Abstract
The most vital aspect of marker-assisted backcross breeding is the recurrent parent genome recovery. This enables the selection of only parents with recovered recipient/recurrent parent genome in addition to the targeted genes. The recurrent parent genome recovery (RPGR) ensures that non-desirable genomic segments are removed while the gene of interest is sustained in the recombined progenies without further segregations. This work was aimed at quantifying the RPGR of backcross populations with introgression of bacterial leaf blight resistance genes. Putra-1, a Malaysian elite variety, high yielding with inherent resistance to blast but susceptible to bacterial leaf blight (BLB), was crossed with IRBB60 which is resistant to BLB disease. The IRBB60 has four Xoo resistance genes—Xa4, xa5, xa13 and Xa21. Tightly linked polymorphic functional and SSR markers were used for foreground selection at every stage of backcrossing to select progenies with introgressed target genes. Background selection was done to quantify the percentage of RPGR in the selected lines using 79 confirmed polymorphic microsatellites. Result obtained showed that the percentage of RPGR was 80.11% at BC1F1, 95.30% at BC2F1 and 95.9% at BC2F2. The introgression of Xa4, xa5, xa13 and Xa21 Xoo resistance genes were faster through the adopted marker-assisted backcross breeding compared to what could be obtained through conventional breeding. All the 16 selected lines displayed resistance to BLB with three lines showing high resistance to the disease. The blast resistance contained in the genetic background of Putra-1 was also sustained in all the selected lines. The newly developed lines were recommended as new rice varieties for commercial cultivation.
1. Introduction
Rice is an important cereal crop that plays a critical role in human diet [1,2]. Most rice production takes place in the Asian continent with over 150 million ha cultivated all over the world [1,3]. Given a favorable environmental condition, the rice crops would grow fast and produce high yields [4]. The crop requires minimal fertilizer application. It does well in saline water with a high enough quantity of micronutrients. The availability of irrigation facilities and its further expansion, provision of subsidy for machineries, fertilizer, seeds, irrigation, as well as new technologies would lead to increased productivity of rice in various agricultural areas [5]. Singh et al. [6] reported that there will be over eight billion people in the world by 2030 and the population is further predicted to reach nine billion by 2050. The growth in population would require increase of 40% in rice production in order to avert hunger. Blast and blight are fungal and bacterial infections, respectively. Both diseases are very destructive to rice production in several rice agro-ecologies round the world. The diseases are responsible for significant yield reduction in rice production. Previous research has focused on breeding new varieties that are resistant to blast disease and high yielding, e.g., Putra-1 rice variety. The need for sustainable crop development and resistance to biotic stress caused by blast and blight are essential due to the emergence of new races of the pathogens such as Xanthomonas oryzae pv oryzae (Xoo) responsible for bacterial leaf blight (BLB) and Magnaporthe oryzae responsible for rice blast [7]. Following the success recorded from conventional breeding over the years, significant progress has been made to develop suitable cultivars that can resist various types of biotic and abiotic stress that affect rice productivity. The emergence of new biotypes necessitated the pyramiding of various resistance genes into cultivars with good agronomic value to ensure durable resistance. This enables the cultivars to resist attacks from different pathogens as well as survival in unfavorable environmental conditions.
Backcrossing is a conventional means of inserting a specific gene controlling a particular trait into an elite variety. It involves the use of two parents known as donor and recipient. The recipient parent is referred to as recurrent parent when it is used repeatedly in the crossing scheme [7]. A disease resistance gene could be transferred from one cultivar (usually not improved) to another cultivar being an elite variety [8]. Marker-assisted backcross breeding offers an efficient and precise method for breeding that preserves the vital characteristics of the recurrent parent, such as a high yielding trait. The underlying principle of marker-assisted backcrossing is to incorporate the specific gene of interest obtained from the donor into the defined locus of the recurrent parent. Marker-assisted backcrossing reduces linkage drag and helps in recovering the recurrent parent genome while simultaneously reducing the donor parent genome. Useful molecular markers could be functional markers and/or simple sequence repeats (SSR/microsatellite) markers, etc. [9]. Backcross breeding aided by marker technique is useful in incorporating disease resistance in rice without sacrificing its genetic background and this is done by several backcrossing to the recurrent parent. Recovery of the recurrent parent genome could be achieved using marker-assisted background selection. However, the high cost of molecular markers and the limitations of microsatellites in detecting polymorphisms, as well as the need for prompt execution of the whole process, are some of the constraints of marker-assisted background selection [10]. Laha et al. [11] also noted that limitations for natural screening of BLB are labor-intensive and time consuming and due to variation in the degree of natural infection. Artificial inoculation of BLB could be the most effective method of screening and it could be performed by a number of strategies such as prick inoculation of the leaves, spraying the plants with bacterial suspension, dipping the seedlings in bacterial suspension before transplanting and cutting the leaves and then spraying with bacterial suspension.
Marker-assisted backcrossing involves the use of marker to select for the target locus and subsequently enhance the recovery of recurrent parent genome [12]. On this basis, marker-assisted backcross breeding basically consists of three levels known as the foreground, background and recombinant selections [13]. The background selection accelerates the recurrent parent genome recovery (RPGR) ratio, thereby saving the breeder some cycles of selection [7]. At every stage of backcrossing, the proportion of donor parent genome is brought down by half. Hence, the RPGR percentage is expressed as a ratio to the donor parent genome recovery percentage [5,8]. Recurrent parent marker alleles could be used to select all genomic regions during background selection and target locus could also be selected using phenotypic screening. Background selection facilitates the recovery of recurrent parent genome during marker-assisted backcross breeding while further backcrossing leads to varietal development and conversion of complete line [14,15]. Therefore, the objective of this study was to quantify the recurrent parent genome recovery of the new introgression lines from the cross Putra-1 × IRBB60 using functional and SSR markers.
2. Results
2.1. Foreground Selection of F1 Hybrids and Backcross Populations
The functional marker Xa21FR revealed a total of 46 true F1 plants with heterozygous alleles from a list of 72 F1 plants produced (Figure 1). However, all the blast resistance genes, Piz, Pi2 and Pi9, and bacterial leaf blight R-genes, Xa21, xa13, xa5 and Xa4, were confirmed in only five F1 plants and were selected as hybrids for use in backcrossing. At BC1F1, a total of 108 plants out of 288 produced were confirmed to carry Xa21 BLB R-gene. Additionally, xa13, xa5 and Xa4 BLB R-genes were incorporated into 125, 118 and 122 plants, respectively. On the other hand, the blast resistance genes were confirmed in 112 plants only. The result obtained on Chi-square (χ2) analysis indicated a goodness of fit (no significant difference) to 1:1 Mendel’s segregation ratio (single gene model) for foreground markers at BC1F1 (Table 1). Selection at BC1F1 was made on nine plants only that were confirmed to carry all BLB and blast resistance genes studied, and were used for subsequent backcrossing. At BC2F1, 106 heterozygous plants out of a total of 268 produced were confirmed to carry dominant Xa21 and recessive xa13 genes each, using the functional markers Xa21FR and Xa13prom, respectively. In the same way, xa5 and Xa4 were confirmed present in 106 and 96 plants, respectively. The blast resistance genes were confirmed in 108 plants only, using the SSR markers RM6836 and RM8225. Both blast and BLB R-genes were found to be incorporated in 14 progenies only at BC2F1. Background selection revealed only nine out of the 14 plants to have sufficiently recovered their recurrent parent genome and these were selected for the next stage of crossing. A goodness of fit to a 1:1 Mendel’s ratio for a single gene model was obtained using chi-square test (Table 2).
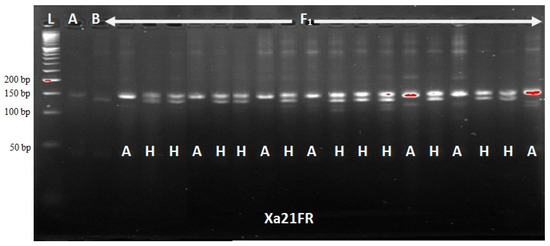
Figure 1.
Foreground selection of F1 hybrids using Xa21FR functional marker.

Table 1.
Foreground marker segregation analysis of the BC1F1 progenies.

Table 2.
Foreground marker segregation analysis of the BC2F1 progenies.
A total of 220 BC2F2 plants were grown from nine selected recurrent parent genome recovered BC2F1 lines. The result obtained from molecular genotyping showed that Xa21 gene was fixed in 17 plants. Additionally, homozygous alleles for xa13, xa5 and Xa4 BLB resistance genes were confirmed in 32, 17 and 115 plants, respectively. For blast resistance genes, homozygous alleles similar to that of recurrent parent (Putra-1) were confirmed in 69 plants only. Final selection was made from homozygous individuals carrying the donor (IRBB60) parent allele with high RPGR percentage (Figure 2 and Figure 3). Contrary to the BC2F2result for Xoo resistance, the ratio of 69:100:49 obtained for blast resistance marker segregation using chi-square analysis indicated a goodness of fit to the Mendelian expected 1:2:1 segregation ratio at BC2F2 (Table 3).
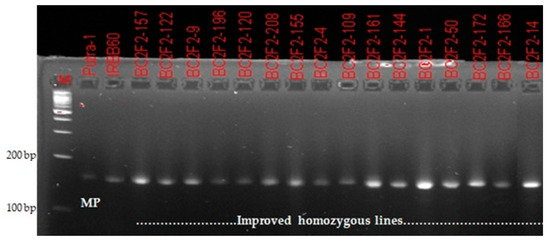
Figure 2.
Confirmation of bacterial leaf blight resistance in homozygous improved lines using the functional marker MP.
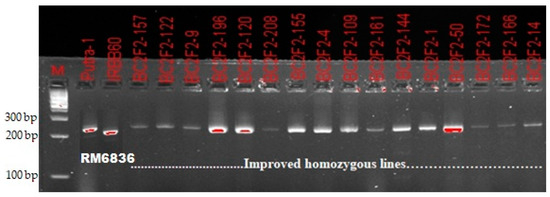
Figure 3.
Confirmation of blast resistance in homozygous improved lines using the SSR marker RM6836.

Table 3.
Foreground marker segregation analysis of the BC2F2 progenies.
2.2. Marker-Assisted Background Selection of Backcross Populations
Out of the nine progenies assessed for RPGR at BC1F1, six individuals (BC1F1-38, BC1F1-328, BC1F1-15, BC1F1-36, BC1F1-97 and BC1F1-105) with a minimum of 80% (mean recorded) RPGR and above were selected and these individuals were preferred for BC2F1 crossing [16,17]. The best progeny in BC1F1 population was BC1F1-38, with an RPGR of 86.40%, a low heterozygous component of 8.70% and a reduced donor genome of 4.90%, in addition to very negligible linkage drag. With the result obtained on RPGR from marker-assisted background selection of BC2F1 after genotyping, coupled with further confirmation through phenotyping, the nine best BC2F1 progenies with minimum RPGR of 95.31% (mean recorded) were selected. These nine best BC2F1 were chosen as recombinant parental seeds and selfed to produce BC2F2 generation.
2.3. Recurrent Parent Genome Recovery of the Selected Improved BC2F2 Lines
The result shows that the RPGR obtained at BC2F2ranged from 93.2% to 98.7% (Figure 4). The line BC2F2–4 recorded the highest RPGR. The 16 selected lines had average RPGR of 95.9% spread across the 12 chromosomes. The percentage of the donor parent genome ranged from 0.2% in BC2F2–50 to 4.3% in BC2F2–122. The mean proportion of donor parent genome recorded was 1.7%. Additionally, the proportion of heterozygous genome ranged from 0.7% in BC2F2–161 to 5.6% in BC2F2–50. This result indicated that recombination after one generation of self-fertilization from BC2F1 to BC2F2 resulted to 0.75% increase in recurrent parent genome recovery, 0.26% reduction of the donor parent genome and 0.50% reduction in the heterozygous genome proportion. The highest chromosome-wise RPGR of the improved selected lines observed in the lines BC2F2–4 is as shown in Figure 5.
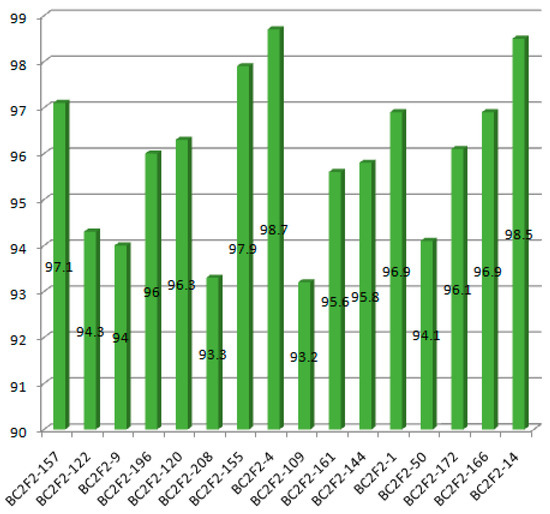
Figure 4.
Recurrent parent genome recovery percentage of the improved selected lines.
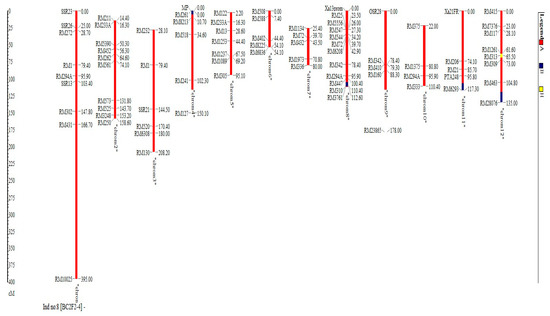
Figure 5.
Highest chromosome-wise recurrent parent genome recovery of the improved selected lines observed in BC2F2-4.
2.4. Genetic Increase of the Recurrent Parent Genome Size in Backcross Generations
The size of recurrent parent genome recorded in BC1F1 ranged from 1198.2 cM to 1566.2 cM while it ranged from 1441.5 cM to 1566.2 cM at BC2F1 generation. However, the mean recurrent parent genome size ranged from 1307.1 cM to 1510.7 cM in BC1F1 and BC2F1, respectively. This is an increase of 203.6 cM after the two successive backcross generations. Additionally, the individual with the highest RPGR at BC1F1 generation (BC1F1-38) recorded a recurrent parent genome size of 1373.9 cM, while the individual with the highest RPGR at BC2F1 (BC2F1-81) had a recurrent parent genome size of 1566.2 cM. This indicated a genetic increase in the recurrent parent genome size of 192.3 cM. These results unveil the potentials of molecular marker-assisted backcross breeding in the progressive restoration of recurrent parent genome size in backcross populations [18].
2.5. Genetic Decrease of the Donor Parent Genome Size in Backcross Generations
The genome size of the heterozygous segment ranged from 7.5 cM (BC1F1-105) to 295.7 cM (BC1F1-521) at BC1F1 generation, while it ranged from 7.5 cM (BC2F1-81) to 116.8 cM (BC2F1-198) at BC2F1 generation. The average heterozygous genome size was 150.9 cM and 42.1 cM at BC1F1 and BC2F1 generations, respectively. Additionally, the individual with the highest RPGR at BC1F1 generation recorded heterozygous genome size of 138.8 cM, while the individual with highest RPGR at BC2F1had a heterozygous genome size 7.5 cM. These results showed reduction in heterozygous genome size from BC1F1 to BC2F1. When the result obtained from the donor genome size is critically looked at, a similar trend of decrease was observed. The donor parent genome size ranged from 17.3 cM to 294.5 cM and 3.8 cM to 118.7 cM at BC1F1 and BC2F1, respectively. Mean donor parent genome sizes were 132.98 cM and 38.22 cM at BC1F1 and BC2F1 generations, respectively. The best progeny at BC1F1 recorded 78.3 cM, while the best individual at BC2F1 recorded 17.3 cM. The decrease or reduction in donor parent genome size as well as the heterozygous segment as the research progressed from BC1F1 to BC2F1 was proof that the essence of backcrossing is to reduce the donor parent genome while the recurrent parent genome is increased or recovered [7,12].
2.6. Agronomic Performance of Selected Backcross Lines
The results obtained on the agro-morphological characteristics of the improved selected lines are as presented in Table 4. The mean agro-morphological characteristics of the selected lines recorded were as follows: plant height (110.65cm), flag leaf length to width ratio (13.14), number of panicles per hill (15.94), number of days to flowering (75.75), number of days to maturity (105.8), number of effective tillers (16.06), panicle length (32.64), total number of grains per panicle (177.6), 1000 grain weight (79.45g), total grain weight per hill (52.74g), seed length to width ratio (3.86) and yield per hectare (8.44 t/ha). The result showed that the selected backcross rice lines performed better than their recurrent parents in most traits of agronomic importance. The improved lines differed significantly with their recurrent parent in all the agro-morphological traits studied.

Table 4.
Comparative agro-morphological performance of the selected improved lines.
3. Discussions
Marker-assisted background selection is useful for obtaining information on the recurrent parent genome recovery. In addition, information pertaining to the donor and heterozygous genome segments are also obtained from background screening. The aim of the plant breeder is to select individuals with the highest recurrent parent genome recovery, as such individuals received the target genes and not sacrificing their recurrent parent genes [19]. Some BC1F1 and BC2F2 progenies recorded a lower percentage of recurrent parent genome recovery compared to the theoretical mean obtained at both stages of backcrossing. Similar results have been reported by Neeraja et al. [20] and Yi et al. [21]. Additionally, Sundaram et al. [22] described a “pull” through an unknown mechanism, which could be exercised by the gene of interest in a research project that favored the transmission of additional loci from the donor gene, which would result in a percentage recurrent parent genome recovery that is less than the theoretical mean. However, most of the progenies at both BC1F1 and BC2F2met the expected MABB theoretical recurrent parent genome recovery of 79% and 92% at the two backcross generations, respectively [7,9]. The result obtained in this present study corresponds with Sabu et al. [23] and Martinez et al. [24], who reported non-significant difference between the progenies and their parents in grain yield. The number of productive tillers is responsible for the number of panicles obtained in rice [25]. There was a significant increase in panicle length and total number of grains per panicle obtained in this study. These could have contributed to the recovery of grain yield in the improved lines. The number of productive tillers and grain number per panicle have been reported to be associated with high grain yield in rice [26,27]. In the same vein, grain length and width are important quantitative traits that have close relationship with outer physical quality [28,29]. Grain length and width have also been reported to determine the shape of grain/seed [30]. On the other hand, grain shape has been reported by Shi and Zhu [31] to be concurrently influenced by triploid endosperm, maternal and cytoplasmic genes.
The highest proportion of heterozygous genome segment obtained in BC1F1 was 18.6% (BC1F1-521) with a recurrent parent genome recovery of 77.9%. The result showed a little background marker deviation of 1.1% from the theoretical 79% expected from MABB. This showed that some of the markers deviated towards the heterozygous genome segment. Lau [17] reported that there could be a preferential inheritance of IRBB60 alleles at some loci that caused the increased heterozygous segment in some progenies. This situation was more prevalent as there were about four plants out of the nine BC1F1 screened progenies that had more than the mean (10.84%) percentage in the heterozygous genome segment. However, the condition was highly reduced in BC2F1 generation, as the highest proportion of heterozygous genome segment recorded was 7.3%, while all BC2F1 progenies met the expected MABB recurrent parent genome recovery of 92.2%. The result of this study was in line with Miah et al. [19], who reported the extent of recurrent parent genome recovery of 75.40–91.3% in BC1F1 and 80.40–96.7% in BC2F2 generations. Reflinur et al. [32] observed that the role played by F1 either as male or female parent could affect the transmission pattern of alleles. They observed transmission ratio distortion at several loci at BC1F1 progenies obtained from F1 cross that involved indica x japonica with reciprocal crosses. Where F1 played the role of female parent, japonica alleles were preferably segregated during F1 meiosis at some loci while when backcrossed to indica, fertilization between the japonica embryo sac and indica pollen was highly probable. This resulted in marker segregation that skewed towards the heterozygous genome segment. In the current study, F1 and BC1F1 selected progenies were used as female plants, while Putra-1 served as the males that donated pollens. The high heterozygous genome segment observed in some BC1F1 progenies was an indication that transmission ratio distortion could have occurred during meiosis. The IRBB60 allele was preferred during meiosis at F1 and BC1F1 which caused more chances of fertilization between the embryo sac that carried the IRBB60 allele and Putra-1 pollen.
Koide et al. [33] described transmission ratio distortion as the preferential transmission of alleles where a pair of alleles is recovered preferentially in the progeny of a heterozygote and such phenomenon causes a deviation in frequency of genotypes expected from Mendelian ratio. Usually, the observed segregation distortion takes place in wide crosses such as the indica x japonica inter-subspecific cross and Basmati x indica inter-group cross [31,34]. Currently, breeders have found the mechanism involved in the transmission ratio distortion of many loci or genes in rice wide crosses. Some examples include sterility gene (S) [33,35], gametophytic gene (ga) [36] and hybrid breakdown genes (hbd) [37].Although some backcross progenies in this breeding program could have suffered from the effects of genes involved in transmission ratio distortion which favored IRBB60 alleles in a backcrossing with Putra-1, not many backcross progenies had high proportion of heterozygous genome segment, especially in BC2F1. The essence of background selection was to increase the recurrent parent genome recovery and reduce the heterozygous and donor genome segment to a substantial level. The BC1F1 and BC2F1 backcross generations could be considered as largely successful, having accelerated the mean recurrent parent genome recovery from a mean of 80.11% at BC1F1 to 95.3% at BC2F1. The selected progenies from BC2F1 population were selfed to produce BC2F2 seeds, which were planted in the next season to produce BC2F2 plants. This was essential, because self-fertilization is capable of increasing the homozygosity of non-carrier chromosomes, reduce the heterozygosity and avoid further segregation in subsequent trials [7,38,39].
4. Materials and Methods
4.1. Source of Germplasm and Breeding Procedure
The F1 hybrid was developed from a cross between Putra-1 and IRBB60 (Figure 6). Putra-1 had inherent Pi9, Pi2 and Piz blast R-genes, while IRBB60 is an IRRI variety with four Xoo R-genes, namely, Xa21, Xa4, xa13 and xa5 [40]. Putra-1 served as female/recipient, and subsequently, the recurrent parent during hybridization and backcrossing, while IRBB60 served as the male parent (donor) during hybridization only, which led to the development of F1 plants. True heterozygous F1 plants were confirmed using foreground tightly linked functional and SSR markers [40]. Putra-1 was used as recurrent parent in all the backcross generations, with the aim of recovering its high yielding trait. At every stage of backcrossing, the foreground markers which included both functional and SSR markers were used for foreground selection of specific genes of interest. Background selection was carried out with a total of 79 polymorphic SSR markers. Progenies with high recurrent parent genome recovery and reduced donor parent genome segment were selected throughout the breeding program. BC2F2 plants were selfed to recombine the genes. Figure 6 shows the MABB breeding scheme adopted in the study.
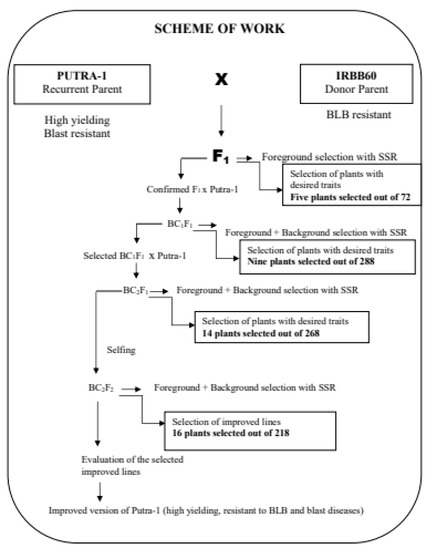
Figure 6.
Marker assisted backcross breeding program for blast and blight resistance.
4.2. Extraction of DNA and Molecular Marker Screening
After two weeks of transplanting in the glass house, samples of fresh young leaves (0.5 g) were excised from growing plants and subsequently used for genomic DNA extraction. The method of CTAB DNA extraction as proposed by Doyle and Doyle [41] and modified by Ashkani et al. [42] was adopted in this experiment. The DNA concentration, quality and purity were checked with the aid of Nanodrop spectrophotometry machine (Product specification: ND1000 Spectrophotometer USA). The protein contamination level of DNA is represented by the A260/280 ratio, while the A260/230 ratio represents the level of organic contaminants present in the nucleic acid. A 260/280 ratio of ~1.8 is generally accepted as ‘pure’ for DNA, while A260/230 from 2.0 to 2.2 is generally accepted as also ‘pure’ DNA at 230 nm absorbence. However, the most suitable samples of DNA selected for polymerase chain reaction (PCR) were those that had A260/280 purity range from 1.8 to 2.0. The presence of DNA in the extracted sample was also confirmed using gel electrophoresis. From the Image doc result displayed on computer screen, a singular high-molecular-weight DNA band was taken to be good DNA, while smeared and/or multiple allelic DNA bands were considered poor-quality DNA, and hence, not suitable for PCR. The foreground and background markers reported to be associated with resistance against blast and bacterial leaf blight were first screened for polymorphism and suitable ones were selected (Table 5). A mixture of 7.5 uL DNA master mix + 4.5 uL of nuclease free water + 1 uL of forward primer + 1 uL of reverse primer + 1 uL of DNA sample was prepared and spun for thorough mixing using the short spinning machine for 12 s. The PCR mixtures were run for 2.5 hours [10].

Table 5.
SSR markers used for background selection.
4.3. DNA Scoring
Based on the banding pattern obtained from the Gel Imager® (GelDocTM XR, Bio-Rad Lab. Inc., Hercules, CA, USA), the progenies were scored with specific reference to their parents. In the banding pattern obtained for the progenies, the progenies that followed the banding pattern of the homozygous recurrent parent was scored as ‘A,’ indicating genotypic resemblance of Putra-1 variety, while progenies that followed the banding pattern of the homozygous donor parent was scored as ‘B,’ indicating a genotypic resemblance of IRBB60 variety. However, progenies that followed heterozygous banding pattern were scored as ‘H,’ indicating a genotypic resemblance of both parents.
4.4. Foreground and Background Selections
Six linked markers out of 15 tested were confirmed to be polymorphic between the two parents for bacterial leaf blight resistance genes, while two linked markers tested were confirmed polymorphic between the two parents for blast resistance genes. The foreground markers were used to select only progenies that carried both Xoo and blast resistance genes. A total of 472 SSR markers spread across the 12 chromosomes in rice were molecularly screened for heterogeneous alleles (polymorphism). Out of the 472 screened, 79 polymorphic rice markers were identified and used for background selection. The 79 polymorphic markers were distributed across the 12 chromosomes, with each chromosome getting minimum of four polymorphic markers (Table 5).
4.5. Phenotypic Selection, Characterization for Agro-Morphological Traits and Data Analysis
At every stage of backcrossing, the whole population was subjected to phenotypic selection after carrying out foreground and background selections. The phenotypic selection was carefully done to ensure that only plants with the Xoo and blast resistance genes with clear visual phenotypic expression were selected. Agro-morphological characteristics were recorded on suitable plants for yield and yield component traits following a standard procedure described by IRRI [43]. Data obtained on foreground marker genotyping were subjected to a chi-square test using SAS software version 9.4 and compared with Mendelian genetics. Additionally, quantitative data obtained from yield and yield component traits were subjected to descriptive statistical analysis using SAS software. Maximum RPGR was determined by subjecting the genotypic data obtained from background selection with the 79 polymorphic markers to further analysis in genetic software called Graphical Genotyper (GGT 2.0) [44].
5. Conclusions
This work showed that marker-assisted backcross breeding is a useful tool for introgression of resistance genes from the donor parent into the recipient. It also revealed the potentials of foreground selection in identifying the target genes for resistance of bacterial leaf blight and blast infections. The ability of background selection in recovering the recurrent parent genome was also exposed in this study. In addition, the capability of backcross breeding method to introgress the resistance genes and also reduce the donor parent genome were evident in this breeding program. The success story of this research is the recovery of the recipient parent genome in Putra-1 and its yield in addition to the introgression of bacterial leaf blight resistance. The introgression of the Xoo R-genes were faster through the adopted marker-assisted backcross breeding compared to what could be obtained through conventional breeding. The high percentage of recurrent parent genome recovery in this experiment is an indication of the available potentials of marker-assisted backcross breeding in recovering the genomes of the recipient parent in rice and other cereal crops. From the available records, this work presents the very first attempt to manipulate the genome of the elite Malaysia rice variety “Putra-1” for bacterial leaf blight and blast resistance without jeopardizing its high yielding characteristic. Therefore, the newly improved lines are recommended as new varieties to farmers in rice growing regions.
Author Contributions
S.C.C. and M.Y.R. conceived the research idea; S.C.C. carried out the research and wrote the initial draft. S.C.C., M.Y.R., S.I.R., S.I.I., Y.O., I.M. (Isma’ila Muhammad), I.M. (Ibrahim Musa), M.A., M.I.J. and B.R.Y. contributed resources. All authors have read and agreed to the published version of the manuscript.
Funding
This experiment was sponsored by the Malaysian Government through the Higher Institution Centre of Excellence (HiCoE) Research Grant award (Grant No: 6369105).
Acknowledgments
The authors are grateful to Mohd Yusop Rafii for effective mentorship and to the Universiti Putra Malaysia for providing enabling environment for research and learning.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Hasan, M.M.; Oladosu, Y.A.; Magaji, U.G.; Akos, I.; Olalekan, K.K. Bacterial leaf blight resistance in rice: A review of conventional breeding to molecular approach. Mol. Biol. Rep. 2019, 46, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.I.; Kolapo, K. Drought Resistance in Rice from Conventional to Molecular Breeding: A Review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, Y.; Rafii, M.Y.; Fatai, A.; Chukwu, S.C.; Muhammad, I.; Kareem, I.; Salisu, M.A.; Arolu, I.W. Submergence tolerance in rice: Review of mechanism, breeding and, future prospects. Sustainability 2020, 12, 1632. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Magaji, U.; Abdullah, N.; Miah, G.; Chukwu, S.C.; Hussin, G.; Ramli, A.; Kareem, I. Genotypic and phenotypic relationship among yield components in rice undertropical conditions. Biomed Res. Int. 2018, 2018, 8936767. [Google Scholar] [CrossRef] [PubMed]
- Sabri, R.S.; Rafii, M.Y.; Ismail, M.R.; Yusuff, O.; Chukwu, S.C.; Hasan, N. Assessment of Agro-Morphologic Performance, Genetic Parameters and Clustering Pattern of Newly Developed Blast Resistant Rice Lines Tested in Four Environments. Agronomy 2020, 10, 1098. [Google Scholar] [CrossRef]
- Singh, A.K.; Sarma, B.K.; Singh, P.K.; Nandan, R. Screening of rice (Oryzasativa L.) germplasms against Xanthomonas oryzae pv. oryzae. J. Eco-Friendly Agric. 2013, 8, 86–88. [Google Scholar]
- Hasan, M.M.; Rafii, M.Y.; Ismail, M.R.; Mahmood, M.; Rahim, H.A.; Alam, M.A.; Ashkani, S.; Malek, M.A.; Latif, M.A. Marker-assisted backcrossing: A useful method for rice improvement. Biotechnol. Biotechnol. Equip. 2015, 29, 237–254. [Google Scholar] [CrossRef]
- Matthew, R. Backcrossing, Backcross (BC) Population and Backcross Breeding. In Plant Breeding and Genomics; The Ohio State University: Columbus, OH, USA, 2012. [Google Scholar]
- Akos, I.S.; Yusop, M.R.; Ismail, M.R.; Ramlee, S.I.; Shamsudin, N.A.; Ramli, A.B.; Haliru, B.S.; Muhammad, I.; Chukwu, S.C. A review on gene pyramiding of agronomic, biotic and abiotic traits in rice variety development. Int. J. Appl. Biol. 2019, 3, 65–96. [Google Scholar]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Oladosu, Y.; Muhammad, I.I.; Ubi, B.E.; Nwokwu, G. Genetic analysis of microsatellites associated with resistance against bacterial leaf blight and blast diseases of rice (Oryzasativa L.). Biotechnol. Biotechnol. Equip. 2020, 34, 898–904. [Google Scholar] [CrossRef]
- Laha, G.S.; Reddy, C.S.; Krishnaveni, D. Bacterial blight of rice and its management. In Technical Bulletin No. 41; Directorate of Rice Research (ICAR): Hyderabad, India, 2009; 37p. [Google Scholar]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Oladosu, Y.; Okporie, E.; Onyishi, G.; Utobo, E.; Ekwu, L.; Swaray, S.; et al. Marker-assisted selection and gene pyramiding for resistance to bacterial leaf blight disease of rice (Oryzasativa L.). Biotechnol Biotechnol. Equip. 2019, 33, 440–455. [Google Scholar] [CrossRef]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the 21st century. Phil. Trans. R. Soc. B 2008, 363, 557–572. [Google Scholar] [CrossRef]
- Hospital, F. Size of donor chromosome segments around introgressed loci and reduction of linkage drag in marker-assisted backcross programs. Genetics 2011, 158, 1363–1379. [Google Scholar]
- Mackill, D. From genes to farmers’ fields. Rice Today 2006, 5, 28–31. Available online: www.irri.org/publications/today/pdfs/5-4/28-31.pdf (accessed on 15 February 2020).
- Cuc, L.M.; Huyen, L.T.; Hien, P.T.; Hang, V.T.; Dam, N.Q.; Mui, P.T.; Quang, V.D.; Ismail, A.M.; Ham, L.H. Application of marker assisted backcrossing to introgress the submergence tolerance QTLSUB1 into the Vietnamelite rice variety-AS996. Am. J. Plantscience 2012, 3, 528. [Google Scholar]
- Lau, W.C.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.; Latif, M.A.; Asfaliza, R.; Miah, G. Development of advanced fragrant rice lines from MR269 × Basmati 370 through marker-assisted backcrossing. Euphytica 2017, 213, 11. [Google Scholar] [CrossRef]
- Kazeem, K.O.; Rafii, M.Y.; Azrul, M.S.; Mahmud, T.M.M.; Khairulmazmi, A.; Azizah, M.; Tanweer, F.A.; Oladosu, Y.; Ibrahim, W.A.; Chukwu, S.; et al. Analysis of Recurrent Parent Genome Recovery in Marker-Assisted Backcross Breeding Program in Watermelon. Int. J. Sci. Technol. Res. 2019, 8, 945–955. [Google Scholar]
- Miah, G.; Rafii, M.Y.; Ismail, M.R.; Puteh, A.B.; Rahim, H.A.; Latif, M.A. Recurrent parent genome recovery analysis in a marker-assisted backcrossing program of rice (Oryzasativa L.). Comptes Rendus Biol. 2015, 338, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Neeraja, C.N.; Maghirang-Rodriguez, R.; Pamplona, A.; Heuer, S.; Collard, B.C.; Septiningsih, E.M.; Vergara, G.; Sanchez, D.; Xu, K.; Ismail, A.M.; et al. A marker-assisted backcross approach for developing submergence-tolerant rice cultivars. Theor. Appl. Genet. 2007, 115, 767–776. [Google Scholar] [CrossRef]
- Yi, M.; Nwe, K.T.; Vanavichit, A.; Chai-arree, W.; Toojinda, T. Marker assisted backcross breeding to improve cooking quality traits in Myanmar rice cultivar Manawthukha. Field Crop. Res. 2009, 113, 178–186. [Google Scholar] [CrossRef]
- Sundaram, R.M.; Vishnupriya, M.R.; Biradar, S.K.; Laha, G.S.; Reddy, G.A.; Rani, N.S.; Sarma, N.P.; Sonti, R.V. Marker assisted introgression of bacterial blight resistance in Samba Mahsuri, an elite indica rice variety. Euphytica 2008, 160, 411–422. [Google Scholar] [CrossRef]
- Sabu, K.K.; Abdullah, M.Z.; Lim, L.S.; Wickneswari, R. Development and evaluation of advanced backcross families of rice for agronomically important traits. Commun. Biometry Crop Sci. 2006, 1, 111–123. [Google Scholar]
- Martínez, C.P.; Tohme, J.; López, J.; Borrero, J.; McCouch, S.R.; Almeida, A. Identification and utilization of genes from wild rice germplasm for the improvement of yield and stress resistance. In Proceedings of the 27th Rice Technical Working Group, Reno, NV, USA, 1–4 March 1998. [Google Scholar]
- Hossain, M.B.; Islam, M.O.; Hasanuzzaman, M. Influence of different nitrogen levels on the performance of four aromatic rice varieties. Int. J. Agric. Biol. 2008, 10, 693–696. [Google Scholar]
- Dutta, R.K.; Mia, M.B.; Khanam, S. Plant architecture and growth characteristics of fine grain and aromatic rice sand the irrelation with grain yield. Int. Rice Comm. Newsl. 2002, 51, 51–55. [Google Scholar]
- Kusutani, A.; Tovata, M.; Asanuma, K.; Cui, J. Studies on the varietal differences of harvest index and morphological characteristics of rice. Jpn. J. Crop Sci. 2000, 69, 359–364. [Google Scholar]
- Shi, C.; Zhu, J.; Wu, J.; Fan, L. Genetic and genotype × environment interaction effects from embryo, endosperm, cytoplasm and maternal plant for rice grain shape traits of indica rice. Field Crops Res. 2000, 68, 191–198. [Google Scholar] [CrossRef]
- Sarif, H.M.; Rafii, M.Y.; Ramli, A.; Oladosu, Y.; Musa, H.M.; Rahim, H.A.; Zuki, Z.M.; Chukwu, S.C. Genetic diversity and variability among pigmented rice germplasm using molecular marker and morphological traits. Biotechnol. Biotechnol. Equip. 2020. [Google Scholar] [CrossRef]
- Juliano, B.O. Rice in Human Nutrition. Food and Agriculture Organization of the United Nations, Rome (FAO), International Rice Research Institute. Available online: http://books.irri.org/9251031495_content.pdf.1993 (accessed on 6 November 2019).
- Shi, C.; Zhu, J. Genetic analysis of endosperm, cytoplasmic and maternal effects for exterior quality traits in indica rice. J. Biomath. 1996, 11, 73–81. [Google Scholar]
- Reflinur, K.B.; Jang, S.M.; Chu, S.H.; Bordiya, Y.; Akter, M.B.; Lee, J.; Chin, J.H.; Koh, H.J. Analysis of segregation distortion and its relationship to hybrid barriers in rice. Rice 2014, 7, 3. [Google Scholar] [CrossRef]
- Koide, Y.; Onishi, K.; Nishimoto, D.; Baruah, A.R.; Kanazawa, A.; Sano, Y. Sex-independent transmission ratio distortion system responsible for reproductive barriers between Asian and African rice species. New Phytol. 2008, 179, 888–900. [Google Scholar] [CrossRef]
- Shanmugavadivel, P.S.; Mithra, S.A.; Dokku, P.; Kumar, K.A.; Rao, G.J.; Singh, V.P.; Singh, A.K.; Singh, N.K.; Mohapatra, T. Mapping quantitative trait loci (QTL) for grain size in rice using a RIL population from Basmati × indica cross showing high segregation distortion. Euphytica 2013, 194, 401–416. [Google Scholar] [CrossRef]
- Chen, J.; Ding, J.; Ouyang, Y.; Du, H.; Yang, J.; Cheng, K.; Zhao, J.; Qiu, S.; Zhang, X.; Yao, J.; et al. Atriallelic system of S5 is a major regulator of the reproductive barrier and compatibility of indica–japonica hybrids in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 11436–11441. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Ko, P.Y.; Lee, W.C.; Wei, F.J.; Kuo, S.C.; Ho, S.W.; Hour, A.L.; Hsing, Y.I.; Lin, Y.R. Comparative analyses of linkage maps and segregation distortion of two F2 populations derived from japonica crossed with indicarice. Hereditas 2010, 147, 225–236. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kimura, T.; Terakami, S.; Nishitani, C.; Sawamura, Y.; Saito, T.; Kotobuki, K.; Hayashi, T. Integrated reference genetic link age maps of pear based on SSR and AFLP markers. Breed. Sci. 2007, 57, 321–329. [Google Scholar] [CrossRef]
- Rajpurohit, D.; Kumar, R.; Kumar, M.; Paul, P.; Awasthi, A.; Basha, P.O.; Puri, A.; Jhang, T.; Singh, K.; Dhaliwal, H.S. Pyramiding of two bacterial blight resistance and a semi dwarfing gene in Type 3 Basmatiusing marker-assisted selection. Euphytica 2011, 178, 111–126. [Google Scholar] [CrossRef]
- Okporie, E.O.; Chukwu, S.C.; Onyishi, G.C. Phenotypic recurrent selection for increase yield and chemical constituents of maize (Zea mays L.). World Appl. Sci. J. 2013, 21, 994–999. [Google Scholar]
- Chukwu, S.C.; Rafii, M.Y.; Ramlee, S.I.; Ismail, S.I.; Oladosu, Y.A.; Olalekan, K.K.; Musa, I.; Halidu, J.; Muhammad, I.; Ahmed, M. Marker-assisted introgression of multiple resistance genes confers broad spectrum resistance against bacterial leaf blight and blast diseases in Putra-1 rice variety. Agronomy 2020, 10, 42. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 39–40. [Google Scholar]
- Ashkani, S.; Rafii, M.Y.; Rusli, I.; Sariah, M.; Abdullah, S.N.A.; Rahim, H.A.; Latif, M.A. SSRs for Marker-Assisted Selection for Blast Resistance in Rice (Oryzasativa L.). Plant Mol. Biol. Report. 2012, 30, 79–86. [Google Scholar] [CrossRef]
- IRRI. Standard Evaluation System for Rice, 4th ed.; International Rice Research Institute: Manila, Philippines, 1996. [Google Scholar]
- van Berloo, R. GGT2.0: Versatile software for visualization and analysis of genetic data. J. Hered. 2008, 99, 232–236. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).