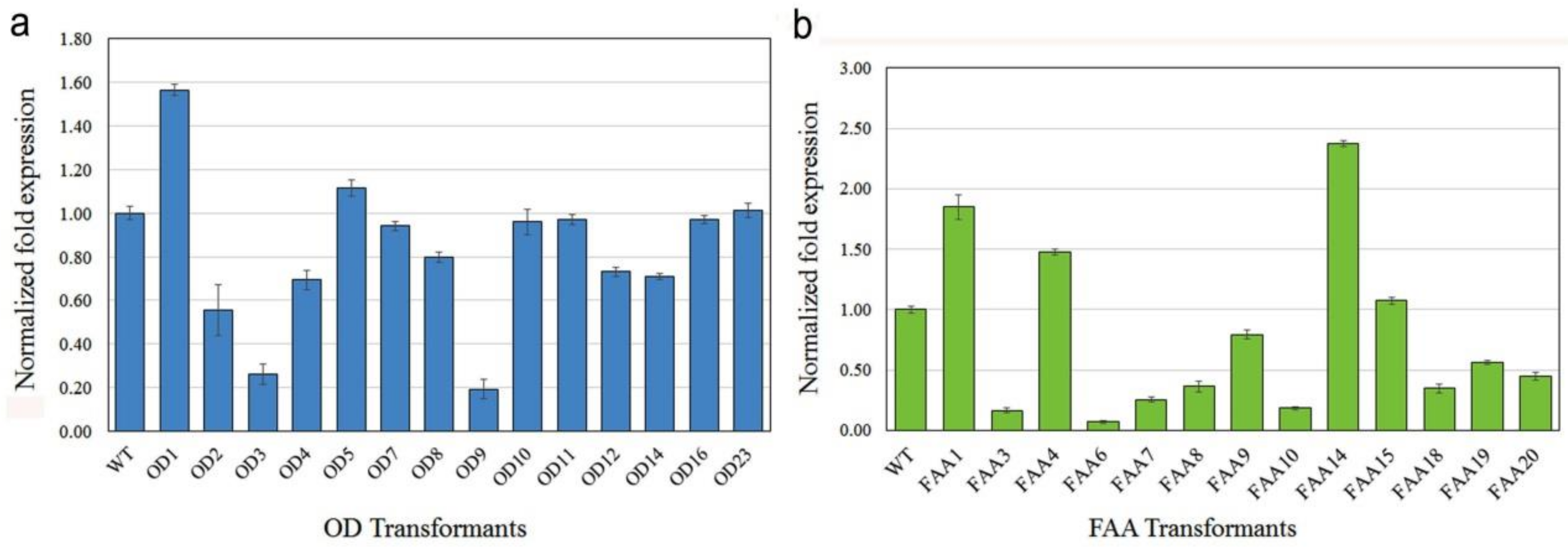
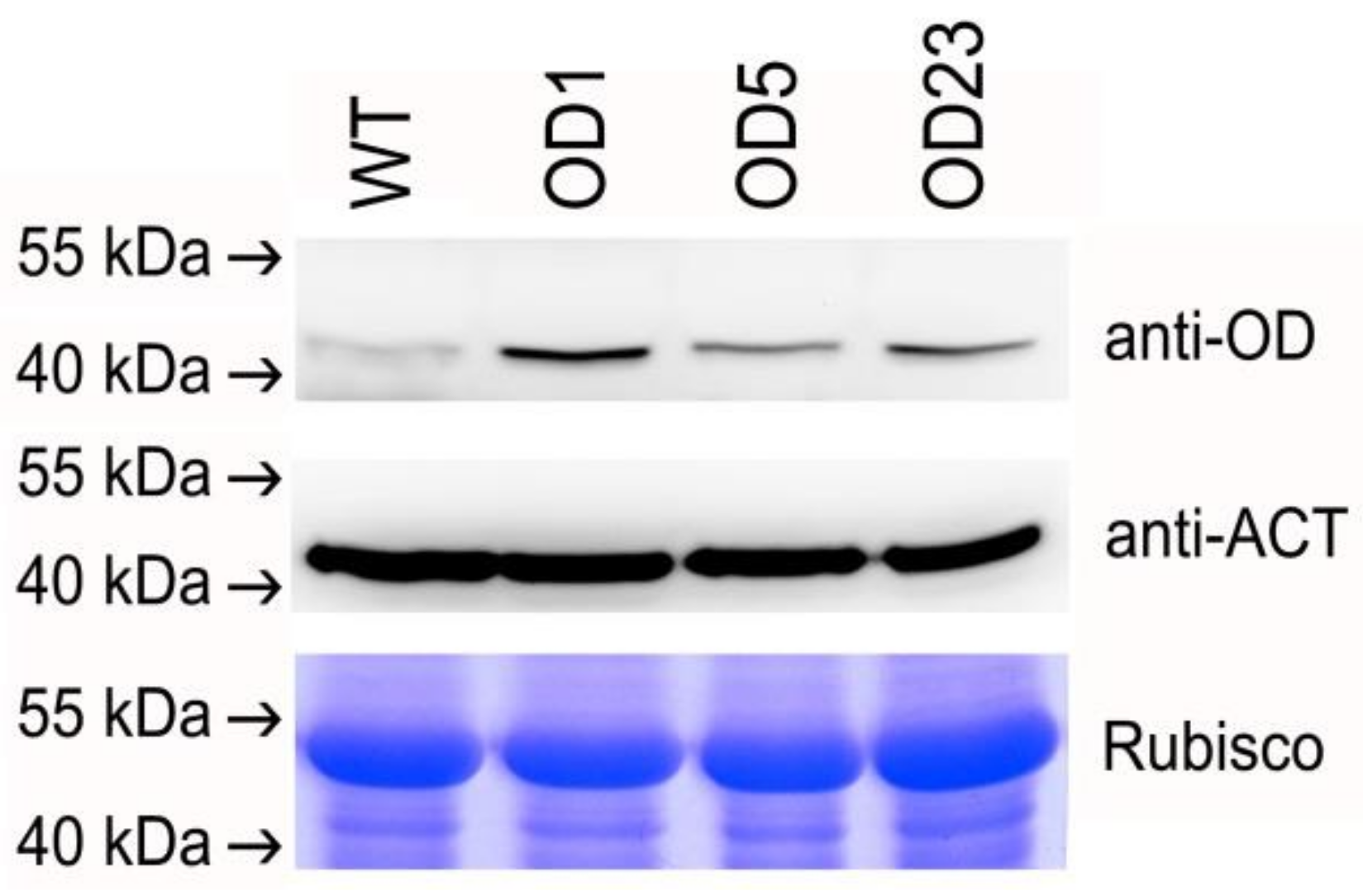
2.4. mRNA and Protein Expression in Transgenic Plants
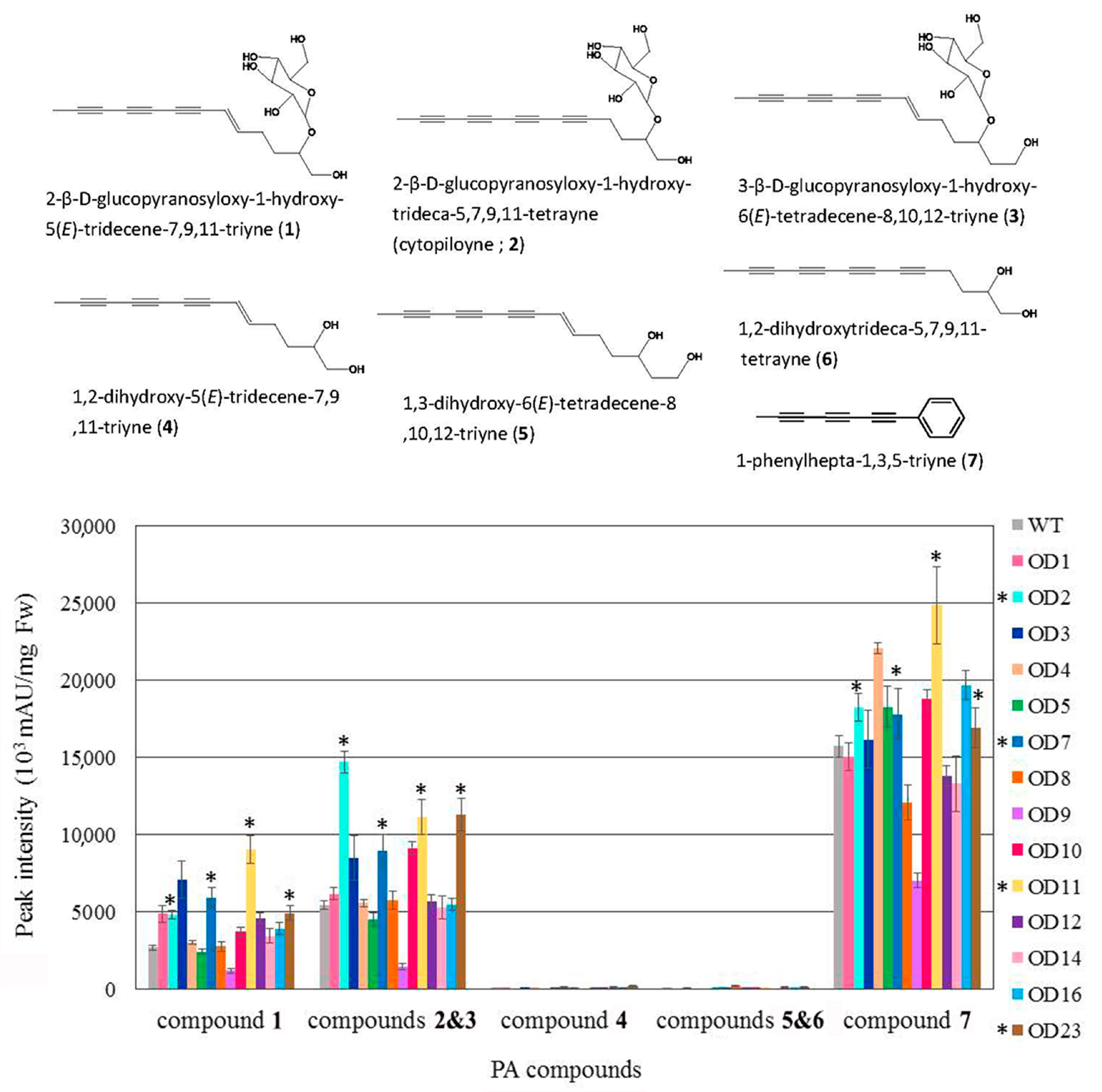
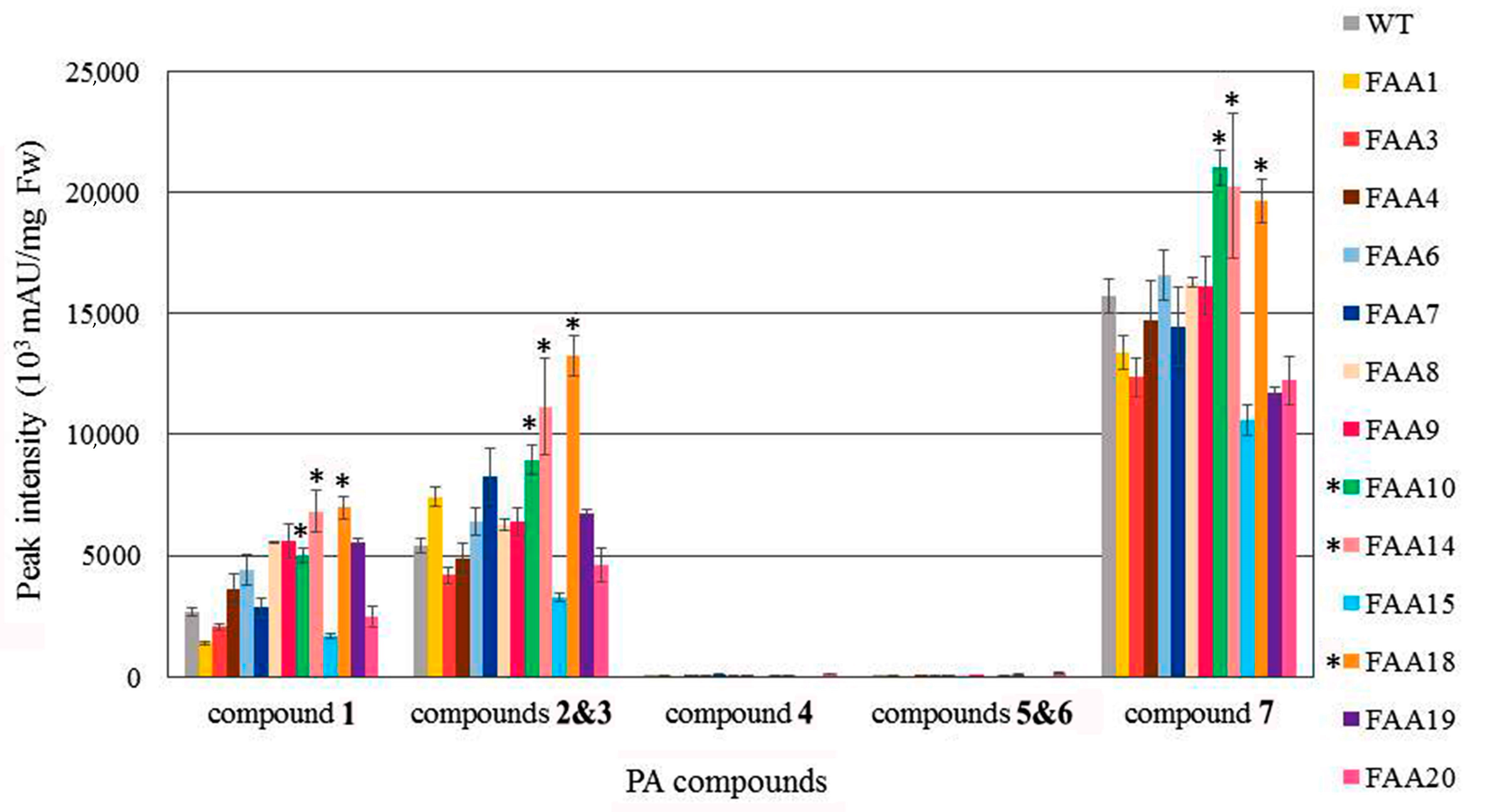
2.5. Polyacetylenic Compound Profiling in Transgenic Plants
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Cloning of Full-Length cDNAs
4.3. Phylogenetic Analysis
4.4. Construction of Expression Vectors and Agrobacterium-mediated Transformation
4.5. Transgenic Plant Verification by Genomic PCR Analysis
4.6. Southern Blot Analysis
4.7. Quantitative Real-Time PCR Analysis
4.8. Western Blot Analysis
4.9. High-Performance Liquid Chromatography Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BA | 6-Benzyladenine |
| BPFAA | Δ12-Fatty acid acetylenase from Bidens pilosa var. radiata |
| BPOD | Δ12-Oleate desaturase from Bidens pilosa var. radiata |
| CaMV | Cauliflower mosaic virus |
| FAD2 | Fatty acid desaturase 2 |
| HPLC | High-performance liquid chromatography |
| hptII | Hygromycin phosphotransferase II |
| IAA | Indole-3-acetic acid |
| nptII | Neomycin phosphotransferase II |
| qRT-PCR | Quantitative real time-PCR |
| WT | Wild-type |
References
- Li, T.S.C. Chinese and Related North American Herbs: Phytopharmacology and Therapeutic Values; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Botsaris, A.S. Plants used traditionally to treat malaria in Brazil: The archives of flora medicinal. J. Ethnobiol. Ethnomed. 2007, 3, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Young, P.H.; Hsu, Y.J.; Yang, W.C. Bidens pilosa and its medicinal use. In Drug Plants II; Awaad, A.S., Singh, V.K., Govil, J.N., Eds.; Studium Press LLC: New Delhi, India, 2010; Volume 28, pp. 443–459. [Google Scholar]
- Chien, S.C.; Young, P.H.; Hsu, Y.J.; Chen, C.H.; Tien, Y.J.; Shiu, S.Y.; Li, T.H.; Young, C.W.; Marimuthu, P.; Tsai, L.F.L.; et al. Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry 2009, 70, 1246–1254. [Google Scholar] [CrossRef]
- Silva, F.L.; Fischer, D.C.H.; Tavares, J.F.; Silva, M.S.; de Athayde-Filho, P.F.; Barbosa-Filho, J.M. Compilation of secondary metabolites from Bidens pilosa L. Molecules 2011, 16, 1070–1102. [Google Scholar] [CrossRef]
- Chang, S.L.; Chang, C.L.T.; Chiang, Y.M.; Hsieh, R.H.; Tzeng, C.R.; Wu, T.K.; Sytwu, H.K.; Shyur, L.F.; Yang, W.C. Polyacetylenic compounds and butanol fraction from Bidens pilosa can modulate the differentiation of helper T cells and prevent autoimmune diabetes in non-obese diabetic mice. Planta Med. 2004, 70, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.W.; Chiang, Y.M.; Chuang, H.C.; Wang, S.Y.; Yang, G.W.; Chen, Y.H.; Lai, L.Y.; Shyur, L.F. Polyacetylenes functions as anti-angiogenic agents. Pharm. Res. 2004, 21, 2112–2119. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.M.; Chang, C.L.T.; Chang, S.L.; Yang, W.C.; Shyur, L.F. Cytopiloyne, a novel polyacetylenic glucoside from Bidens pilosa, functions as a T helper cell modulator. J. Ethnopharmacol. 2007, 110, 532–538. [Google Scholar] [CrossRef]
- Wu, L.W.; Chiang, Y.M.; Chuang, H.C.; Lo, C.P.; Yang, K.Y.; Wang, S.Y.; Shyur, L.F. A novel polyacetylene significantly inhibits angiogenesis and promotes apoptosis in human endothelial cells through activation of the CDK inhibitors and caspase-7. Planta Med. 2007, 73, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Negri, R. Polyacetyenes from terrestrial plants and fungi: Recent phytochemical and biological advances. Fitoterapia 2015, 106, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Yang, Y.; Busta, L.; Cahoon, E.B.; Wang, H.; Lü, S. FAD2 gene radiation and selection contributed to polyacetylene metabolism evolution in campanulids. Plant Physiol. 2019, 181, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef] [PubMed]
- Okuley, J.; Lightner, J.; Feldmann, K.; Yadav, N.; Lark, E.; Browse, J. Aradidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 1994, 6, 147–158. [Google Scholar] [PubMed]
- Cao, S.; Zhou, X.R.; Wood, C.C.; Green, A.G.; Singh, S.P.; Liu, L.; Liu, Q. A large and functionally diverse family of Fad2 in safflower (Carthamus tinctorius L.). BMC Plant Biol. 2013, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Zhang, L.; Hu, X.; Nan, S.; Chen, X.; Fu, H. Cloning and functional analysis of the FAD2 gene family from desert shrub Artemisia sphaerocephala. BMC Plant Biol. 2019, 19, 481. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.L.; Mancha, M.; Martínez-Rivas, J.M. Molecular cloning and characterization of genes encoding two microsomaloleate desaturases (FAD2) from olive. Phytochemistry 2005, 66, 1417–1426. [Google Scholar] [CrossRef]
- Lee, M.W.; Padilla, C.S.; Gupta, C.; Galla, A.; Pereira, A.; Li, J.; Goggin, F.L. The FATTY ACID DESATURASE 2 family in tomato contributes to primary metabolism and stresses. Plant Physiol. 2020, 182, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Heppard, E.P.; Kinney, A.J.; Stecca, K.L.; Miao, G.H. Developmental and growth temperature regulation of two different microsomal ω-6 desaturase genes in soybeans. Plant Physiol. 1996, 110, 311–319. [Google Scholar] [CrossRef]
- Martínez-Rivas, J.M.; Sperling, P.; Lühs, W.; Heinz, E. Spatial and temporal regulation of three different microsomal oleate desaturase genes (FAD2) from normal-type and high-oleic varieties of sunflower (Helianthus annuus L.). Mol. Breed. 2001, 8, 159–168. [Google Scholar]
- Zhang, Z.; Wei, X.; Liu, W.; Min, X.; Jin, X.; Ndayambaza, B.; Wang, Y. Genome-wide identification and expression analysis of the fatty acid desaturase genes in Medicago truncatula. Biochem. Biophys. Res. Comm. 2018, 499, 361–367. [Google Scholar] [CrossRef]
- Pirtle, I.L.; Kongcharoensuntorn, W.; Nampaisansuk, M.; Knesek, J.E.; Chapman, K.D.; Pirtle, R.M. Molecular cloning and functional expression of the gene for a cotton Δ-12 fatty acid desaturase (FAD2). Biochim. Biophys. Acta 2001, 1522, 122–129. [Google Scholar] [CrossRef]
- Zhang, D.; Pirtle, I.L.; Park, S.J.; Nampaisansuk, M.; Neogi, P.; Wanjie, S.W.; Pirtle, R.M.; Chapman, K.D. Identification and expression of a new delta-12 fatty acid desaturase (FAD2-4) gene in upland cotton and its functional expression in yeast and Arabidopsis thaliana plants. Plant Physiol. Biochem. 2009, 47, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.R.; Sohn, S.I.; Jin, H.J.; Sun, H.K.; Roh, K.H.; Kim, J.B.; Mi, C.S.; Kim, H.U. Functional analysis and tissue-differential expression of four FAD2 genes in amphidiploid Brassica napus derived from Brassica rapa and Brassica oleracea. Gene 2013, 531, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Zhao, Y.; Prakash, C.S.; He, G.; Yin, D. Insights into the novel members of the FAD2 gene family involved in high-oleate fluxes in peanut. Genome 2015, 58, 375–383. [Google Scholar] [CrossRef]
- Lee, M.; Lenman, M.; Banaś, A.; Bafor, M.; Singh, S.; Schweizer, M.; Nilsson, R.; Liljenberg, C.; Dahlqvist, A.; Gummeson, P.O.; et al. Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation. Science 1998, 280, 915–918. [Google Scholar] [CrossRef]
- Leonard, A.E.; Pereira, S.L.; Sprecher, H.; Huang, Y.S. Elongation of long-chain fatty acids. Prog. Lipid Res. 2004, 43, 36–54. [Google Scholar] [CrossRef]
- Shanklin, J.; Whittle, E.; Fox, B.G. Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry 1994, 33, 12787–12794. [Google Scholar] [CrossRef] [PubMed]
- Cahoon, E.B.; Schnurr, J.A.; Huffman, E.A.; Minto, R.E. Fungal responsive fatty acid acetylenases occur widely in evolutionarily distant plant families. Plant J. 2003, 34, 671–683. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar]
- Wang, C.K.; Hsu, S.Y.; Chen, P.Y.; To, K.Y. Transformation and characterization of transgenic Bidens pilosa L. Plant Cell Tiss. Organ Cult. 2012, 109, 457–464. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Chen, P.Y.; To, K.Y. Plant regeneration and stable transformation in the floricultural plant Cleome spinosa, a C3 plant closely related to the C4 plant C. gynandra. Plant Cell Rep. 2012, 31, 1189–1198. [Google Scholar] [CrossRef]
- Wang, H.M.; Jeng, S.T.; To, K.Y. In vitro regeneration, Agrobacterium-mediated transformation, and genetic assay of chalcone synthase in the medicinal plant Echinacea pallida. Plant Cell Tiss. Organ Cult. 2017, 130, 117–130. [Google Scholar] [CrossRef]
- Wu, H.; Spark, C.A.; Jones, H.D. Characterisation of T-DNA loci and vector backbone sequences in transgenic wheat produced by Agrobacterium-mediated transformation. Mol. Breed. 2006, 18, 195–208. [Google Scholar] [CrossRef]
- Kim, S.R.; Lee, J.; Jun, S.H.; Park, S.; Kang, H.G.; Kwon, S.; An, G. Transgene structures in T-DNA-inserted rice plants. Plant Mol. Biol. 2003, 52, 761–773. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, B.; Wang, R.; Win, A.N.; Li, J.; Chai, Y. Genome-wide survey and characterization of fatty acid desaturase gene family in Brassica napus and its parental species. Appl. Biochem. Biotechnol. 2018, 184, 582–598. [Google Scholar] [CrossRef]
- Okuzaki, A.; Ogawa, T.; Koizuka, C.; Kaneko, K.; Inaba, M.; Imamura, J.; Koizuka, N. CRISPR/Cas9-mediated genome editing of the fatty acid desaturase 2 gene in Brassica napus. Plant Physiol. Biochem. 2018, 131, 63–69. [Google Scholar] [CrossRef]
- Yuan, M.; Zhu, J.; Gong, L.; He, L.; Lee, C.; Han, S.; Chen, C.; He, G. Mutagenesis of FAD2 genes in peanut with CRISPR/Cas9 based gene editing. BMC Biotechnol. 2019, 19, 24. [Google Scholar] [CrossRef]
- Abe, K.; Araki, E.; Suzuki, Y.; Toki, S.; Saika, H. Production of high oleic/low linoleic rice by genome editing. Plant Physiol. Biochem. 2018, 131, 58–62. [Google Scholar] [CrossRef]
- Huang, H.; Cui, T.; Zhang, L.; Yang, Y.; Xie, K.; Fen, C.; Zhou, Y. Modifications of fatty acid profile through targeted mutation at BnaFAD2 gene with CRISPR/Cas9-mediated gene editing in Brassica napus. Theoret. Appl. Genet. 2020, 133, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Lu, Q.; Wang, P.; Zhang, Q.; Zhang, J.; Qu, J.; Wang, N. Construction and analysis of GmFAD2-1A and GmFAD2-2A soybean fatty acid desaturase mutants based on CRISPR/Cas9 technology. Int. J. Mol. Sci. 2020, 21, 1104. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, K.; Li, X.; Zheng, Y.; Chen, F. Design of high-oleic tobacco (Nicotiana tabacum L.) seed oil by CRISPR-Cas9-mediated knockout of NtFAD2-2. BMC Plant Biol. 2020, 20, 233. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for the rapid growth and bioassays with tobacco culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, S. Isolation of total genomic DNA. In Plant Molecular Biology-A Laboratory Manual; Clark, M.S., Ed.; Springer: Berlin, Germany, 1997; pp. 3–15. [Google Scholar]
- Chen, P.Y.; Wang, C.K.; Soong, S.C.; To, K.Y. Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insertion from transgenic plants. Mol. Breed. 2003, 11, 287–293. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Chiang, Y.M.; Chuang, D.Y.; Wang, S.Y.; Kuo, Y.H.; Tsai, P.W.; Shyur, L.F. Metabolic profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol. 2004, 95, 409–419. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-Y.; Hsieh, M.-J.; Tsai, Y.-T.; Chung, H.-H.; Shyur, L.-F.; Hsieh, C.-H.; To, K.-Y. Transformation and Characterization of Δ12-Fatty Acid Acetylenase and Δ12-Oleate Desaturase Potentially Involved in the Polyacetylene Biosynthetic Pathway from Bidens pilosa. Plants 2020, 9, 1483. https://doi.org/10.3390/plants9111483
Chen P-Y, Hsieh M-J, Tsai Y-T, Chung H-H, Shyur L-F, Hsieh C-H, To K-Y. Transformation and Characterization of Δ12-Fatty Acid Acetylenase and Δ12-Oleate Desaturase Potentially Involved in the Polyacetylene Biosynthetic Pathway from Bidens pilosa. Plants. 2020; 9(11):1483. https://doi.org/10.3390/plants9111483
Chicago/Turabian StyleChen, Po-Yen, Mi-Jou Hsieh, Yung-Ting Tsai, Hsiao-Hang Chung, Lie-Fen Shyur, Cheng-Han Hsieh, and Kin-Ying To. 2020. "Transformation and Characterization of Δ12-Fatty Acid Acetylenase and Δ12-Oleate Desaturase Potentially Involved in the Polyacetylene Biosynthetic Pathway from Bidens pilosa" Plants 9, no. 11: 1483. https://doi.org/10.3390/plants9111483
APA StyleChen, P.-Y., Hsieh, M.-J., Tsai, Y.-T., Chung, H.-H., Shyur, L.-F., Hsieh, C.-H., & To, K.-Y. (2020). Transformation and Characterization of Δ12-Fatty Acid Acetylenase and Δ12-Oleate Desaturase Potentially Involved in the Polyacetylene Biosynthetic Pathway from Bidens pilosa. Plants, 9(11), 1483. https://doi.org/10.3390/plants9111483





