Do Compositions of Lipid Fraction Correspond to Species Differentiation in Bupleurum L. (Apiaceae)?
Abstract
1. Introduction
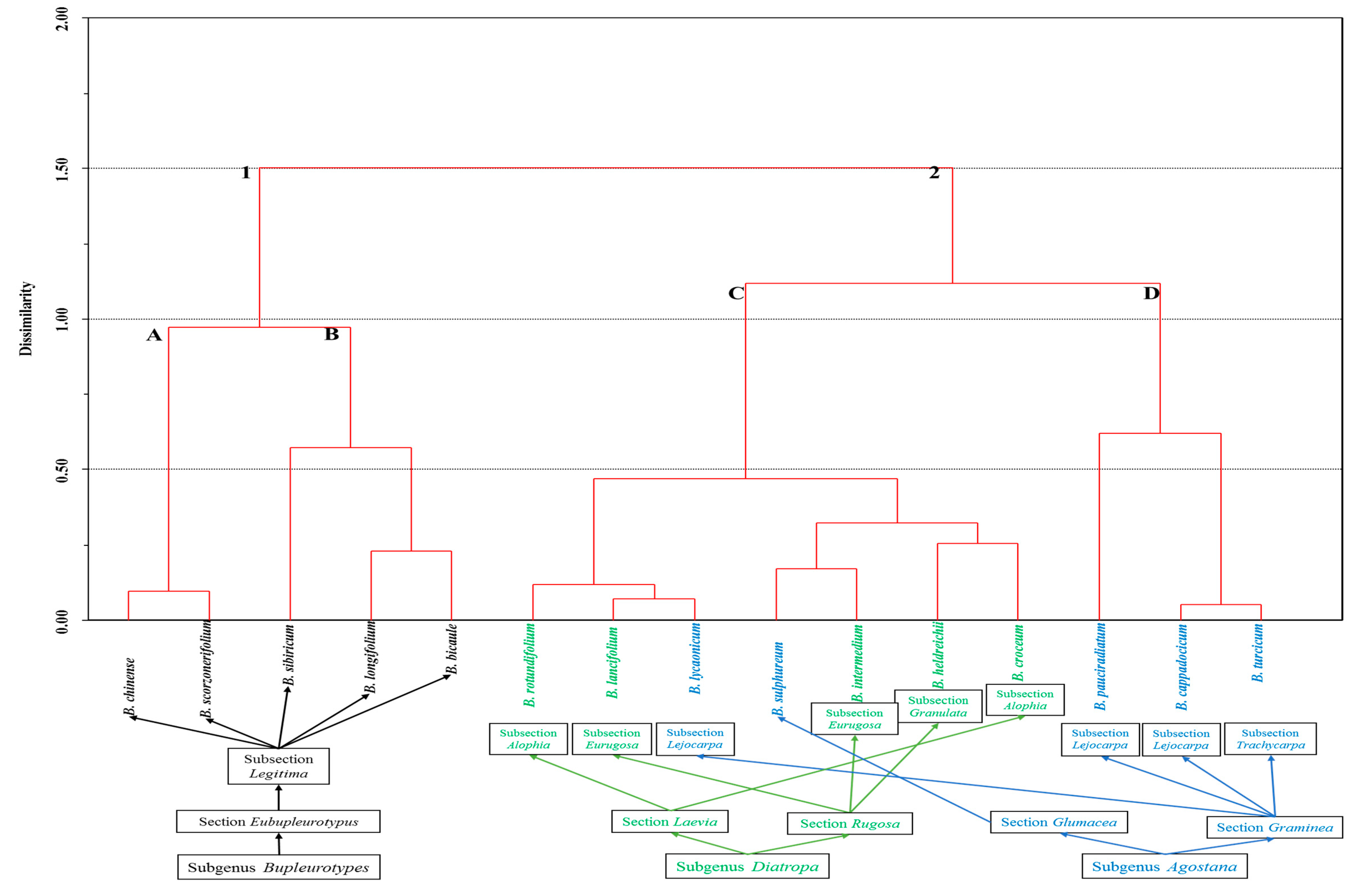
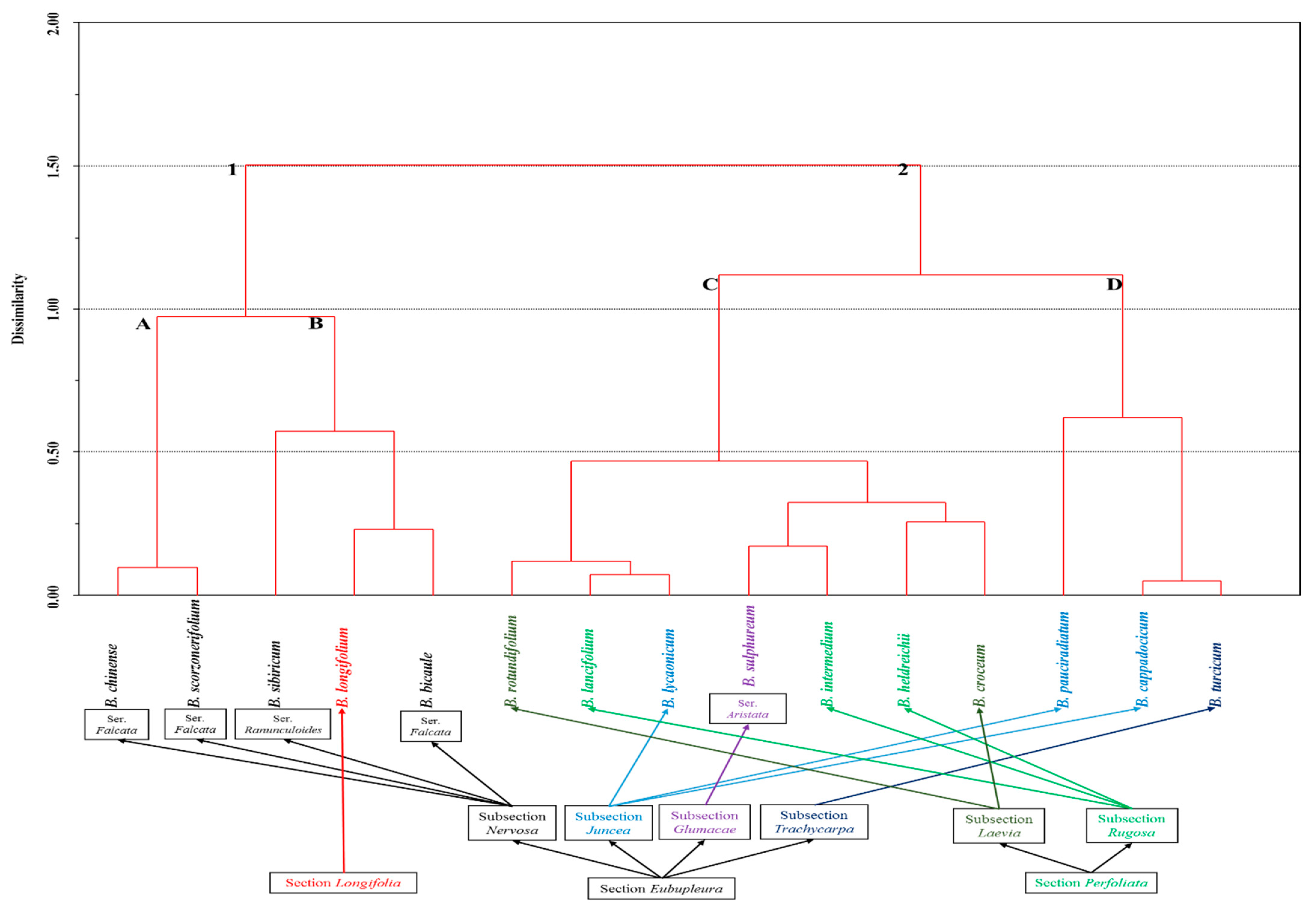
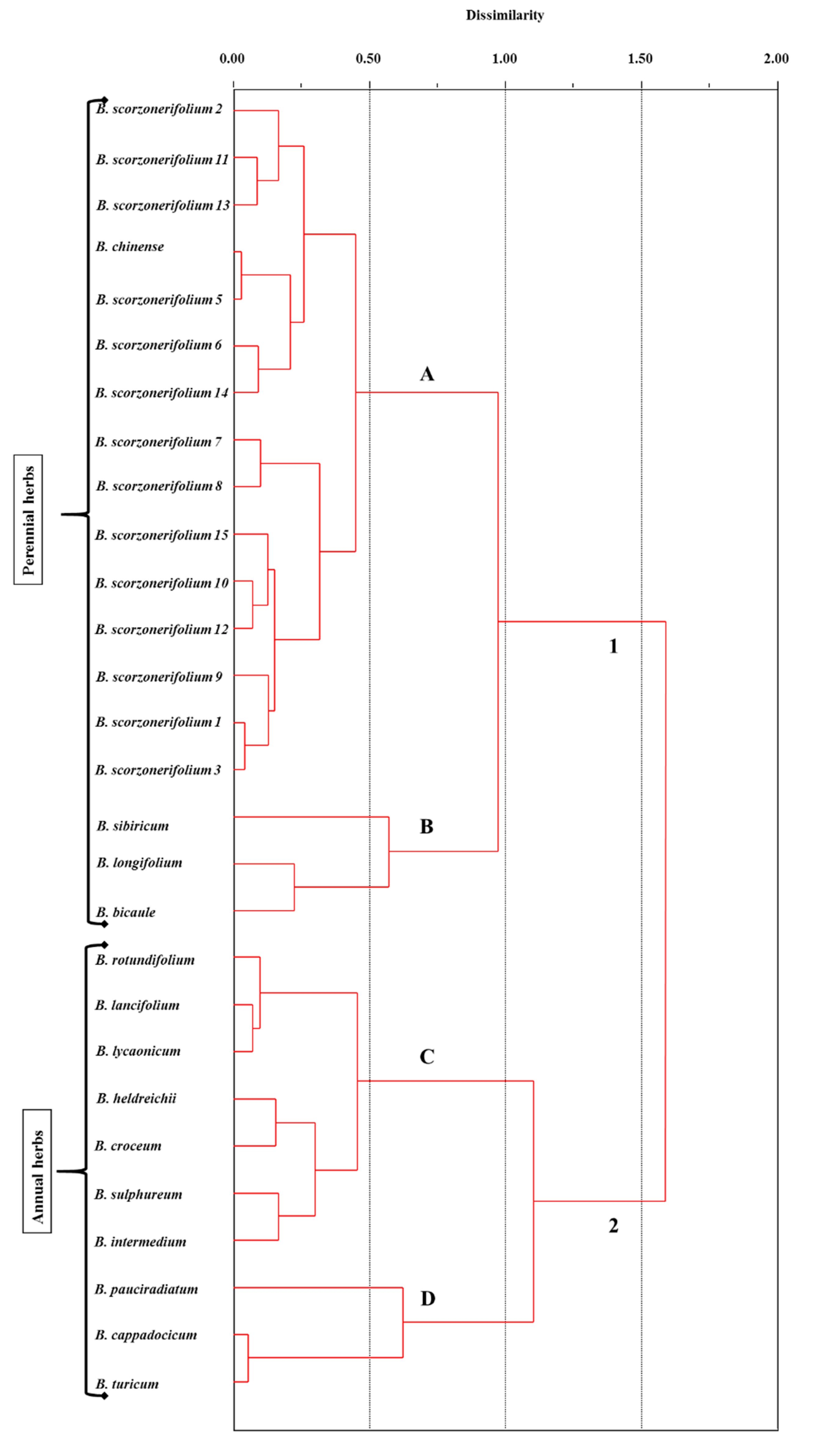
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Species | Voucher Number or Mark or Sample No | Country | Locality | Analyzed Parts | Collection Period | Source of Data |
|---|---|---|---|---|---|---|
| B. longifolium | UUH018720, UUH018721, UUH018722 | Russia | Headstream of Bolshoy Mamay river, Kabanskiy district, Buryatia | Aerial part | August 2019 | Present study |
| B. chinense | TZA-2015-8 | China | 49 km northeast of Xining, Qinghai province | Aerial part | July 2015 | Present study |
| Roots | July 2015 | [17] | ||||
| – * | Liaoning province, Shenyang | Roots | – | [19] | ||
| B. bicaule | – | Russia | Mukhorshibirskiy district, Buryatia | Aerial part and roots | July 2018 | [15] |
| B. cappadocicum | HT1008 KNYA | Turkey | Karaman: Karaman–Mut road, Central Anatolia region | Aerial part | May and June 2009 | [21] |
| B. croceum | HT1007 KNYA | Turkey | Konya: Altinapa, around Değirmenkoy, Central Anatolia region | Aerial part | May and June 2009 | [21] |
| – | Native populations distributed in C5, C2, A1, and C2 squares, according to the grid system of Turkey | Fruits | 2011–2012 | [14] | ||
| TU42168 | – | – | Seed and pericarp | – | [22] | |
| B. falcatum | – | – | – | Fruits | – | [23] |
| – | – | – | Seeds | – | [18] | |
| B. flavum | – | Turkey | Native populations distributed in C5, C2, A1, and C2 squares, according to the grid system of Turkey | Fruits | – | [14] |
| B. heldreichii | HT1006 KNYA | Turkey | Central Anatolia region Saracoglu, | Aerial part | May and June 2009 | [20] |
| B. beldreichii ** | TU41276 | – | – | Seed and pericarp | – | [22] |
| B. intermedium | HT1010 KNYA | Turkey | Karaman, Ermenek–Kazanci road, Central Anatolia region | Aerial part | May and June 2009 | [21] |
| B. lanceolatum | PA44128 | – | – | Seed and pericarp | – | [22] |
| B. lancifolium | HT1009 KNYA | Turkey | Karaman, Ermenek–Kazanci road, Central Anatolia region | Aerial part | May and June 2009 | [21] |
| B-412 | Iran | Khalkhal–Ardabil road, Ardabil province | Leaf and seed | August 2010 | [24] | |
| IS42967TU46887 | – | – | Seed and pericarp | – | [22] | |
| B. longiradiatum | K044420 | – | – | Seed and pericarp | – | [22] |
| B. lycaonicum | HT1004 KNYA | Turkey | Central Anatolia region | Aerial part | May and June 2009 | [20] |
| B. odontites | TU46886 | – | – | Seed and pericarp | – | [22] |
| B. pauciradiatum | HT1002 KNYA | Turkey | Central Anatolia region | Aerial part | May and June 2009 | [20] |
| B. rotundifolium | HT1001 KNYA | Turkey | Konya: in the center of Bozku village, Central Anatolia region | Aerial part | May and June 2009 | [21] |
| – | Native populations distributed in C5, C2, A1, and C2 squares, according to the grid system of Turkey | Fruits | – | [14] | ||
| – | – | - | Fruits | – | [23] | |
| B. scorzonerifolium | TZA-2014-1 | Russia | 1. Surroundings of the Sotnikovo village, Ivolginsky district, Buryatia | Aerial part and roots | July 2014 | [16,17] |
| TZA-2015-1 | 2. Surroundings of the Sotnikovo village, Ivolginsky district, Buryatia | Aerial part | June 2015 | [16] | ||
| UUH017463 | 3. Surroundings of the Sotnikovo village, Ivolginsky district, Buryatia | Aerial part | August 2017 | [16] | ||
| TZA-2015-2 | 5. Surroundings of the Zagustay arbor, Selengisky district, Buryatia | Aerial part and roots | June 2015 | [16,17] | ||
| TZA-2015-3 | 6. Surroundings of the Georgievka village, Khorinsk district, Buryatia | Aerial part and roots | July 2015 | [16,17] | ||
| UUH017680 | 7. Surroundings of the Oninoborsk village, Khorinsk district, Buryatia | Aerial part | August 2016 | [16] | ||
| UUH017349 | 8. Surroundings of the Shiringa village, Eravninsky district, Buryatia | Aerial part | July 2016 | [16] | ||
| TZA-2014-3 | 9. Surroundings of the Nizhniy Tsasuchey village, Ononsky district, Trans-Baikal region | Aerial part | July 2014 | [16] | ||
| TZA-2014-4 | Mongolia | 10. Surroundings of the Bayan Ulaan Uul mountain, Khentii aimag | Aerial part | August 2014 | [16] | |
| TZA-2015-4 | 11. Surroundings of the Bayan Ulaan Uul mountain, Khentii aimag | Aerial part and roots | August 2015 | [16,17] | ||
| TZA-2014-5 | 12. Surroundings of the Berkh territory, Khentii aimag | Aerial part | August 2014 | [16] | ||
| TZA-2015-5 | 13. Surroundings of the Berkh territory, Khentii aimag | Aerial part and roots | August 2015 | [16,17] | ||
| TZA-2014-6 | 14. In 20 km to the northeast from the Berkh territory, Khentii aimag | Aerial part | July 2014 | [16] | ||
| TZA-2014-7 | 15.Surroundings of the Huh nuur Lake, Khentii aimag | Aerial part | July 2014 | [16] | ||
| B. sulphureum | HT1003 KNYA | Turkey | Central Anatolia region | Aerial part | May and June 2009 | [20] |
| B. tenue *** | PA44272 | – | – | Seed and pericarp | – | [22] |
| B. turcicum | HT1005 KNYA | Turkey | Central Anatolia region | Aerial part | May and June 2009 | [20] |
References
- Yao, R.-Y.; Zou, Y.-F.; Chen, X.-F. Traditional Use, Pharmacology, Toxicology, and Quality Control of Species in Genus Bupleurum L. Chin. Herb. Med. 2013, 5, 245–255. [Google Scholar] [CrossRef]
- Pimenov, M.G.; Ostroumova, T.A. Umbelliferae of Russia; KMK Scientific Press: Moscow, Russia, 2012; pp. 48–69. [Google Scholar]
- Zhang, T.; Zhou, J.; Wang, Q. Flavonoids from aerial part of Blupleurum chinense DC. Biochem. Syst. Ecol. 2007, 35, 801–804. [Google Scholar] [CrossRef]
- Yuan, B.; Yang, R.; Ma, Y.; Zhou, S.; Zhang, X.; Liu, Y. A systematic review of the active saikosaponins and extracts isolated from Radix Bupleuri and their applications. Pharm. Biol. 2017, 55, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Minaeva, V.G. Lekarstvennye Rasteniya Sibiri (Medicinal Plants of Siberia); Publ. Nauka (Sib. Office): Novosibirsk, Russia, 2012; pp. 56–58. [Google Scholar]
- Panin, V.P.; Martynchik, I.A.; Panina, M.I.; Ferubko, E.V. The pharmacological studying of the influence of Bupleurum aureum L. on cardiovascular and nervous systems. Probl. Biol. Med. Pharm. Chem. 2018, 21, 16–21. [Google Scholar] [CrossRef]
- Kurmanova, E.N.; Ferubko, E.V.; Strelkova, L.B.; Panin, V.P.; Kurmanov, R.K.; Panina, M.I. The hepatoprotective activity of Bupleurum aureum L. at the experimental model operation of liver pathology. Probl. Biol. Med. Pharm. Chem. 2018, 21, 45–51. [Google Scholar] [CrossRef]
- Finley, J.W.; Shahidi, F. The Chemistry, Processing, and Health Benefits of Highly Unsaturated Fatty Acids: An Overview; ACS Symp. Ser.: Washington, DC, USA, 2001; Volume 788, pp. 2–11. [Google Scholar]
- Koso-Polljanski, B. Epitome Bupleurorum Rossiae. Tabilus 1–14. Acta Horti. Petropolitani. 1915, 30, 135–333. [Google Scholar]
- Wolff, H. Bupleurum. In Das Pflanzenreich Regnis Vegetabilis Conspectus; Engler, A., Ed.; Forgotten Books: London, UK, 1910; Volume IV, p. 228. [Google Scholar]
- Li, D.; Yue, D.; Liu, D.; Liu, X.; Song, S. Chemical constituents from Bupleurum chinense and their chemotaxonomic significance. Biochem. Syst. Ecol. 2019, 86, e103929. [Google Scholar] [CrossRef]
- Tian, R.; Xie, P.; Liu, H. Evaluation of traditional Chinese herbal medicine: Chaihu (Bupleuri Radix) by both high-performance liquid chromatographic and high-performance thin-layer chromatographic fingerprint and chemometric analysis. J. Chromatogr. A 2009, 1216, 2150–2155. [Google Scholar] [CrossRef]
- Gorovoy, P.G.; Ulanova, K.P. Systematika i chemotaxonomiya dal’nevostochnykh vydov Bupleurum L. Komar. Chteniya 1969, XV–XVII, 143–154. [Google Scholar]
- Ozcan, T. Patterns of lipid biomarkers in the fruit of Bupleurum L. growing wild in Turkey. Plant Biosyst. 2016, 150, 459–467. [Google Scholar] [CrossRef]
- Tykheev, Z.A.; Taraskin, V.V.; Chimitov, D.G.; Anenkhonov, O.A.; Zhigzhitzhapova, V.S.; Radnaeva, L.D. Composition of Lipid Fraction from Bupleurum bicaule and B. sibiricum. Chem. Nat. Compd. 2019, 55, 712–713. [Google Scholar] [CrossRef]
- Tykheev, Z.A.; Zhigzhitzhapova, S.V.; Zhang, F.; Taraskin, V.V.; Anenkhonov, O.A.; Radnaeva, L.D.; Chen, S. Constituents of Essential Oil and Lipid Fraction from the Aerial Part of Bupleurum scorzonerifolium Willd. (Apiaceae) from Different Habitats. Molecules 2018, 23, 1496. [Google Scholar] [CrossRef]
- Tykheev, Z.A.; Taraskin, V.V.; Radnaeva, L.D.; Zhang, F.Q.; Chen, S.L. Composition of Lipids from Roots of Bupleurum scorzonerifolium and B. chinense. Chem. Nat. Compd. 2017, 53, 937–938. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Wang, H.; Yang, C. Chemical constituents analysis on the seeds of Bupleurum falcatum L. Chin. J. Anal. Chem. 2000, 28, 1083–1084. [Google Scholar]
- Li, X.; Song, A.; Li, W.; Chen, X.; Bi, K. Analysis of the fatty acid from Bupleurum chinense DC. in China by GC-MS and GC-FID. Chem. Pharm. Bull. 2005, 53, 1613–1617. [Google Scholar] [CrossRef]
- Saracoglu, H.T.; Zengin, G.; Akin, M.; Aktumsek, A. Evaluation of oil content and fatty acid composition of five endemic Bupleurum species growing in the Central Anatolia region of Turkey. Nat. Prod. Res. 2012, 26, 1188–1194. [Google Scholar] [CrossRef]
- Saracoglu, H.T.; Zengin, G.; Akin, M.; Aktumsek, A. A comparative study on the fatty acid composition of the oils from five Bupleurum species collected from Turkey. Turk. J. Biol. 2012, 36, 527–532. [Google Scholar] [CrossRef]
- Kleiman, R.; Spencer, G.F. Search for new industrial oils: XVI. Umbelliflorae—Seed oils rich in petroselinic acid. J. Am. Oil Chem. Soc. 1982, 59, 29–38. [Google Scholar] [CrossRef]
- Azimova, S.S.; Glushenkova, A.I.; Vinogradova, V.I. Lipids, Lipophilic Components and Essential Oils from Plant Sources; Springer: London, UK, 2012; pp. 14–16. [Google Scholar] [CrossRef]
- Shafaghat, A. Antioxidant, antimicrobial activities and fatty acid components of leaf and seed of Bupleurum lancifolium Hornem. J. Med. Plants Res. 2011, 5, 3758–3762. [Google Scholar]
- Tevini, M. Light, Function, and Lipids During Plastid Development; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Galston, A.W.; Davies, P.J.; Satter, R.L. The Life of the Green Plant; Benjamin Cummings: San Francisco, CA, USA, 1980. [Google Scholar]
- Gurr, M.I.; Harwood, J.L. Lipid Biochemistry; Springer: New York, NY, USA, 1991. [Google Scholar]
- Grechkin, A.N.; Tarchevsky, I.A. The lipoxygenase signaling system. Russ. J. Plant Physiol. 1999, 46, 114–123. [Google Scholar]
- Neves, S.S.; Watson, M.F. Phylogenetic relationships in Bupleurum (Apiaceae) based on nuclear ribosomal DNA ITS sequence data. Ann. Bot. 2004, 93, 379–398. [Google Scholar] [CrossRef]
- Neves, S.S. Taxonomy and Evolution in Bupleurum L. (Umbelliferae) in the West Mediterranean and Macaronesia. Ph.D. Thesis, University of Edinburgh, Edinburgh, UK, 2000. [Google Scholar]
- Linchevskii, L.A. Rod Volodushka—Bupleurum L. In Flora of the USSR (Flora SSSR); Israel Program for Scientific Translations: Jerusalem, Israel, 1950; Volume 16, pp. 275–349. [Google Scholar]
- Chubarov, I. Genus Bupleurum L. in the Altay mountain country. Turczaninowia 2004, 7, 53–70. [Google Scholar]
- Korolyuk, Y.A. Syntaxonomy of steppe vegetation of the Republic of Buryatia. Veg. Russ. 2017, 31, 2–32. [Google Scholar]
- Korolyuk, Y.A. Steppes of the class Cleistogenetea squarrosae Mirkin et al. ex Korotkov et al. 1991 in Eastern Transbaikalia. Veg. Russ. 2019, 35, 28–60. [Google Scholar] [CrossRef]
- Tutin, T.G. Bupleurum L. In Flora Europea, Vol. 2: Rosaceae to Umbelliferae; Cambridge University Press: Cambridge, UK, 1968; pp. 345–350. [Google Scholar]
- Kates, M.; Works, T.S. Techniques of lipidology: Isolation, analysis, and identification of lipids. Lab. Tech. Biochem. Mol. Biol. 1972, 3, 151–155. [Google Scholar]
- Kvalheim, O.M.; Karstang, T.V. A general-purpose program for multivariate data analysis. Chemom. Intell. Lab. Syst. 1987, 2, 235–237. [Google Scholar] [CrossRef]
| B. chinense | B. longifolium | |
|---|---|---|
| Yield of Lipids, w/w (%) | 9.73 | 3.02 |
| Compound | Peak Area % (Percentage) | |
| Saturated fatty acids (SFA) | ||
| 10:0 | 0.12 | |
| Nonanedioic acid | 0.39 | 0.36 |
| 12:0 | 0.55 | 0.90 |
| 14:0 | 1.10 | 3.31 |
| 15:0 | 1.59 | |
| 16:0 | 18.32 | 20.61 |
| 17:0 | 0.83 | |
| 18:0 | 1.98 | 3.35 |
| 19:0 | 0.18 | |
| 20:0 | 0.52 | 1.19 |
| 21:0 | 0.33 | |
| 22:0 | 1.00 | 2.67 |
| 23:0 | 0.37 | 2.24 |
| 24:0 | 1.18 | 3.02 |
| 25:0 | 0.55 | |
| 26:0 | 0.64 | 4.65 |
| ∑SFA | 26.05 | 45.91 |
| Monounsaturated fatty acids (MUFA) | ||
| 16:1n7 | 1.85 | |
| 16:1n9 | 1.54 | 1.14 |
| 17:1n10 | 0.19 | |
| cis18:1n9 | 25.30 | 16.14 |
| trans18:1n9 | 0.88 | |
| 20:1n1 | 0.50 | |
| ∑MUFA | 28.22 | 19.32 |
| Polyunsaturated fatty acids (PUFA) | ||
| 16:3n7 | 2.53 | |
| 18:3n6 | 0.50 | |
| 18:2n9 | 33.82 | 21.83 |
| 20:3n8 | 0.52 | |
| ∑PUFA | 36.85 | 22.35 |
| Others | ||
| 6-Tridecene-4-yne | 0.78 | |
| 10-Nonadecanon | 7.12 | 12.42 |
| Stigmasterol | 0.62 | |
| β-Sitosterol | 0.36 | |
| ∑Others | 8.88 | 12.42 |
| Total identified | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tykheev, Z.A.; Anenkhonov, O.A.; Zhigzhitzhapova, S.V.; Taraskin, V.V.; Radnaeva, L.D.; Zhang, F. Do Compositions of Lipid Fraction Correspond to Species Differentiation in Bupleurum L. (Apiaceae)? Plants 2020, 9, 1407. https://doi.org/10.3390/plants9111407
Tykheev ZA, Anenkhonov OA, Zhigzhitzhapova SV, Taraskin VV, Radnaeva LD, Zhang F. Do Compositions of Lipid Fraction Correspond to Species Differentiation in Bupleurum L. (Apiaceae)? Plants. 2020; 9(11):1407. https://doi.org/10.3390/plants9111407
Chicago/Turabian StyleTykheev, Zhargal Alexandrovich, Oleg Arnoldovich Anenkhonov, Svetlana Vasilievna Zhigzhitzhapova, Vasiliy Vladimirovich Taraskin, Larisa Dorzhievna Radnaeva, and Faqi Zhang. 2020. "Do Compositions of Lipid Fraction Correspond to Species Differentiation in Bupleurum L. (Apiaceae)?" Plants 9, no. 11: 1407. https://doi.org/10.3390/plants9111407
APA StyleTykheev, Z. A., Anenkhonov, O. A., Zhigzhitzhapova, S. V., Taraskin, V. V., Radnaeva, L. D., & Zhang, F. (2020). Do Compositions of Lipid Fraction Correspond to Species Differentiation in Bupleurum L. (Apiaceae)? Plants, 9(11), 1407. https://doi.org/10.3390/plants9111407






