Breaking the Dormancy of Snake’s Head Fritillary (Fritillaria meleagris L.) In Vitro Bulbs—Part 1: Effect of GA3, GA Inhibitors and Temperature on Fresh Weight, Sprouting and Sugar Content
Abstract
1. Introduction
2. Results
2.1. Effect of GA3, GA Inhibitors and Temperature on Fresh Weight of Bulbs
2.2. Effect of GA3 and GA Inhibitors and Temperature on Sprouting of Bulbs
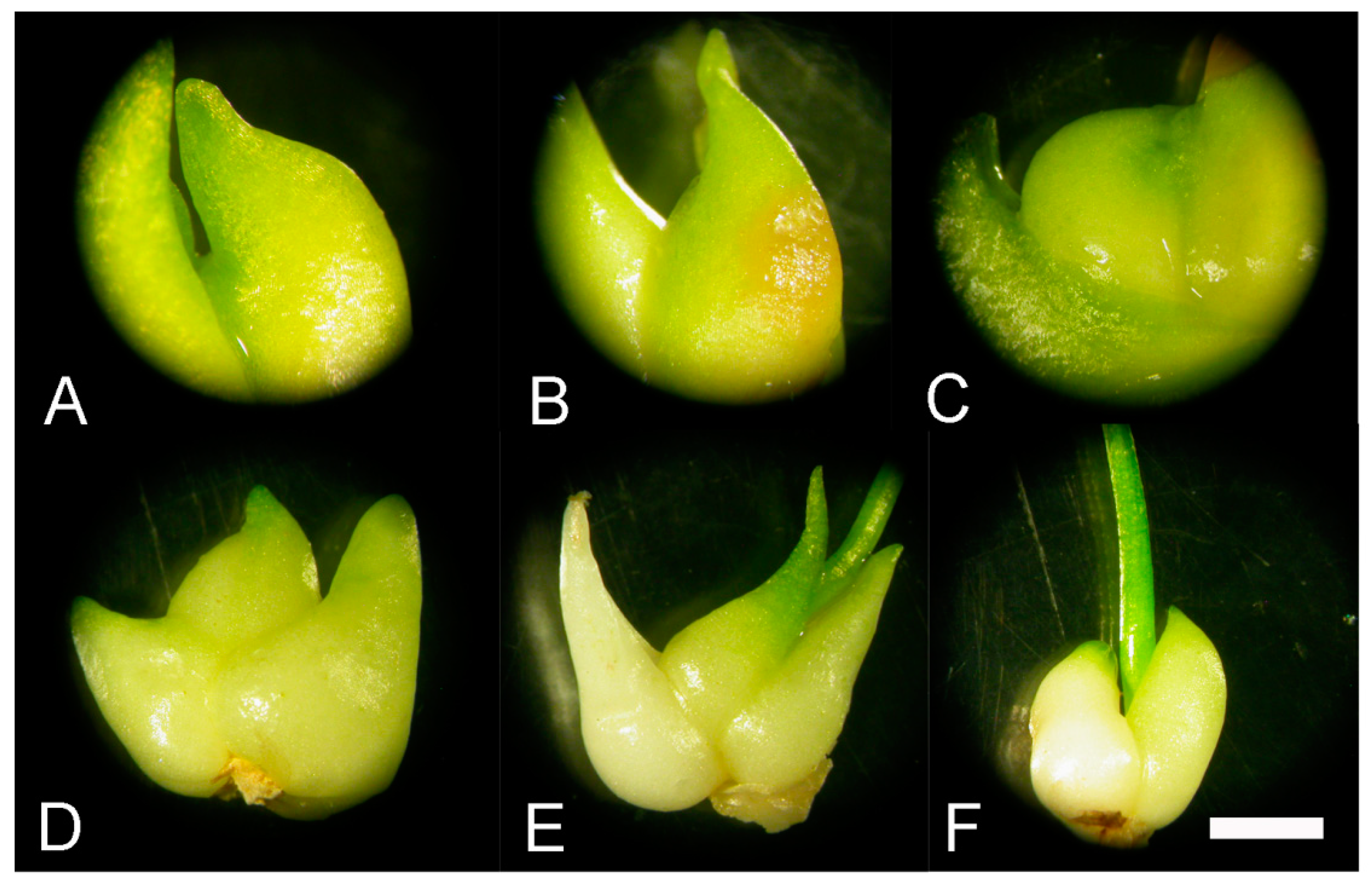
2.3. Morpho-Anatomical Analysis of the Effect of GA3, GA Inhibitors and Temperatures on Sprouting of F. meleagris Bulbs
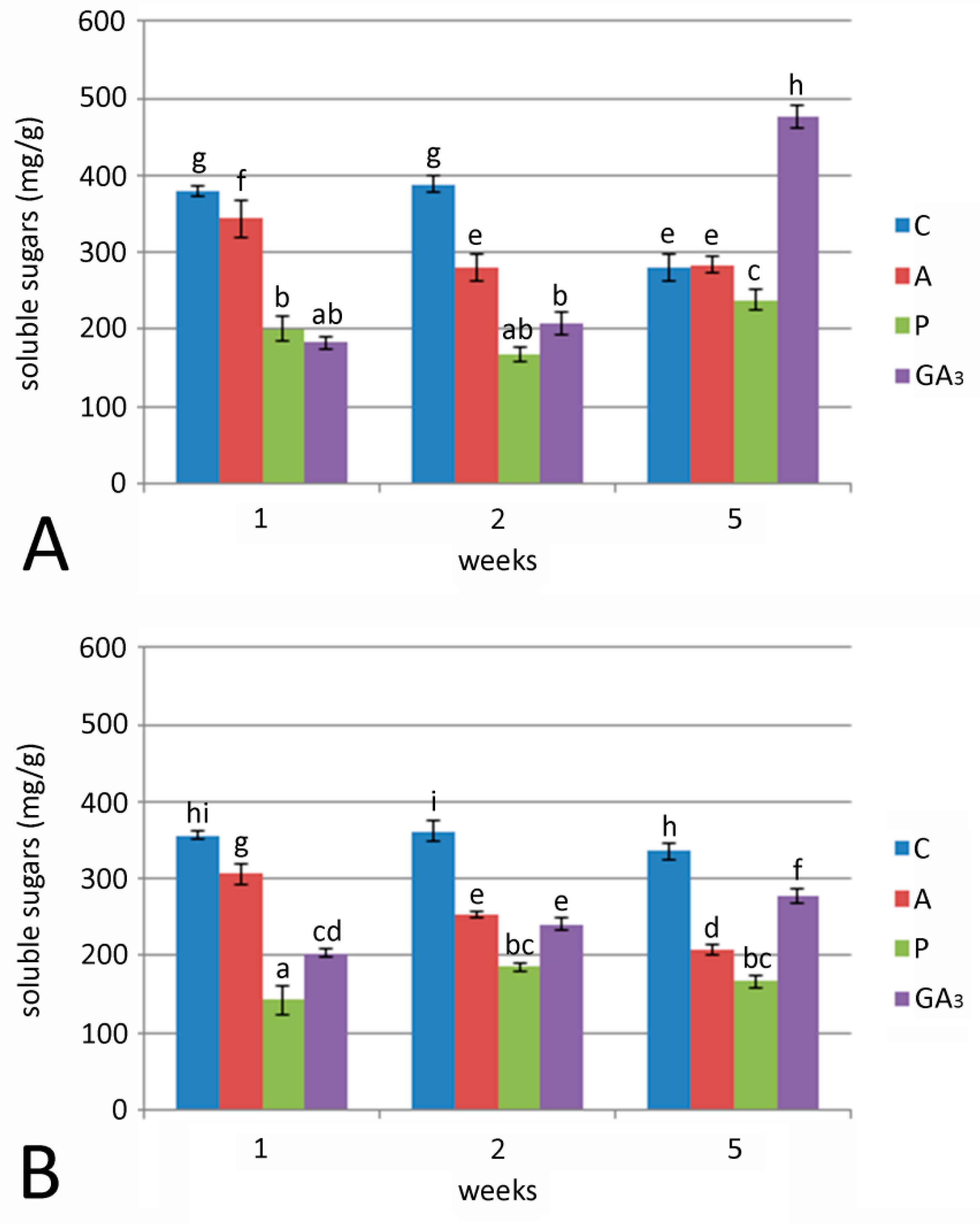
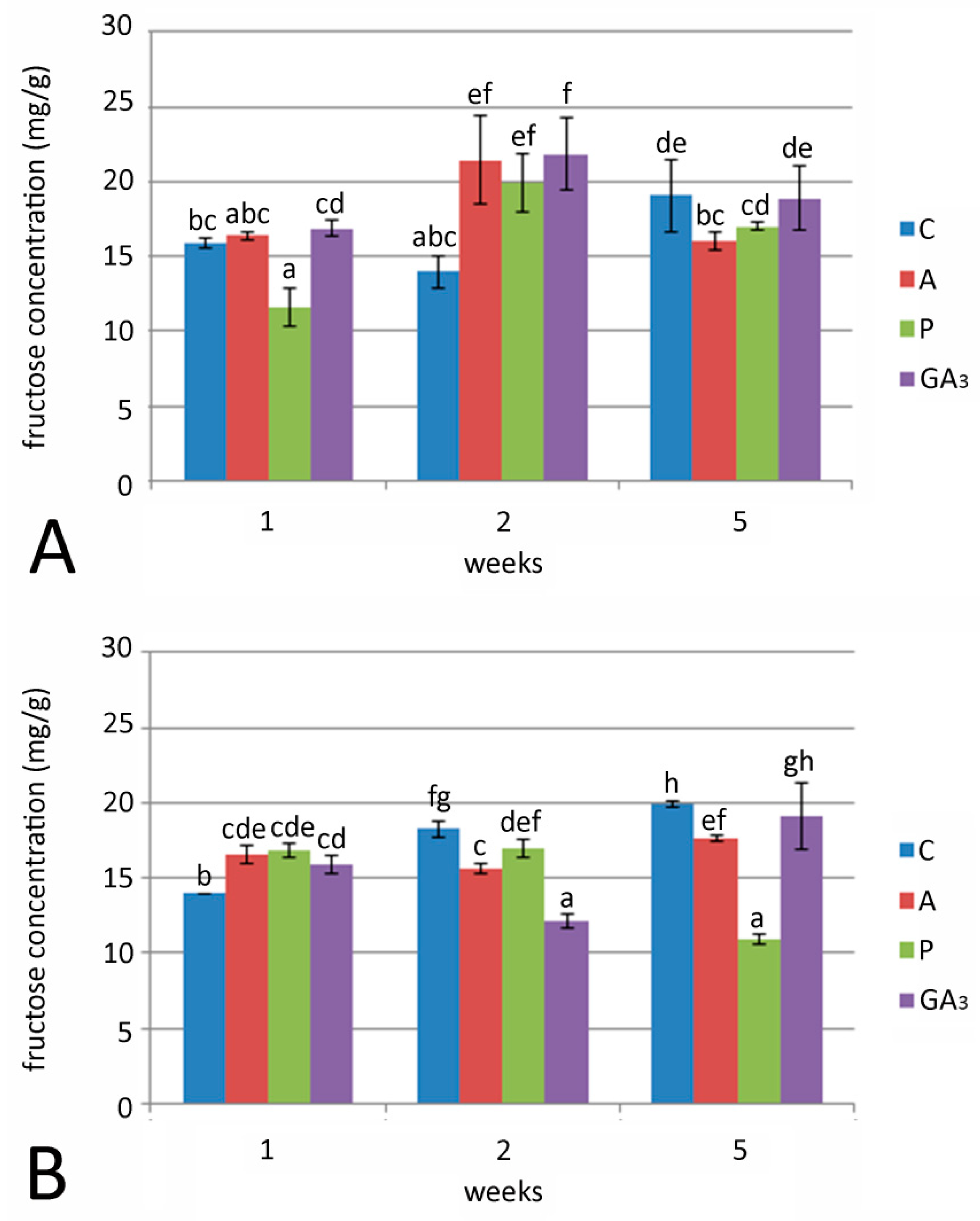
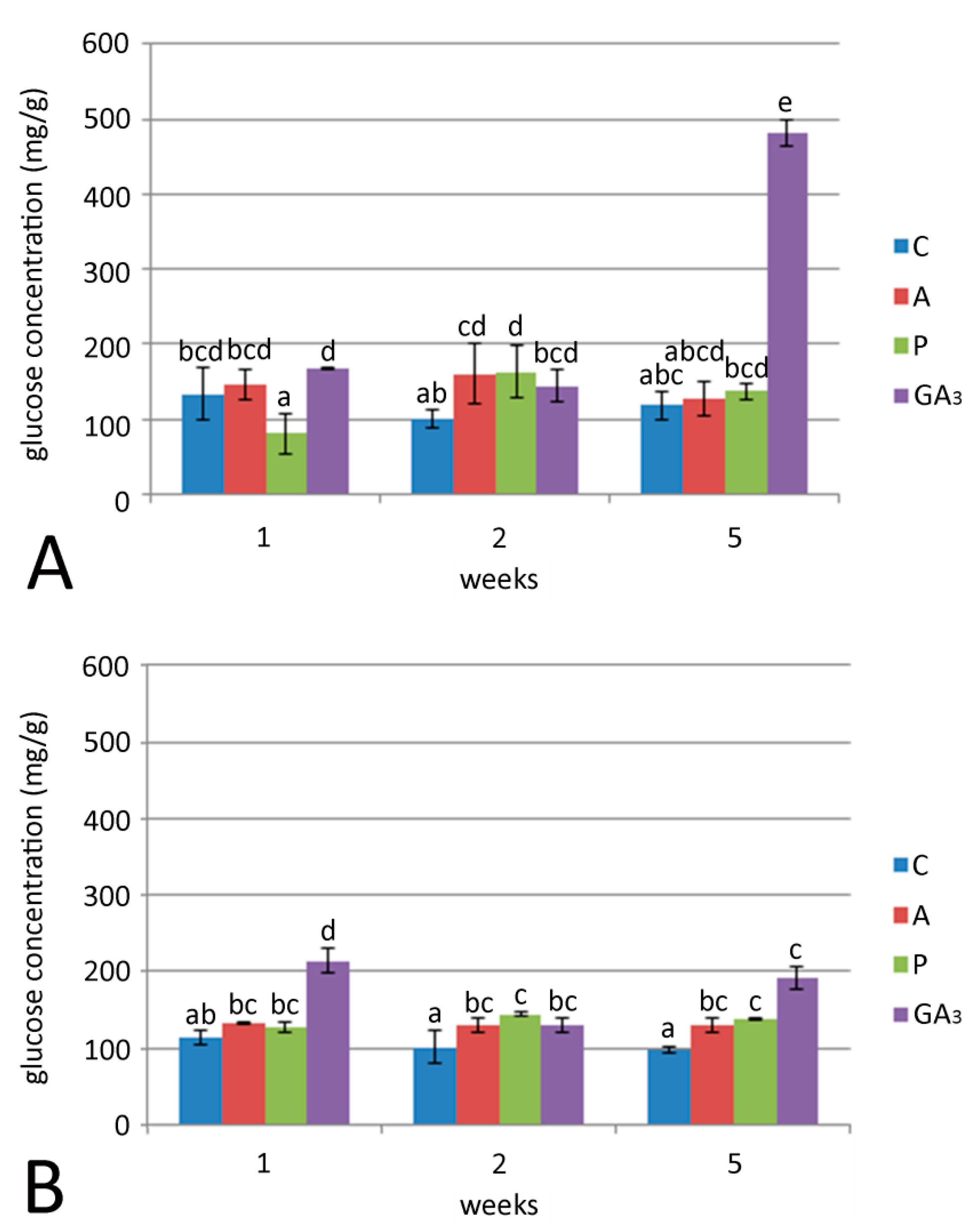
2.4. Effect of GA3 and GA Inhibitors and Temperature on Total Sugar, Fructose and Glucose Content
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Culture Media and Growth Conditions
4.3. Morpho-Anatomical Study
4.4. Sugar Analysis
4.4.1. Determination of Total Sugars Content
4.4.2. Fructose Determination
4.4.3. Glucose Determination
4.5. Statistical Analysis of Data
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paek, K.Y.; Murthy, H.N. High frequency of bulblet regeneration from bulb scale section of Fritillaria thunbergii. Plant Cell Tissue Organ Cult. 2002, 68, 247–252. [Google Scholar]
- Kukulczanka, K.; Kromer, K.; Czastka, B. Propagation of Fritillaria meleagris L. through tissue culture. Acta Hortic 1989, 251, 147–153. [Google Scholar] [CrossRef]
- Subotić, A.; Trifunović, M.; Jevremović, S.; Petrić, M. Morpho-histological study of direct somatic embryogenesis in endangered species Fritillaria meleagris. Biol. Plant. 2010, 54, 592–596. [Google Scholar]
- Langens-Gerrits, M.M.; Klerk, G.J.; Croes, A.F. Phase change in lily bulblets regenerated in vitro. Physiol. Plant. 2003, 119, 590–597. [Google Scholar] [CrossRef]
- Petrić, M.; Jevremović, S.; Trifunović, M.; Tadić, V.; Milošević, S.; Dragićević, M.; Subotić, A. The effect of low temperature and GA3 treatments on dormancy breaking and activity of antioxidant enzymes in Fritillaria meleagris bulblets cultured in vitro. Acta Physiol. Plant. 2013, 35, 3223–3236. [Google Scholar] [CrossRef]
- Kamenetsky, R.; Zemah, H.; Ranwala, A.P.; Vergeldt, F.; Ranwala, N.K.; Miller, W.B.; Van As, H.; Bendel, P. Water status and carbohydrate pools in tulip bulbs during dormancy release. New Phytol. 2003, 158, 109–118. [Google Scholar] [CrossRef]
- De Hertogh, A.A.; Le Nard, M. Physiological and biochemical aspects of flower bulbs. In The Physiology of Flower Bulbs; De Hertogh, A.A., Le Nard, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 53–69. [Google Scholar]
- Bewley, J.D. Seed gemination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Le Nard, M. Physiology and storage of bulbs. In Post Harvest Physiology and Crop Preservation, NATO Advanced Study Institute, Series A: Life Science; Liberman, M., Ed.; Plenum Press: New York, NY, USA, 1983; pp. 191–230. [Google Scholar]
- Atif, J.M.; Ahanger, M.A.; Amin, B.; Ghani, M.I.; Ali, M.; Cheng, Z. Mechanism of Allium crops bulb enlargement in response to photoperiod: A review. Int. J. Mol. Sci. 2010, 11, 2694. [Google Scholar] [CrossRef]
- Knyple, J.S. Increasing bulb growth in onion with growth retardants and reversal of the effect by gibberellin. Plant Sci. Lett. 1979, 14, 193–198. [Google Scholar] [CrossRef]
- Le-Guen-Le Saos, F.L.; Hourmant, A.; Esnault, F.; Chauvin, J.E. In vitro bulb development in shallot (Allium cepa L. Aggregatum Group): Effect of anti-gibberellins, sucrose and light. Ann. Bot. 2002, 89, 419–425. [Google Scholar]
- Yamazaki, H.; Shiraiwa, N.; Itai, A.; Honda, I. Involvement of gibberellins in the regulation of tillering in welsh onion (Allium fistulosum L.). Hortic. J. 2015, 84, 334–341. [Google Scholar] [CrossRef]
- Liu, H.; Deng, R.; Huang, C.; Cheng, Z.; Meng, H. Exogenous gibberellins alter morphology and nutritional traits of garlic (Allium sativum L.) bulb. Sci. Hortic. 2019, 246, 298–306. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Yang, F.; Qi, X.; Ahmad, H.; Wu, C.; Cheng, Z. Effect of the mode and time of gibberellic acid treatment on plant architecture and bulb structure in garlic (Allium sativum L.). Sci. Hortic. 2019, 257, 108723. [Google Scholar] [CrossRef]
- Rademacher, W. Growth retardants: Effect on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, W. Chemical regulators of gibberellin status and their application in plant production. Annu. Plant Rev. Line 2016, 49, 359–403. [Google Scholar]
- Galiba, G.; Kerepesi, I.; Snape, J.W.; Sutka, J. Location of a gene regulating cold-induced carbohydrate production on chromosome 5A of wheat. Theor. Appl. Genet. 1997, 95, 265–270. [Google Scholar] [CrossRef]
- Wu, C.; Wang, M.; Dong, Y.; Cheng, Z.; Meng, H. Effect of plant age and vernalisation on bolting, plant growth and enzyme activity of garlic (Allium sativum L.). Sci. Hortic. 2016, 201, 295–305. [Google Scholar] [CrossRef]
- Ranwala, A.P.; Miller, W.B. Analysis of nonstructural carbohydrates in storage organs of 30 ornamental geophytes by high-performance anion exchange chromatography with pulsed amperometric detection. New Phytol. 2008, 180, 421–433. [Google Scholar] [CrossRef]
- Shin, K.S.; Chakrabarty, D.; Paek, K.Y. Sprouting rate, change of carbohydrate contents and related enzymes during cold treatment of lily bulblets regenerated in vitro. Sci. Hortic. 2002, 96, 195–204. [Google Scholar] [CrossRef]
- Nikolić, M.; Mišić, D.; Maksimović, V.; Jevremović, S.; Trifunović, M.; Subotić, A. Effect of low temperature on rooting rate and carbohydrate content of Fritillaria meleagris bulbs formed in culture in vitro. Arch. Biol. Sci. 2008, 60, 5–6. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Lou, Y.; Wang, L.; Slovin, J.P.; Xu, W.; Wang, S.; Zhang, C. Proteomic analysis of the effect of gibberellin on increased fruit sink strength in Asian pear (Pyrus pyrifolia). Sci. Hortic. 2015, 195, 25–36. [Google Scholar] [CrossRef]
- Erogul, D.; Sen, F. Effects of gibberellic acid tretatment on fruit thinning and fruit quality in Japanese plum (Prunus salicina Lindl). Sci. Hortic. 2015, 186, 137–142. [Google Scholar] [CrossRef]
- Thakur, R.; Sood, A.; Nagar, P.K.; Pandey, S.; Sobti, R.C.; Ahuja, P.S. Regulation of growth of Lilium plantlets in liquid medium by application of paclobutrazol or ancymidol, for its amenability in a bioreactor system: Growth parameters. Plant Cell Rep. 2005, 25, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Petrić, M.; Jevremović, S.; Trifunović, M.; Tadić, V.; Milošević, S.; Subotić, A. Effect of antioxidant enzyme during induction of morphogenesis of Fritillaria meleagris in bulb scale culture. Turk. J Biol. 2014, 38, 328–338. [Google Scholar] [CrossRef]
- Wakchaure, G.C.; Minhas, P.S.; Meena, K.K.; Singh, N.P.; Hegade, P.M.; Sorty, A.M. Growth, bulb yield, water productivity and quality of onion (Allium cepa L.) as affecetd by deficit irrigation regimes and exogenous application of plant bio-regulators. Agric. Water Manag. 2018, 199, 1–10. [Google Scholar] [CrossRef]
- Shive, J.B.; Sisler, H.D. Effects of ancymidol (a growth retardant) and triarimol (a fungicide) on growth, sterols, and gibberellins of Phaseolus vulgaris (L.). Plant Physiol. 1976, 57, 640–644. [Google Scholar] [CrossRef]
- Ziv, M. Simple bioreactor for mass propagation of plants. Plant Cell Tissue Organ Cult. 2005, 81, 277–285. [Google Scholar] [CrossRef]
- Petrić, M.; Subotić, A.; Jevremović, S.; Trifunović-Momčilov, M.; Tadić, V.; Grujić, M.; Vujčić, Z. Esterase and peroxidase isoforms in different stages of morphogenesis in Fritillaria meleagris L. in bulb-scale culture. Comptes Rendus Biol. 2015, 12, 793–802. [Google Scholar] [CrossRef]
- Coolbaugh, R.C.; Swanson, D.I.; West, C.A. Comparative effects of ancymidol and its analogs on growth of peas and ent-kaurene oxidation in cell-free extracts of immature Marah macrocarpus endosperm. Plant Physiol. 1982, 69, 707–711. [Google Scholar] [CrossRef]
- Hofmannová, J.; Schwarzerová, K.; Havelková, L.; Boříková, P.; Petrášek, J.; Opatrný, Z. A novel, cellulose synthesis inhibitory action of ancymidol impairs plant cell expansion. J. Exp. Bot. 2008, 59, 3963–3974. [Google Scholar] [CrossRef]
- Norman, S.M.; Bennett, R.D.; Poling, S.M.; Maier, V.P.; Nelson, M.D. Paclobutrazol inhibits abscisic acid biosynthesis in Cercospora rosicola. Plant Physiol. 1986, 80, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Ziv, M. Enchaced shoot and cormlet proliferation in liquid cultured gladiolus buds by growth retardants. Plant Cell Tissue Organ Cult. 1989, 17, 101–110. [Google Scholar] [CrossRef]
- Fernie, A.R.; Willmitzer, L. Molecular and biochemical trrigers of potato tuber development. Plant Physiol. 2001, 127, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Petrić, M.; Subotić, A.; Jevremović, S.; Trifunović, M. Somatic embryogenesis and bulblet regeneration in snakehaed fritillary (Fritillaria meleagris L.). Afr. J. Biotechnol. 2011, 10, 16181–16188. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco culture. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry: Principles and Practices; WH Freeman and Company: San Francisco, CA, USA, 1962. [Google Scholar]
- Dreywood, R. Qualitative test for carbohydrate materials. Ind. Eng. Chem. Anal. Ed. 1946, 18, 499–504. [Google Scholar] [CrossRef]
- Messineo, L.; Musarra, E. Sensitive spectrophotometric determination of fructose, sucrose, and inulin without interference from aldohexoses, aldopentoses, and ketopentoses. Int. J. Biochem. 1972, 3, 691–699. [Google Scholar] [CrossRef]
- Amaral, L.I.V.; Gaspar, M.; Costa, P.M.F.; Aidar, M.P.M.; Buckeridge, M.S. Novo método enzimático rápido e sensível de extração e dosagem de amido em materiais vegetais. Hoehnea 2007, 34, 425–431. [Google Scholar] [CrossRef]

| Culture Medium | Time (Weeks) | ||
|---|---|---|---|
| 1 | 2 | 5 | |
| MS | 95.34 ± 2.81 a* | 104.13 ± 2.48 a | 124.47 ± 2.13 b |
| MS + A | 100.35 ± 1.39 ab | 104.16 ± 1.54 a | 125.12 ± 1.72 b |
| MS + P | 98.25 ± 1.64 ab | 103.16 ± 1.75 a | 118.06 ± 2.05 a |
| MS + GA3 | 102.61 ± 1.89 b | 111.96 ± 1.73 b | 142.81 ± 2.06 c |
| Culture Medium | Time (Weeks) | ||
|---|---|---|---|
| 1 | 2 | 5 | |
| MS | 86.57 ± 2.02 a* | 88.53 ± 2.31 a | 92.69 ± 2.47 a |
| MS + A | 94.9 ± 1.47 b | 95.86 ± 1.46 b | 100.26 ± 1.39 b |
| MS + P | 93.2 ± 1.3 b | 93.96 ± 1.34 b | 97.46 ± 1.47 ab |
| MS + GA3 | 90.97 ± 2.14 b | 96.06 ± 2.47 b | 107.03 ± 2.49 c |
| Culture Medium | Time (Weeks) | ||
|---|---|---|---|
| 1 | 2 | 5 | |
| MS | 0.00 ± 0.00 a* | 6.44 ± 1.86 b | 8.32 ± 2.64 a |
| MS + A | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 7.52 ± 1.07 a |
| MS + P | 0.00 ± 0.00 a | 1.07 ± 1.07 a | 6.45 ± 0.00 a |
| MS + GA3 | 11.82 ± 1.07 b | 12.09 ± 0.00 c | 29.02 ± 1.86 b |
| Culture Medium | Time (Weeks) | ||
|---|---|---|---|
| 1 | 2 | 5 | |
| MS | 0.00 ± 0.00 a* | 0.00 ± 0.00 a | 5.37 ± 1.07 a |
| MS + A | 0.00 ± 0.00 a | 1.07 ±1.07 a | 1.01 ± 1.07 a |
| MS + P | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 2.00 ± 2.00 a |
| MS + GA3 | 8.59 ± 1.07 b | 18.27 ± 2.15 b | 19.35 ± 1.86 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, M.; Trifunović Momčilov, M.; Uzelac, B.; Cingel, A.; Milošević, S.; Jevremović, S.; Subotić, A. Breaking the Dormancy of Snake’s Head Fritillary (Fritillaria meleagris L.) In Vitro Bulbs—Part 1: Effect of GA3, GA Inhibitors and Temperature on Fresh Weight, Sprouting and Sugar Content. Plants 2020, 9, 1449. https://doi.org/10.3390/plants9111449
Marković M, Trifunović Momčilov M, Uzelac B, Cingel A, Milošević S, Jevremović S, Subotić A. Breaking the Dormancy of Snake’s Head Fritillary (Fritillaria meleagris L.) In Vitro Bulbs—Part 1: Effect of GA3, GA Inhibitors and Temperature on Fresh Weight, Sprouting and Sugar Content. Plants. 2020; 9(11):1449. https://doi.org/10.3390/plants9111449
Chicago/Turabian StyleMarković, Marija, Milana Trifunović Momčilov, Branka Uzelac, Aleksandar Cingel, Snežana Milošević, Slađana Jevremović, and Angelina Subotić. 2020. "Breaking the Dormancy of Snake’s Head Fritillary (Fritillaria meleagris L.) In Vitro Bulbs—Part 1: Effect of GA3, GA Inhibitors and Temperature on Fresh Weight, Sprouting and Sugar Content" Plants 9, no. 11: 1449. https://doi.org/10.3390/plants9111449
APA StyleMarković, M., Trifunović Momčilov, M., Uzelac, B., Cingel, A., Milošević, S., Jevremović, S., & Subotić, A. (2020). Breaking the Dormancy of Snake’s Head Fritillary (Fritillaria meleagris L.) In Vitro Bulbs—Part 1: Effect of GA3, GA Inhibitors and Temperature on Fresh Weight, Sprouting and Sugar Content. Plants, 9(11), 1449. https://doi.org/10.3390/plants9111449









