Optimization of a Green Ultrasound-Assisted Extraction of Different Polyphenols from Pistacia lentiscus L. Leaves Using a Response Surface Methodology
Abstract
1. Introduction
- Evaluate the effect of different variables (solvent ratio, temperature, extraction time, and ethanol volume) on the UAE of P. lentiscus leaves using a first-step screening design;
- Optimize the extraction process, using a Box–Behnken design, in order to obtain extracts with higher amounts of different classes of polyphenols (quantified by high performance liquid chromatography coupled to diode array detection, HPLC-DAD) and applying a greener method than those conventionally used for the extraction of leaves of the species;
- Characterize the major compounds present in the extract with the highest content in polyphenols using liquid chromatography-mass spectrometry (LC-MS/MS).
2. Results and Discussions
2.1. Screening Design and Determination of the Important Factors
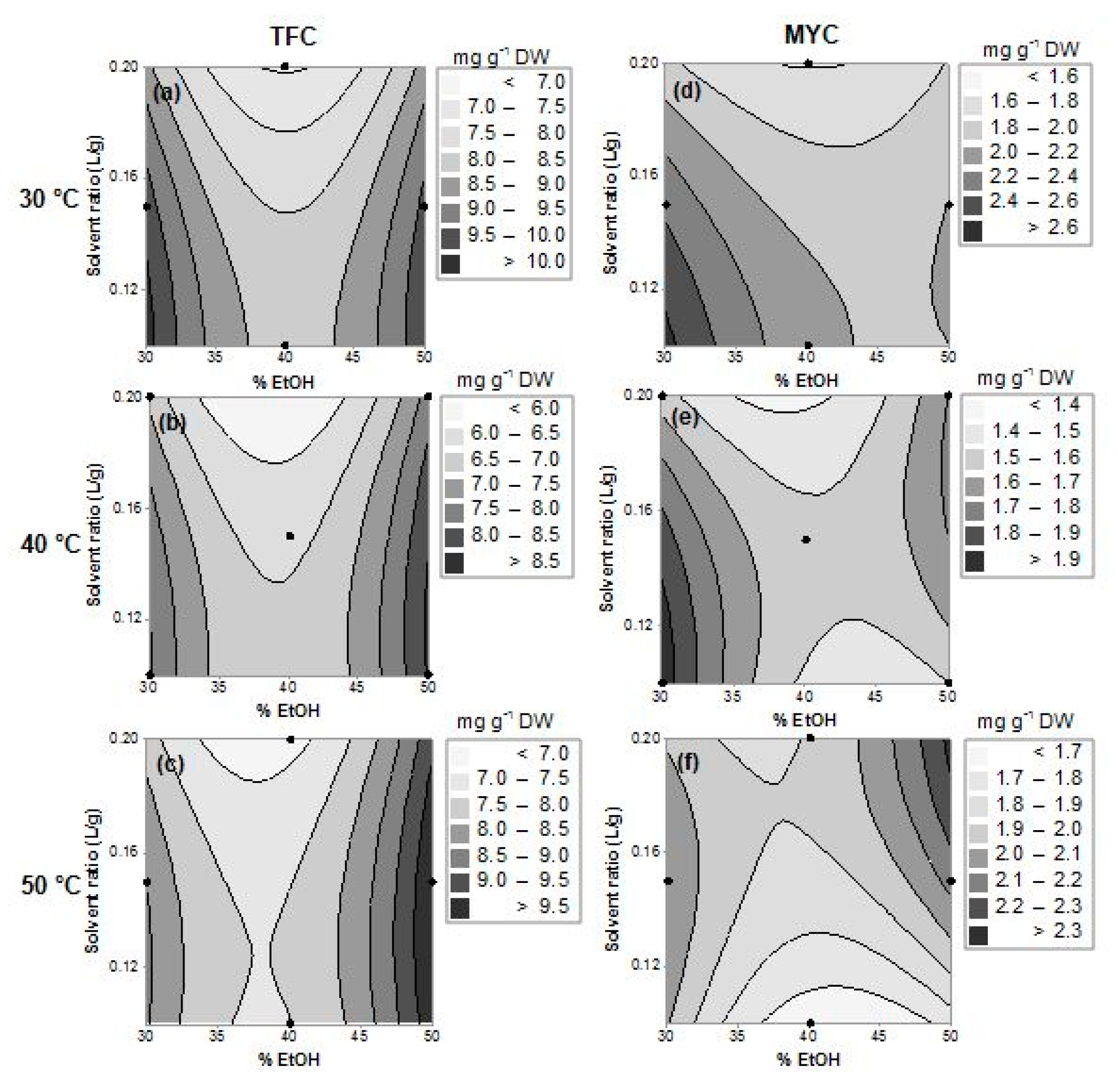
2.2. Optimization Design: Models and Response Surfaces Analysis
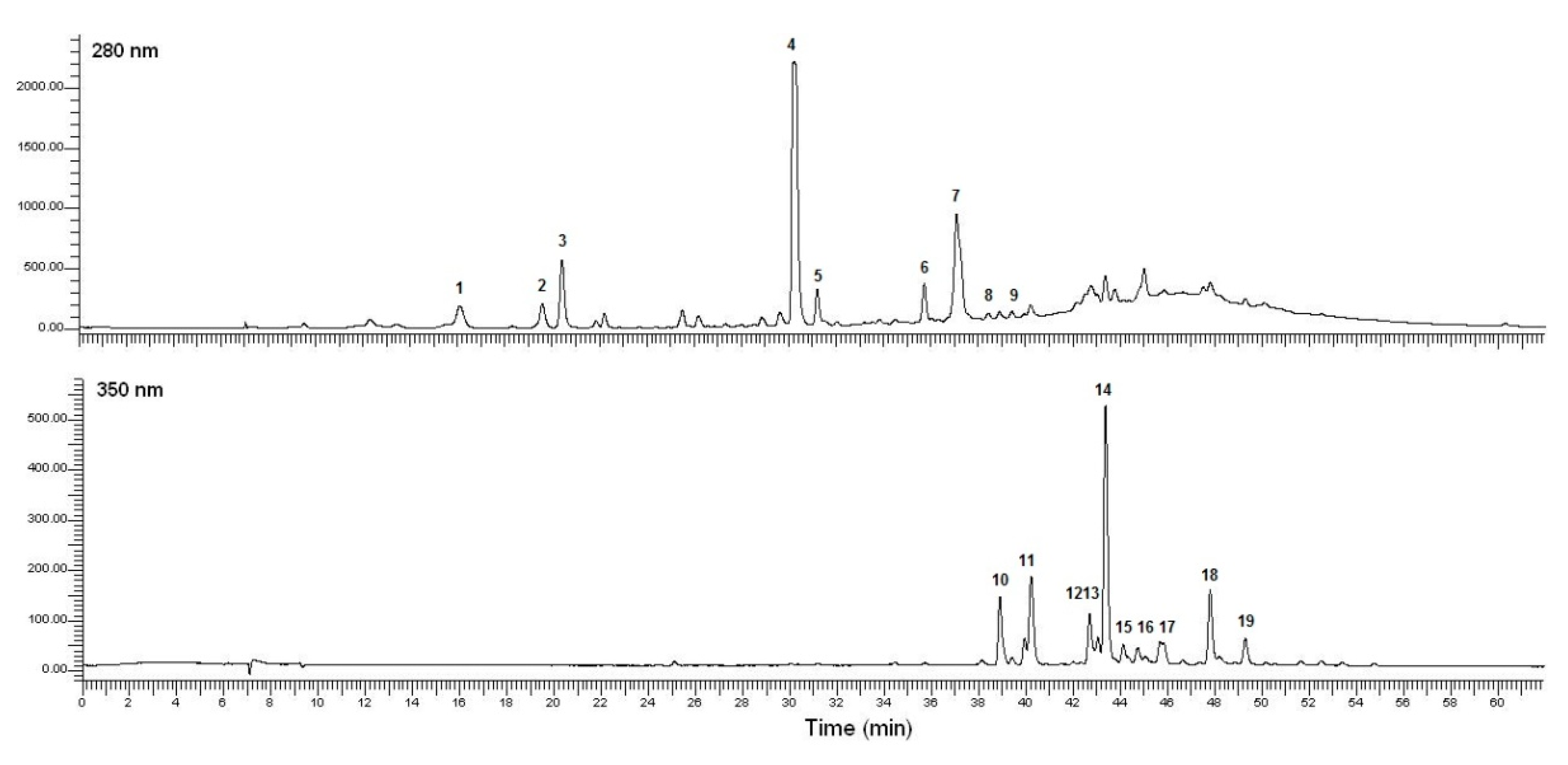
2.3. Polyphenolic Composition of the Richest P. lentiscus Extract
3. Materials and Methods
3.1. Plant Material
3.2. Ultrasound-Assisted Extraction (UAE) Procedure
3.3. HPLC-DAD Quantification and LC-MS/MS Characterization of the Extracts
3.4. Experimental Designs: Optimization Procedure and Data Analysis
3.4.1. Screening Fractional Factorial Design: Selection of the Important Variables for the Extraction Optimization
3.4.2. Box–Behnken Design for Optimization of the Extraction Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zrira, S.; Elamrani, A.; Benjilali, B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco-a seasonal variation. Flavour. Fragr. J. 2003, 18, 475–480. [Google Scholar] [CrossRef]
- Bampouli, A.; Kyriakopoulou, K.; Papaefstathiou, G.; Louli, V.; Aligiannis, N.; Magoulas, K.; Krokida, M. Evaluation of total antioxidant potential of Pistacia lentiscus var. chia leaves extracts using UHPLC–HRMS. J. Food. Eng. 2015, 167, 25–31. [Google Scholar]
- Margaris, N.S. Adaptive strategies in plants dominating Mediterranean-type ecosystems. In Ecosystems of the World; FAO: Roma, Italy, 1981. [Google Scholar]
- Marzano, M.C.; Gori, A.; Baratto, M.C.; Lamponi, S.; Tattini, M.; Brunetti, C.; Ferrini, F. Antioxidant capacity and cytotoxicity of different polyphenolic extracts of Pistacia lentiscus. Planta Med. 2016, 82, P364. [Google Scholar] [CrossRef]
- Nahida, A.; Siddiqui, A.N. Pistacia lentiscus: A review on phytochemistry and pharmacological properties. Int. J. Pharm. 2012, 4, 16–20. [Google Scholar]
- Gori, A.; Nascimento, L.B.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and Diurnal Variation in Leaf Phenolics of Three Medicinal Mediterranean Wild Species: What Is the Best Harvesting Moment to Obtain the Richest and the Most Antioxidant Extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef]
- Remila, S.; Atmani-Kilani, D.; Delemasure, S.; Connat, J.L.; Azib, L.; Richard, T.; Atmani, D. Antioxidant, cytoprotective, anti-inflammatory and anticancer activities of Pistacia lentiscus (Anacardiaceae) leaf and fruit extracts. Eur. J. Integr. Med. 2015, 7, 274–286. [Google Scholar] [CrossRef]
- Azaizeh, H.; Halahleh, F.; Abbas, N.; Markovics, A.; Muklada, H.; Ungar, E.D.; Landau, S.Y. Polyphenols from Pistacia lentiscus and Phillyrea latifolia impair the exsheathment of gastro-intestinal nematode larvae. Vet. Parasitol. 2013, 191, 44–50. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Galardi, C.; Mulinacci, N.; Tattini, M. Identification and quantification of galloyl derivatives, flavonoid glycosides and anthocyanins in leaves of Pistacia lentiscus L. Phytochem. Anal. 2002, 13, 79–86. [Google Scholar] [CrossRef]
- Ruiz-Cruz, S.; Chaparro-Hernández, S.; Hernández-Ruiz, K.L.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Gassos Ortega, L.E.; Lopez Mata, M.A. Flavonoids: Important biocompounds in food. In Flavonoids: From Biosynthesis to Human Health; Goncalo, C.J.J., Ed.; IntechOpen: London, UK, 2017; pp. 353–370. [Google Scholar]
- Brodowska, K.M. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Pretorius, J.C. Flavonoids: A review of its commercial application potential as anti-infective agents. Curr. Med. Chem. 2003, 2, 335–353. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentao, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food. Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Bozorgi, M.; Memariani, Z.; Mobli, M.; Salehi Surmaghi, M.H.; Shams-Ardekani, M.R.; Rahimi, R. Five Pistacia species (P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus): A review of their traditional uses, phytochemistry, and pharmacology. Sci. World. J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Ljubuncic, P.; Song, H.; Cogan, U.; Azaizeh, H.; Bomzon, A. The effects of aqueous extracts prepared from the leaves of Pistacia lentiscus in experimental liver disease. J. Ethno. Pharmacol. 2005, 100, 198–204. [Google Scholar] [CrossRef]
- Landau, S.; Muklada, H.; Markovics, A.; Azaizeh, H. Traditional uses of Pistacia lentiscus in veterinary and human medicine. In Medicinal and Aromatic Plants of the Middle-East; Medicinal and Aromatic Plants of the World; Zohara, Y., Nativ, D., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 2, pp. 163–180. [Google Scholar]
- Moeini, R.; Memariani, Z.; Asadi, F.; Bozorgi, M.; Gorji, N. Pistacia Genus as a Potential Source of Neuroprotective Natural Products. Planta Med. 2019, 85, 1326–1350. [Google Scholar] [CrossRef]
- El Bishbishy, M.H.; Gad, H.A.; Aborehab, N.M. Chemometric discrimination of three Pistacia species via their metabolic profiling and their possible in vitro effects on memory functions. J. Pharm. Biomed. Anal. 2020, 177, 112840. [Google Scholar] [CrossRef] [PubMed]
- Dellai, A.; Souissi, H.; Borgi, W.; Bouraoui, A.; Chouchane, N. Antiinflammatory and antiulcerogenic activities of Pistacia lentiscus L. leaves extracts. Ind. Crops. Prod. 2013, 49, 879–882. [Google Scholar] [CrossRef]
- Gacem, M.A.; El Hadj-Khelil, A.O.; Boudjemaa, B.; Gacem, H. Phytochemistry, Toxicity and Pharmacology of Pistacia lentiscus, Artemisia herba-alba and Citrullus colocynthis. In Sustainable Agriculture Reviews 39; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2020; Volume 39, pp. 57–93. [Google Scholar]
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014, 61, 31–40. [Google Scholar] [CrossRef]
- Oreopoulou, A.; Tsimogiannis, D.; Oreopoulou, V. Extraction of polyphenols from aromatic and medicinal plants: An overview of the methods and the effect of extraction parameters. In Polyphenols in Plants, 2nd ed.; Watson, R.R., Ed.; Academic Press: London, UK, 2019; pp. 243–259. [Google Scholar]
- Nkhili, E.; Tomao, V.; El Hajji, H.; El Boustani, E.S.; Chemat, F.; Dangles, O. Microwave-assisted water extraction of green tea polyphenols. Phytochem. Anal. 2009, 20, 408–415. [Google Scholar] [CrossRef]
- Farhat, M.B.; Jordán, M.J.; Chaouch-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Changes in phenolic profiling and antioxidant capacity of Salvia aegyptiaca L. by-products during three phenological stages. LWT 2015, 63, 791–797. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food. Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green extraction methods for polyphenols from plant matrices and their by-products: A review. Compr. Rev. Food. Sci. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Curr. Green. Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Panja, P. Green extraction methods of food polyphenols from vegetable materials. J. Food. Sci. 2018, 23, 173–182. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. Hyphenated techniques and their applications in natural products analysis. In Natural Products Isolation Methods and Protocols, 3rd ed.; Sarker, S.D., Nahar, L., Eds.; Humana Press: Mumbai, India; Springer: Mumbai, India, 2009; Volume 864, pp. 75–88. [Google Scholar]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 2003, A1012, 119–128. [Google Scholar] [CrossRef]
- Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Jambrak, A.R.; Lorenzo, J.M.; Barba, F.J. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules 2020, 25, 1718. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food. Eng. 2003, 117, 426–436. [Google Scholar] [CrossRef]
- Ben Ahmed, Z.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Mangelings, D.; Vander Heyden, Y. Seasonal, gender and regional variations in total phenolic, flavonoid, and condensed tannins contents and in antioxidant properties from Pistacia atlantica ssp. leaves. Pharm. Biol. 2017, 55, 1185–1194. [Google Scholar] [CrossRef]
- Teng, H.; Choi, Y.H. Optimization of ultrasonic-assisted extraction of bioactive alkaloid compounds from rhizomacoptidis (Coptis chinensis Franch.) using response surface methodology. Food. Chem. 2014, 142, 299–305. [Google Scholar] [CrossRef]
- Abd El-Salam, E.A.; Morsy, N.F. Optimization of the extraction of polyphenols and antioxidant activity from Malva parviflora L. leaves using Box–Behnken design. Prep. Biochem. Biotech. 2019, 49, 876–883. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Khuri, A.I.; Cornell, J.A. Response Surfaces: Designs and Analyses; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Dahmoune, F.; Remini, H.; Dairi, S.; Aoun, O.; Moussi, K.; Bouaoudia-Madi, N.; Mouni, L. Ultrasound assisted extraction of phenolic compounds from P. lentiscus L. leaves: Comparative study of artificial neural network (ANN) versus degree of experiment for prediction ability of phenolic compounds recovery. Ind. Crop. Prod. 2015, 77, 251–261. [Google Scholar] [CrossRef]
- Alessandri, S.; Ieri, F.; Romani, A. Minor polar compounds in extra virgin olive oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu spectrophotometric method. J. Agric. Food. Chem. 2014, 62, 826–835. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Seabra, I.J.; Dias, A.M.A.; De Sousa, H.C. Recent trends and perspectives for the extraction of natural products. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; RSC Publishing: Cambridge, UK, 2013; pp. 231–275. [Google Scholar]
- Nascimento, L.B.S.N.; de Aguiar, P.F.; Leal-Costa, M.V.; Coutinho, M.A.S.; Borsodi, M.P.G.; Rossi-Bergmann, B.; Costa, S.S. Optimization of aqueous extraction from Kalanchoe pinnata leaves to obtain the highest content of an anti-inflammatory flavonoid using a response surface model. Phytochem. Anal. 2018, 29, 308–315. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Rivera-Mondragón, A.; Broeckx, G.; Bijttebier, S.; Naessens, T.; Fransen, E.; Kiekens, F.; Foubert, K. Ultrasound-assisted extraction optimization and validation of an HPLC-DAD method for the quantification of polyphenols in leaf extracts of Cecropia species. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Anandharamakrishnan, C. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, J.G.; Li, W.F.; Chen, J.; Wang, D.Y.; Zhu, L. Optimum extraction process of polyphenols from the bark of Phyllanthus emblica L. based on the response surface methodology. J. Sep. Sci. 2009, 32, 1437–1444. [Google Scholar] [CrossRef]
- Jovanović, A.A.; Đorđević, V.B.; Zdunić, G.M.; Pljevljakušić, D.S.; Šavikin, K.P.; Gođevac, D.M.; Bugarski, B.M. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat-and ultrasound-assisted techniques. Sep. Purif. Technol. 2017, 179, 369–380. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Li, D.; Wang, L.J.; Ozkan, N.; Chen, X.D.; Mao, Z.H.; Yang, H.Z. Optimization of ethanol–water extraction of lignans from flaxseed. Sep. Purif. Technol. 2007, 57, 17–24. [Google Scholar] [CrossRef]
- Gam, D.H.; Yi Kim, S.; Kim, J.W. Optimization of Ultrasound-Assisted Extraction Condition for Phenolic Compounds, Antioxidant Activity, and Epigallocatechin Gallate in Lipid-Extracted Microalgae. Molecules 2020, 25, 454. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of ultrasound-assisted extraction. In Water Extraction of Bioactive Compounds, From Plant to Drug Development; Gonzàlez, H.D., Muñoz, M.J.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Optimization of extraction parameters of total phenolics from Annona crassiflora Mart. (Araticum) fruits using response surface methodology. Food. Anal. Methods 2017, 10, 100–110. [Google Scholar] [CrossRef]
- Zhao, S.; Baik, O.D.; Choi, Y.J.; Kim, S.M. Pretreatments for the efficient extraction of bioactive compounds from plant-based biomaterials. Crit. Rev. Food. Sci. Nutr. 2014, 54, 1283–1297. [Google Scholar] [CrossRef]
- Rusu, M.E.; Fizeșan, I.; Pop, A.; Gheldiu, A.M.; Mocan, A.; Crișan, G.; Tomuta, I. Enhanced recovery of antioxidant compounds from hazelnut (Corylus avellana L.) involucres based on extraction optimization: Phytochemical profile and biological activities. Antioxidants 2019, 8, 460. [Google Scholar] [CrossRef]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Emeko, H.A.; Olugbogi, A.O.; Betiku, E. Appraisal of artificial neural network and response surface methodology in modeling and process variable optimization of oxalic acid production from cashew apple juice: A case of surface fermentation. Bioresources 2015, 10, 2067–2082. [Google Scholar] [CrossRef]
- Joglekar, A.M.; May, A.T.; Graf, E.; Saguy, I. Product excellence through experimental design. Cereal Food World 1987, 32, 857–868. [Google Scholar]
- Wu, J.; Yu, D.; Sun, H.; Zhang, Y.; Zhang, W.; Meng, F.; Du, X. Optimizing the extraction of anti-tumor alkaloids from the stem of Berberis amurensis by response surface methodology. Ind. Crops. Prod. 2015, 69, 68–75. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Silva, E.M.; Rogez, H.; Larondelle, Y. Optimization of extraction of phenolics from Inga edulis leaves using response surface methodology. Sep. Purif. Technol. 2007, 55, 381–387. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, C.; Quirantes-Piné, R.; Amessis-Ouchemoukh, N.; Madani, K.; Segura-Carretero, A.; Fernández-Gutierrez, A. A metabolite-profiling approach allows the identification of new compounds from Pistacia lentiscus leaves. J. Pharm. Biomed. Anal. 2013, 77, 167–174. [Google Scholar] [CrossRef]
- Barbouchi, M.; Elamrani, K.; El Idrissi, M. A comparative study on phytochemical screening, quantification of phenolic contents and antioxidant properties of different solvent extracts from various parts of Pistacia lentiscus L. J. King Saud Univ. Sci. 2020, 32, 302–306. [Google Scholar] [CrossRef]
- Pacifico, S.; Piccolella, S.; Marciano, S.; Galasso, S.; Nocera, P.; Piscopo, V.; Monaco, P. LC-MS/MS profiling of a mastic leaf phenol enriched extract and its effects on H2O2 and Aβ (25–35) oxidative injury in SK-B-NE (C)-2 cells. J. Agric. Food. Chem. 2014, 62, 11957–11966. [Google Scholar] [CrossRef]
- Saliha, D.; Seddik, K.; Djamila, A.; Abdrrahmane, B.; Lekhmici, A.; Noureddine, C. Antioxidant proprieties of Pistacia lentiscus L. leaves extracts. Pharmacogn. Commun. 2013, 3, 28. [Google Scholar] [CrossRef]
- Cherbal, A.; Kebieche, M.; Madani, K.; El-Adawi, H. Extraction and valorization of phenolic compounds of leaves of Algerian Pistacia lentiscus. Asian. J. Plant Sci. 2012, 11, 131–136. [Google Scholar] [CrossRef][Green Version]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Baratto, M.C.; Tattini, M.; Galardi, C.; Pinelli, P.; Romani, A.; Visioli, F.; Pogni, R. Antioxidant activity of galloyl quinic derivatives isolated from P. lentiscus leaves. Free Radic. Res. 2003, 37, 405–412. [Google Scholar] [CrossRef]
- Beghlal, D.; El Bairi, K.; Marmouzi, I.; Haddar, L.; Mohamed, B. Phytochemical, organoleptic and ferric reducing properties of essential oil and ethanolic extract from Pistacia lentiscus (L.). Asian. Pac. J. Trop. Dis. 2016, 6, 305–310. [Google Scholar] [CrossRef]
- Mehenni, C.; Atmani-Kilani, D.; Dumarçay, S.; Perrin, D.; Gérardin, P.; Atmani, D. Hepatoprotective and antidiabetic effects of Pistacia lentiscus leaf and fruit extracts. J. Food Drug. Anal. 2016, 24, 653–669. [Google Scholar] [CrossRef]
- Foddai, M.; Kasabri, V.; Afifi, F.U.; Azara, E.; Petretto, G.L.; Pintore, G. In vitro inhibitory effects of Sardinian Pistacia lentiscus L. and Pistacia terebinthus L. on metabolic enzymes: Pancreatic lipase, α-amylase, and α-glucosidase. Starch-Stärke 2015, 67, 204–212. [Google Scholar] [CrossRef]
- Neszmelyi, A.; Kreher, B.; Müller, A.; Dorsch, W.; Wagner, H. Tetragalloylquinic acid, the major antiasthmatic principle of Galphimia glauca. Planta Med. 1993, 59, 164–167. [Google Scholar] [CrossRef]
- Bouchet, N.; Barrier, L.; Fauconneau, B. Radical scavenging activity and antioxidant properties of tannins from Guiera senegalensis (Combretaceae). Phytother. Res. 1998, 12, 159–162. [Google Scholar] [CrossRef]
- Karas, D.; Ulrichová, J.; Valentová, K. Galloylation of polyphenols alters their biological activity. Food Chem. Toxicol. 2017, 105, 223–240. [Google Scholar] [CrossRef]
- Hussain, S.; Schönbichler, S.A.; Güzel, Y.; Sonderegger, H.; Abel, G.; Rainer, M.; Bonn, G.K. Solid-phase extraction of galloyl-and caffeoylquinic acids from natural sources (Galphimia glauca and Arnicae flos) using pure zirconium silicate and bismuth citrate powders as sorbents inside micro spin columns. J. Pharm. Biomed. Anal. 2013, 84, 148–158. [Google Scholar] [CrossRef]
- Lin, S.; Zhu, Q.; Wen, L.; Yang, B.; Jiang, G.; Gao, H.; Chen, F.; Jiang, Y. Production of quercetin, kaempferol and their glycosidic derivatives from the aqueous organic extracted residue of litchi pericarp with Aspergillus awamori. Food Chem. 2014, 145, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.M. Luteolin a flavonoid with potential for cancer prevention and therapy. Curr. Cancer. Drug. Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Mazaki, T.; Kitajima, T.; Shiozaki, Y.; Sato, M.; Mino, M.; Yoshida, A.; Nakamura, M.; Yoshida, Y.; Tanaka, M.; Ozaki, T.; et al. In vitro and in vivo enhanced osteogenesis by kaempferol found by a high-through put assay using human mesenchymal stromal cells. J. Funct. Foods 2014, 6, 241–247. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Azib, L.; Debbache-Benaida, N.; Da Costa, G.; Atmani-Kilani, D.; Saidene, N.; Ayouni, K.; Atmani, D. Pistacia lentiscus L. leaves extract and its major phenolic compounds reverse aluminium-induced neurotoxicity in mice. Ind. Crop. Prod. 2019, 137, 576–584. [Google Scholar] [CrossRef]
- Adebayo, H.A.; Abolaji, A.O.; Kela, R.; Ayepola, O.O.; Olorunfemi, T.B.; Taiwo, O.S. Antioxidant activities of the leaves of Chrysophyllum Albidum G. Pak. J. Pharm. Sci. 2011, 24, 545–551. [Google Scholar] [PubMed]
- Ahangarpour, A.; Oroojan, A.A.; Khorsandi, L.; Kouchak, M.; Badavi, M. Antioxidant effect of myricitrin on hyperglycemia-induced oxidative stress in C2C12 cell. Cell Stress Chaperones 2018, 23, 773–781. [Google Scholar] [CrossRef]
- Chen, W.; Zhuang, J.; Li, Y.; Shen, Y.; Zheng, X. Myricitrin protects against peroxynitrite-mediated DNA damage and cytotoxicity in astrocytes. Food Chem. 2013, 141, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.N. Basic statistical methods for analytical chemistry. Part 2. Calibration and regression methods. A review. Analyst 1991, 116, 3–14. [Google Scholar] [CrossRef]

| Experimental Trials | Independent Variables (x) | |||||||
|---|---|---|---|---|---|---|---|---|
| x1 (Temperature, in °C) | x2 (Time, in min) | x3 (Solvent Ratio, in L g−1) | x4 (Ethanol Fraction, in % v/v) | |||||
| 1 | 5 | (−) | 15 | (−) | 0.06 | (−) | 50 | (−) |
| 2 | 25 | (+) | 15 | (−) | 0.06 | (−) | 75 | (+) |
| 3 | 5 | (−) | 30 | (+) | 0.06 | (−) | 75 | (+) |
| 4 | 25 | (+) | 30 | (+) | 0.06 | (−) | 50 | (−) |
| 5 | 5 | (−) | 15 | (−) | 0.1 | (+) | 75 | (+) |
| 6 | 25 | (+) | 15 | (−) | 0.1 | (+) | 50 | (−) |
| 7 | 5 | (−) | 30 | (+) | 0.1 | (+) | 50 | (−) |
| 8 | 25 | (+) | 30 | (+) | 0.1 | (+) | 75 | (+) |
| 9 | 15 | (0) | 22.5 | (0) | 0.08 | (0) | 62.5 | (0) |
| Answers (y) | Calculated coefficients | |||||||
| TTC | b1 | 0.338 | b2 | −0.104 | b3 | 0.571 * | b4 | −0.604 * |
| TFC | b1 | −0.092 | b2 | −0.058 | b3 | 0.258 * | b4 | 0.008 |
| TPC | b1 | 0.375 | b2 | −0.108 | b3 | 1.267 * | b4 | −0.700 * |
| Independent Variables (Factors) | Dependent Variables (Responses) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trials | x1 (Ethanol Fraction, in % v/v) | x2 (Solvent Ratio, in L g−1) | x3 (Temperature, in °C) | TTC | TFC | MYC | TPC | |||
| 1 | 30 | (−) | 0.1 | (−) | 40 | (0) | 30.5 | 8.6 | 2.0 | 42.6 |
| 2 | 50 | (+) | 0.1 | (−) | 40 | (0) | 30.1 | 8.9 | 1.4 | 39.9 |
| 3 | 30 | (−) | 0.2 | (+) | 40 | (0) | 24.3 | 6.5 | 1.6 | 33.2 |
| 4 | 50 | (+) | 0.2 | (+) | 40 | (0) | 26.5 | 7.1 | 1.6 | 35.6 |
| 5 | 30 | (−) | 0.15 | (0) | 30 | (−) | 31.9 | 9.5 | 2.2 | 44.0 |
| 6 | 50 | (+) | 0.15 | (0) | 30 | (−) | 33.6 | 9.6 | 2.1 | 46.0 |
| 7 | 30 | (−) | 0.15 | (0) | 50 | (+) | 35.7 | 8.5 | 1.9 | 45.9 |
| 8 | 50 | (+) | 0.15 | (0) | 50 | (+) | 36.6 | 10.1 | 2.2 | 49.4 |
| 9 | 40 | (0) | 0.1 | (−) | 30 | (−) | 37.6 | 8.0 | 2.0 | 49.0 |
| 10 | 40 | (0) | 0.2 | (+) | 30 | (-) | 37.8 | 7.5 | 1.6 | 48.6 |
| 11 | 40 | (0) | 0.1 | (−) | 50 | (+) | 37.7 | 6.9 | 1.6 | 48.6 |
| 12 | 40 | (0) | 0.2 | (+) | 50 | (+) | 34.5 | 7.1 | 1.9 | 44.6 |
| 13 | 40 | (0) | 0.15 | (0) | 40 | (0) | 34.9 ± 1.3 | 5.8 ± 0.60 | 1.4 ± 0.15 | 44.3 ± 1.7 |
| Responses | Analysis of the Model | Lack of Fit (LOF) | ||||
|---|---|---|---|---|---|---|
| R2 | R2adj | F-Value of Model | p-Value of Model | F-Value of Lack of Fit | p-Value of Lack of Fit | |
| TTC | 0.95 | 0.74 | 5.32 | 0.040 * | 3.56 | 0.23 |
| TFC | 0.90 | 0.73 | 5.13 | 0.043 * | 1.52 | 0.42 |
| MYC | 0.89 | 0.71 | 4.79 | 0.049 * | 1.25 | 0.47 |
| TPC | 0.90 | 0.71 | 4.80 | 0.049 * | 3.07 | 0.26 |
| Peak | tR, (min) | λ max, (nm) | Collision Energy, (V) | [M − H]−,(m/z) | MS2, (m/z) | Peak Assignment |
|---|---|---|---|---|---|---|
| 1 | 16.23 | 234,270 | 10 | 331 | 169,151,125 | Monogalloyl glucose |
| 2 | 19.71 | 234,272 | 10 | 169 | 125 | Gallic acid |
| 3 | 20.53 | 236,272 | 15 | 343 | 191 | Monogalloyl quinic acid |
| 4 | 30.24 | 236,276 | 15 | 495 | 343,191,169 | Digalloyl quinic acid (isomer 1) |
| 5 | 31.22 | 236,276 | 15 | 495 | 343,191,169 | Digalloyl quinic acid (isomer 2) |
| 6 | 35.72 | 256,356 | 20 | 647 | 495,343,191,169 | Trigalloyl quinic acid (isomer 1) |
| 7 | 37.09 | 256,356 | 20 | 647 | 343,191,169 | Trigalloyl quinic acid (isomer 2) |
| 8 | 38.47 | 265,355 | 20 | 799 | 495,343,191,169 | Tetragalloyl quinic acid (isomer 1) |
| 9 | 38.59 | 265,355 | 20 | 799 | 495,191,169 | Tetragalloyl quinic acid (isomer 2) |
| 10 | 38.83 | 264,314,346 sh | 10 | 479 | 317,316 | Myricetin-3-O-galactoside |
| 11 | 40.15 | 268,314,348sh | 15 | 625 | 479,316,317 | Myricetin-3-O-rutinoside |
| 12 | 42.62 | 256,350 | 10 | 493 | 301 | Quercetin derivative |
| 13 | 42.94 | 256,350 | 10 | 463 | 381,300,301 | Quercetin-O-hexoside 1 |
| 14 | 43.29 | 260,358,346 sh | 10 | 463 | 316,271,179 | Myricitrin (Myricetin-3-O-rhamnoside) |
| 15 | 44.23 | 270,350,300 sh | 10 | 463 | 381,300,301 | Quercetin-O-hexoside 2 |
| 16 | 44.97 | 256,350 | 15 | 585 | 525,301,179 | Quercetin-O-galloyl-pentoside |
| 17 | 45.57 | 256,350,300 sh | 10 | 433 | 300,301 | Quercetin-3-O-arabinoside |
| 18 | 47.69 | 266,350,300 sh | 15 | 447 | 300,301 | Quercitrin (Quercetin-3-O-rhamnoside) |
| 19 | 49.19 | 265,348 | 15 | 447 | 415,365,285 | Kaempferol-O-hexoside |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detti, C.; dos Santos Nascimento, L.B.; Brunetti, C.; Ferrini, F.; Gori, A. Optimization of a Green Ultrasound-Assisted Extraction of Different Polyphenols from Pistacia lentiscus L. Leaves Using a Response Surface Methodology. Plants 2020, 9, 1482. https://doi.org/10.3390/plants9111482
Detti C, dos Santos Nascimento LB, Brunetti C, Ferrini F, Gori A. Optimization of a Green Ultrasound-Assisted Extraction of Different Polyphenols from Pistacia lentiscus L. Leaves Using a Response Surface Methodology. Plants. 2020; 9(11):1482. https://doi.org/10.3390/plants9111482
Chicago/Turabian StyleDetti, Cassandra, Luana Beatriz dos Santos Nascimento, Cecilia Brunetti, Francesco Ferrini, and Antonella Gori. 2020. "Optimization of a Green Ultrasound-Assisted Extraction of Different Polyphenols from Pistacia lentiscus L. Leaves Using a Response Surface Methodology" Plants 9, no. 11: 1482. https://doi.org/10.3390/plants9111482
APA StyleDetti, C., dos Santos Nascimento, L. B., Brunetti, C., Ferrini, F., & Gori, A. (2020). Optimization of a Green Ultrasound-Assisted Extraction of Different Polyphenols from Pistacia lentiscus L. Leaves Using a Response Surface Methodology. Plants, 9(11), 1482. https://doi.org/10.3390/plants9111482










