Expression Analysis of Key Auxin Biosynthesis, Transport, and Metabolism Genes of Betula pendula with Special Emphasis on Figured Wood Formation in Karelian Birch
Abstract
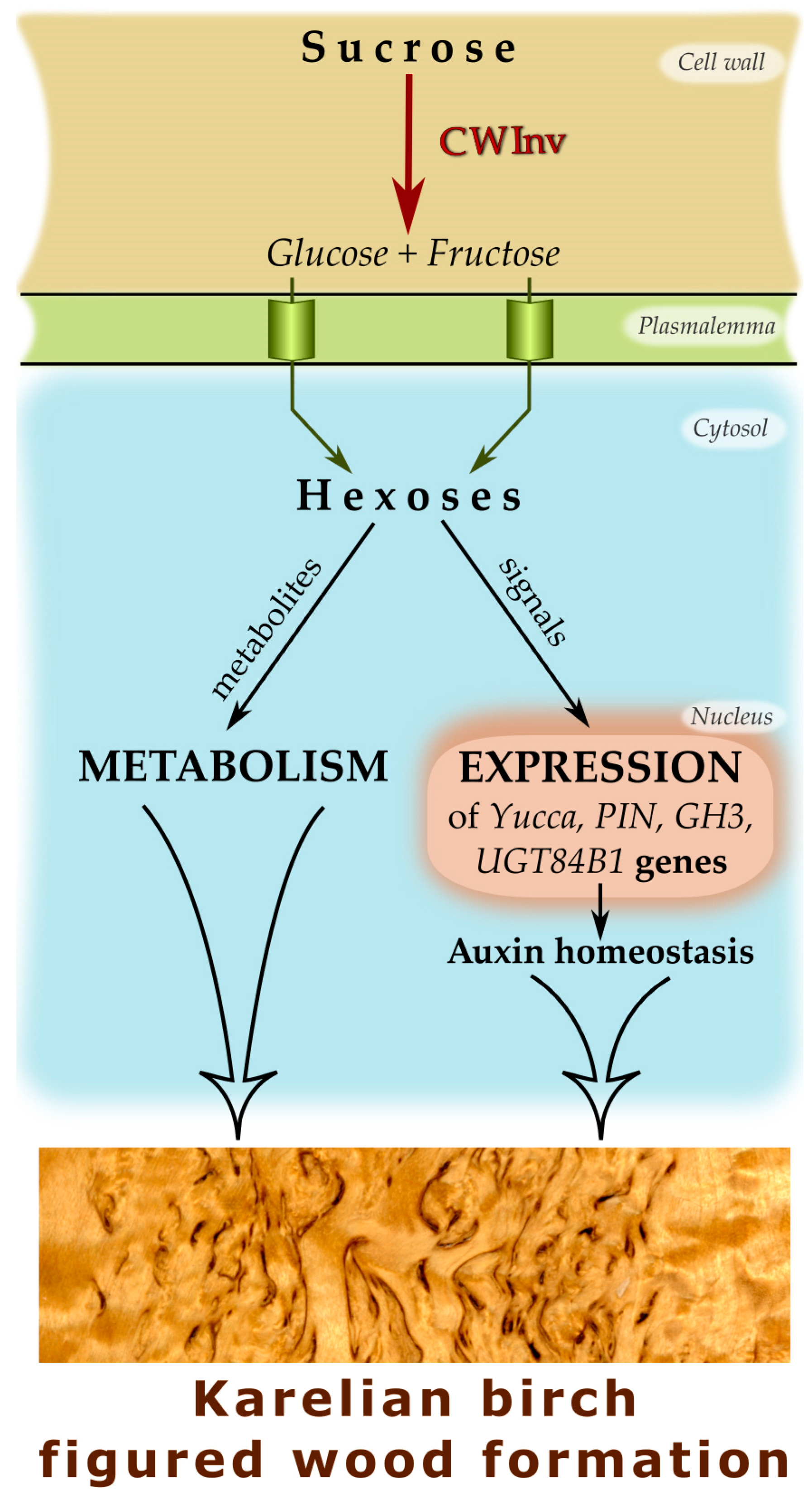
1. Introduction
2. Results
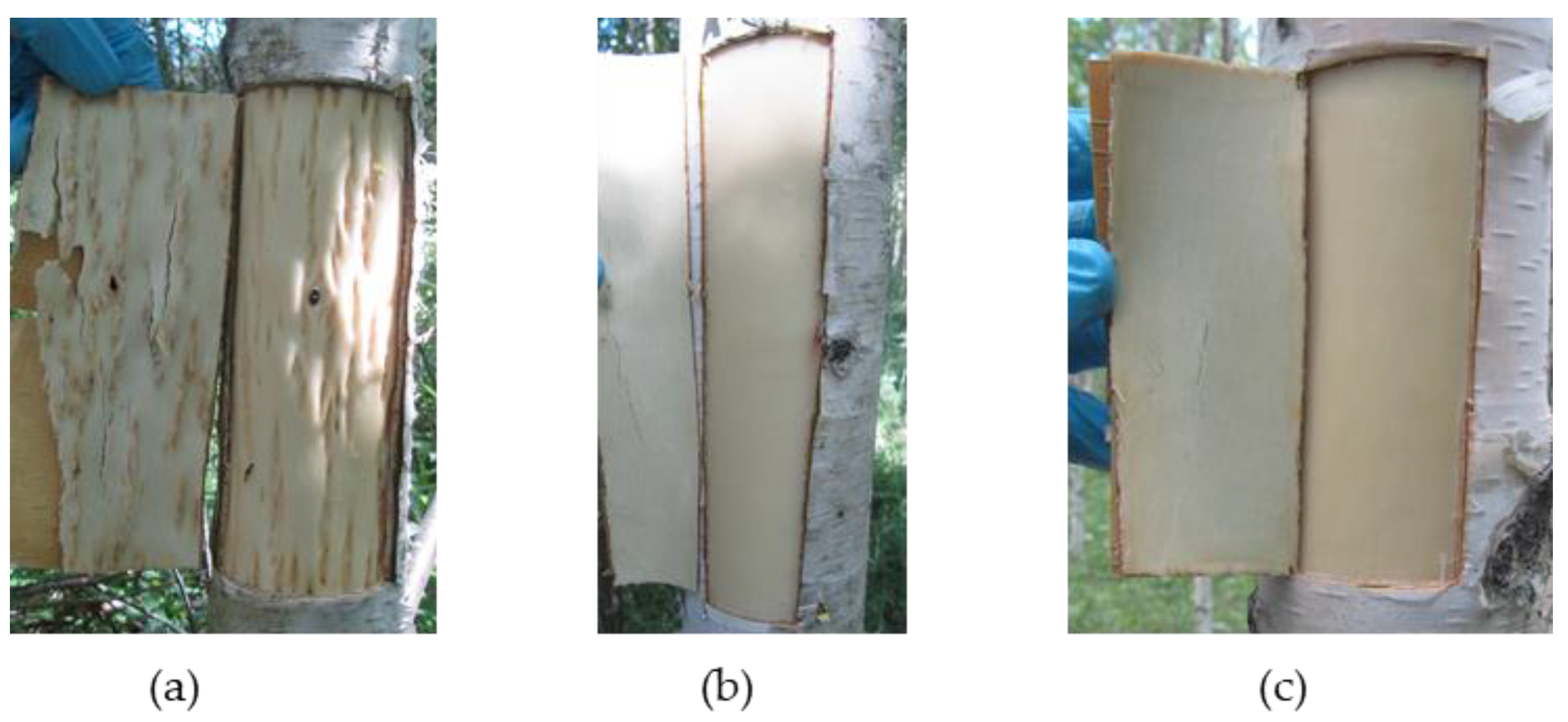
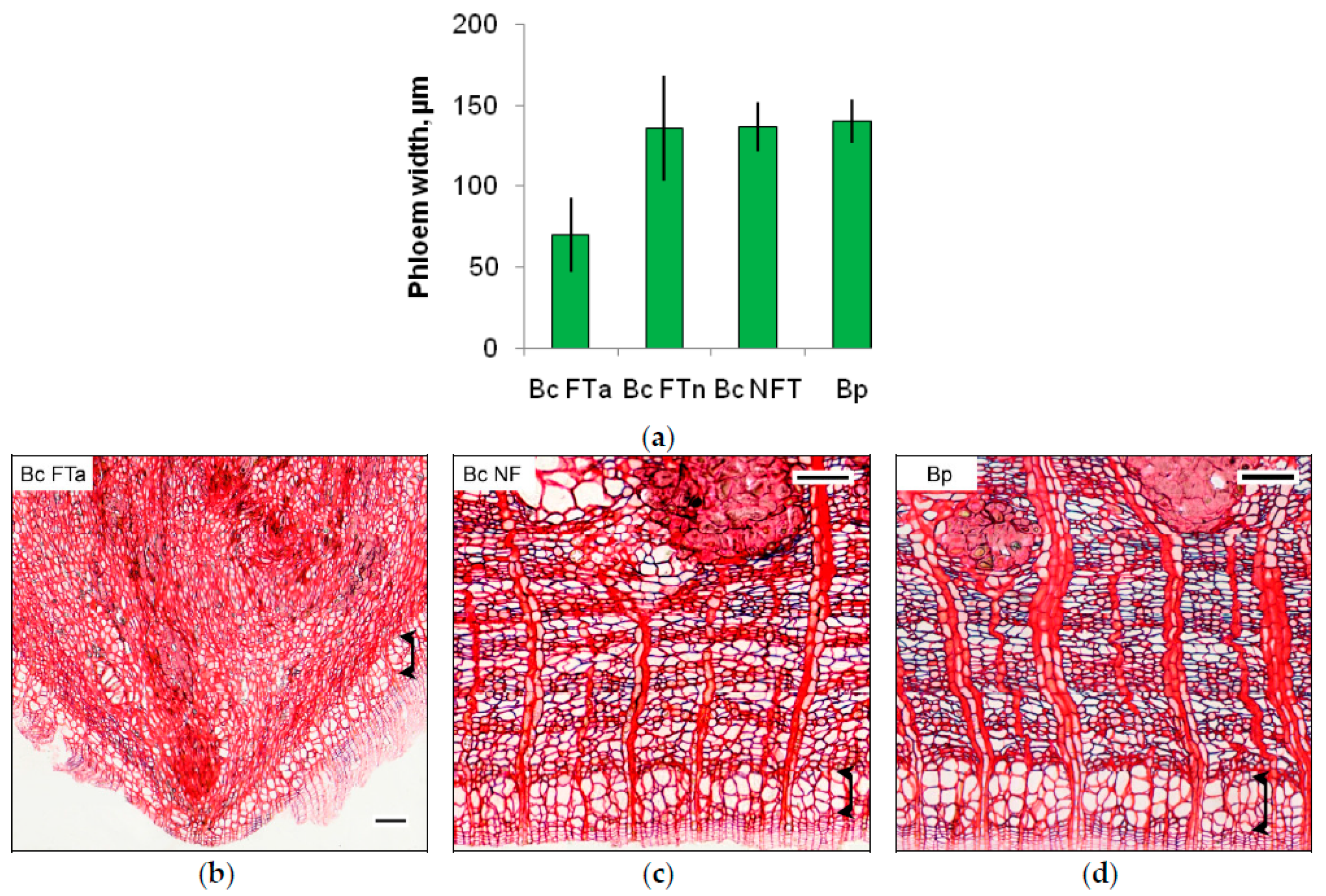
2.1. The Anatomy of Conducting Tissues in Sample Tree Trunks
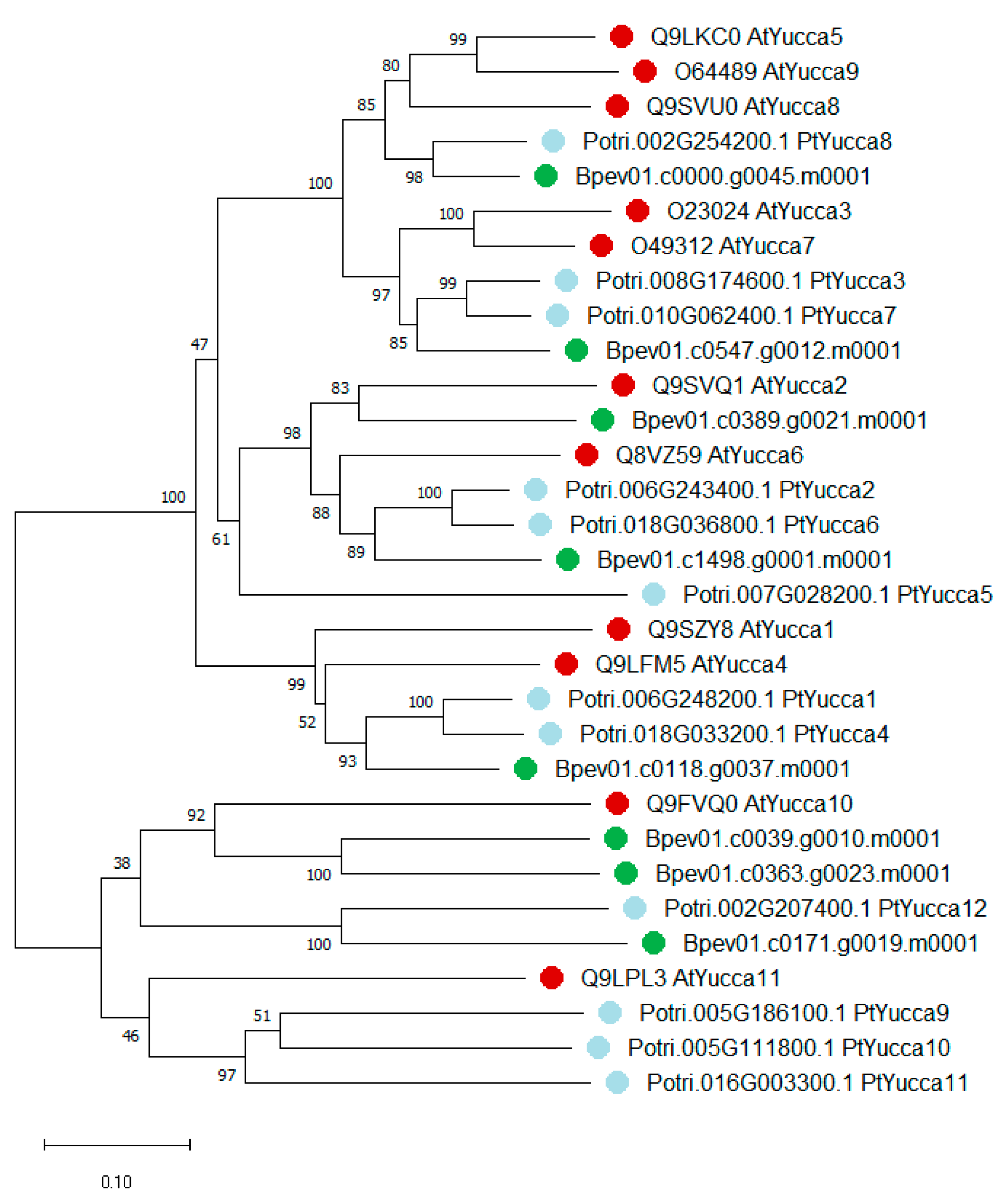
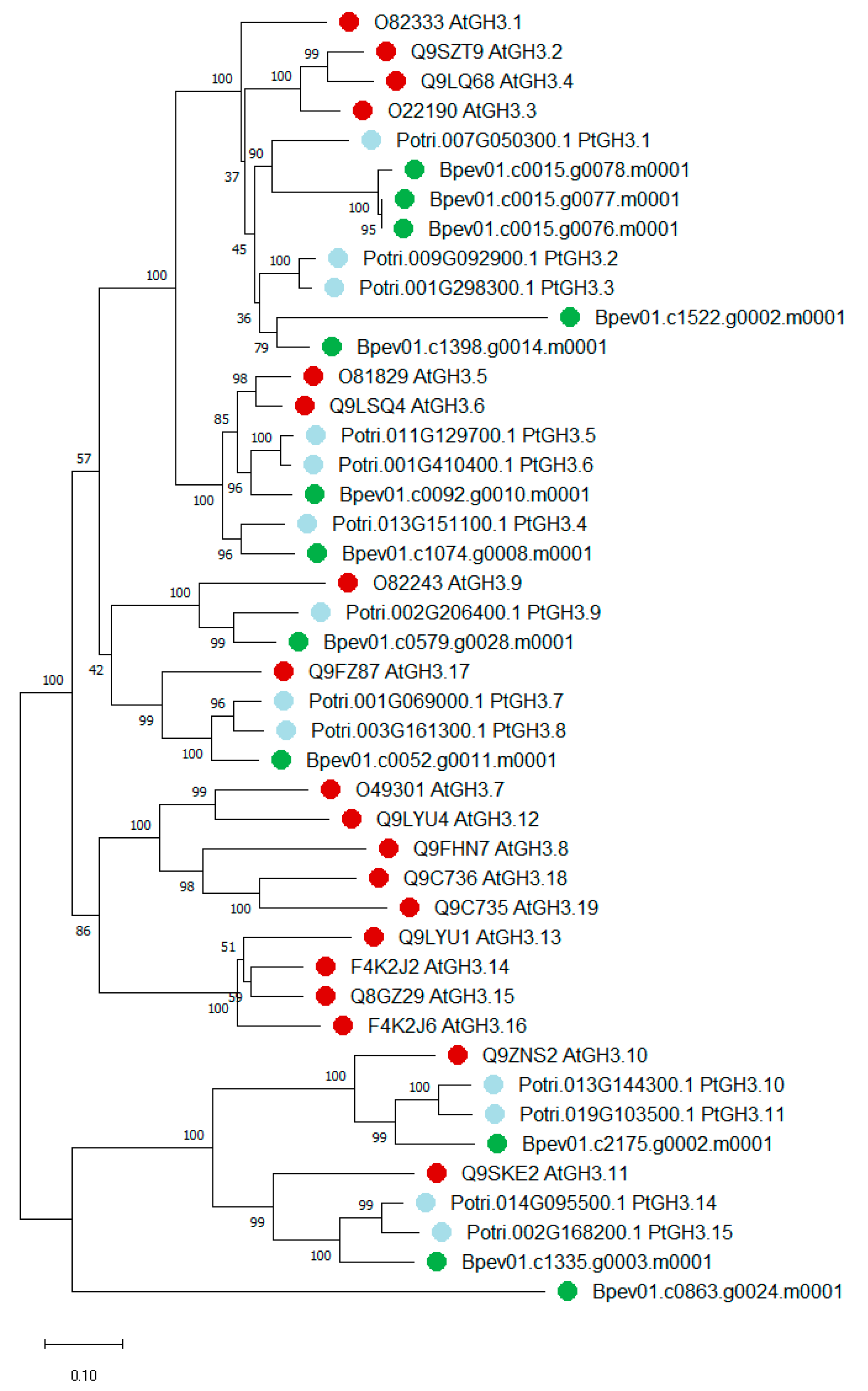
2.2. Yucca and GH3 Identification in the Silver Birch Genome
2.2.1. Yucca Family Genes
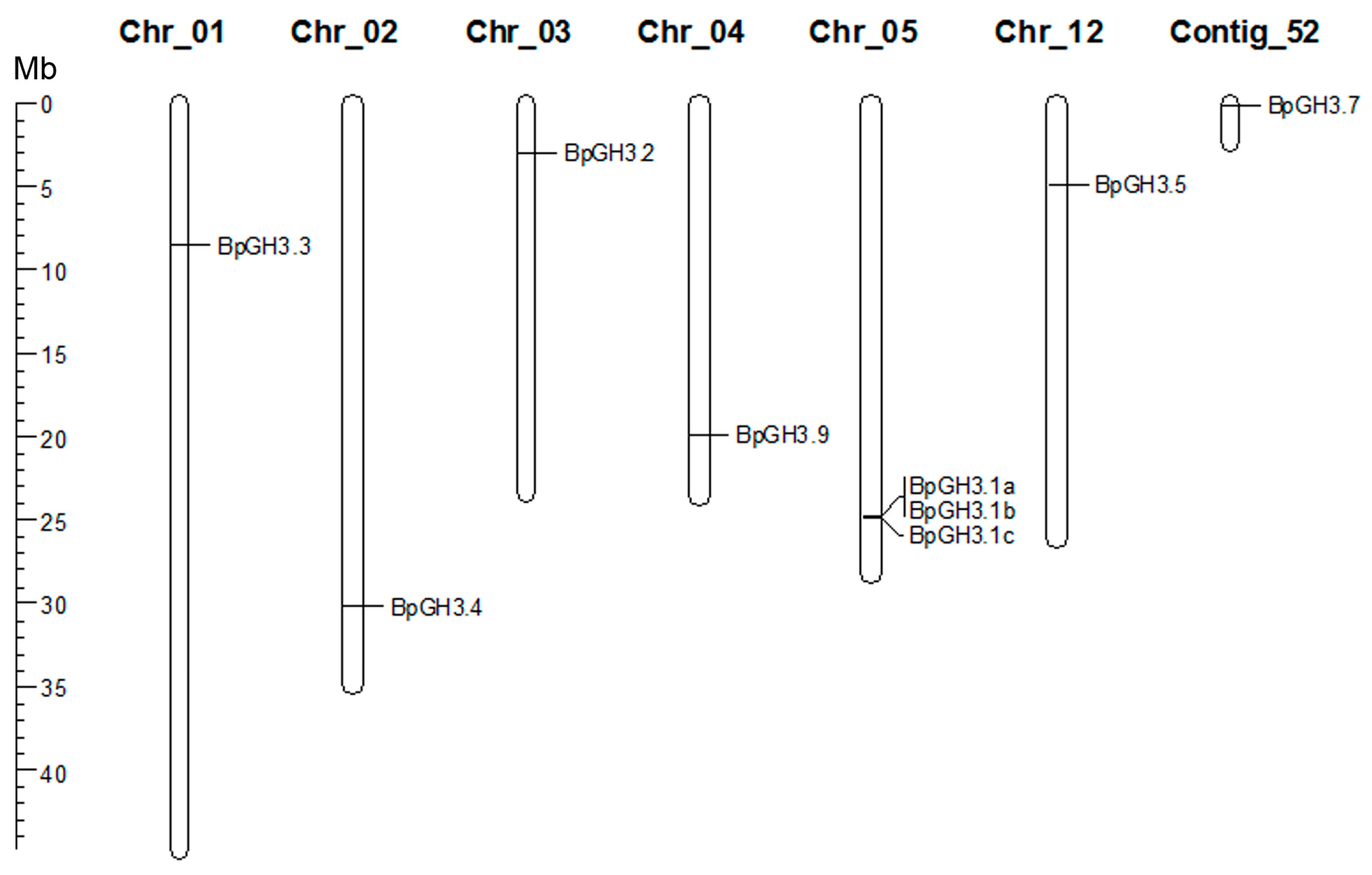
2.2.2. GH3 Family Genes
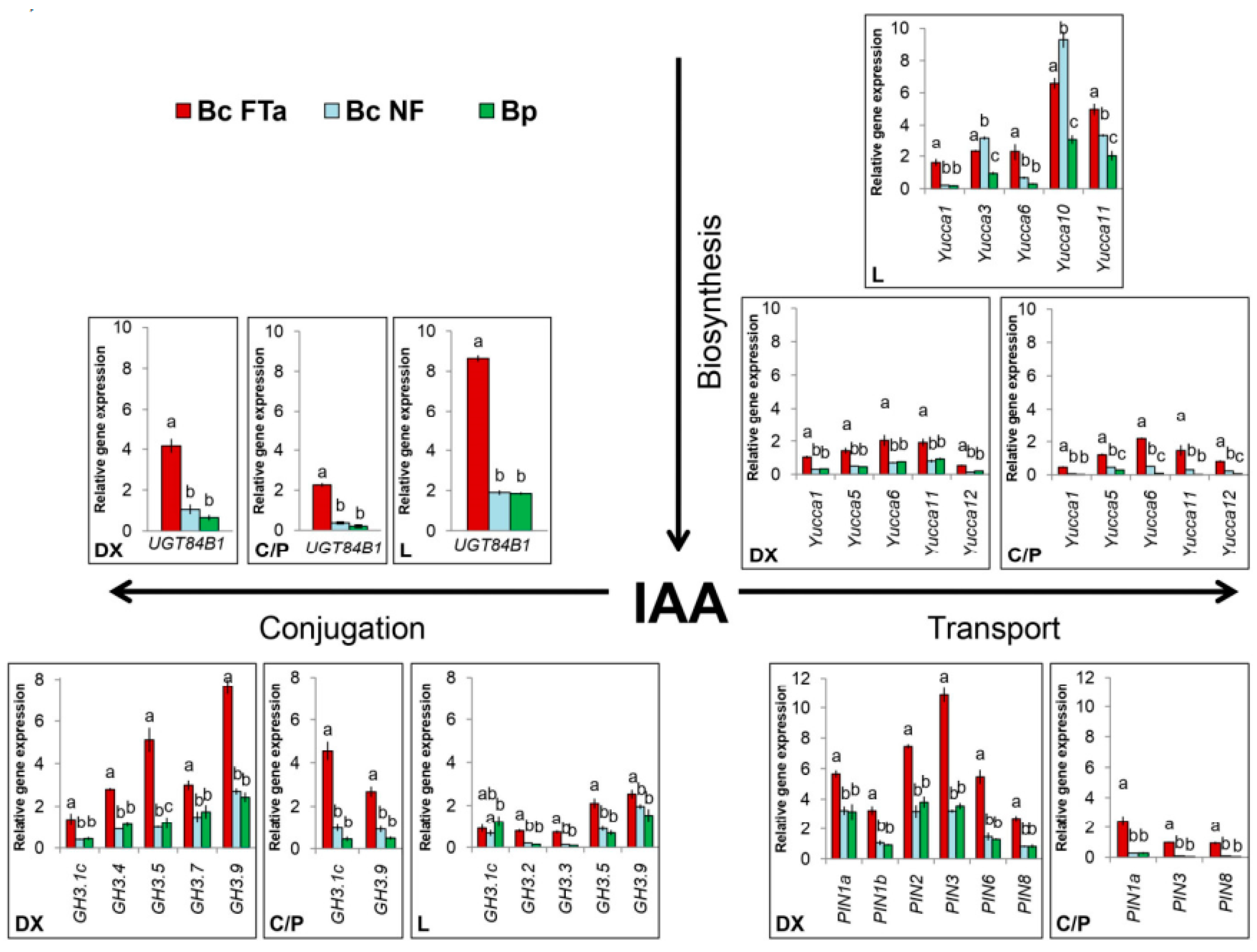
2.3. Expression of the Genes Involved in Auxin Biosynthesis, Transport and Conjugation
2.4. Analysis of Promoters
3. Discussion
3.1. Yucca and GH3 Gene Families in the Silver Birch Genome
3.2. Conducting Tissue Structure in Karelian Birch Trunk Indicates a Reduced Free Auxin Level
3.3. Expression of the Main Genes Involved in Auxin Biosynthesis, Transport and Conjugation during the Formation of Wood Typical of Birch Species
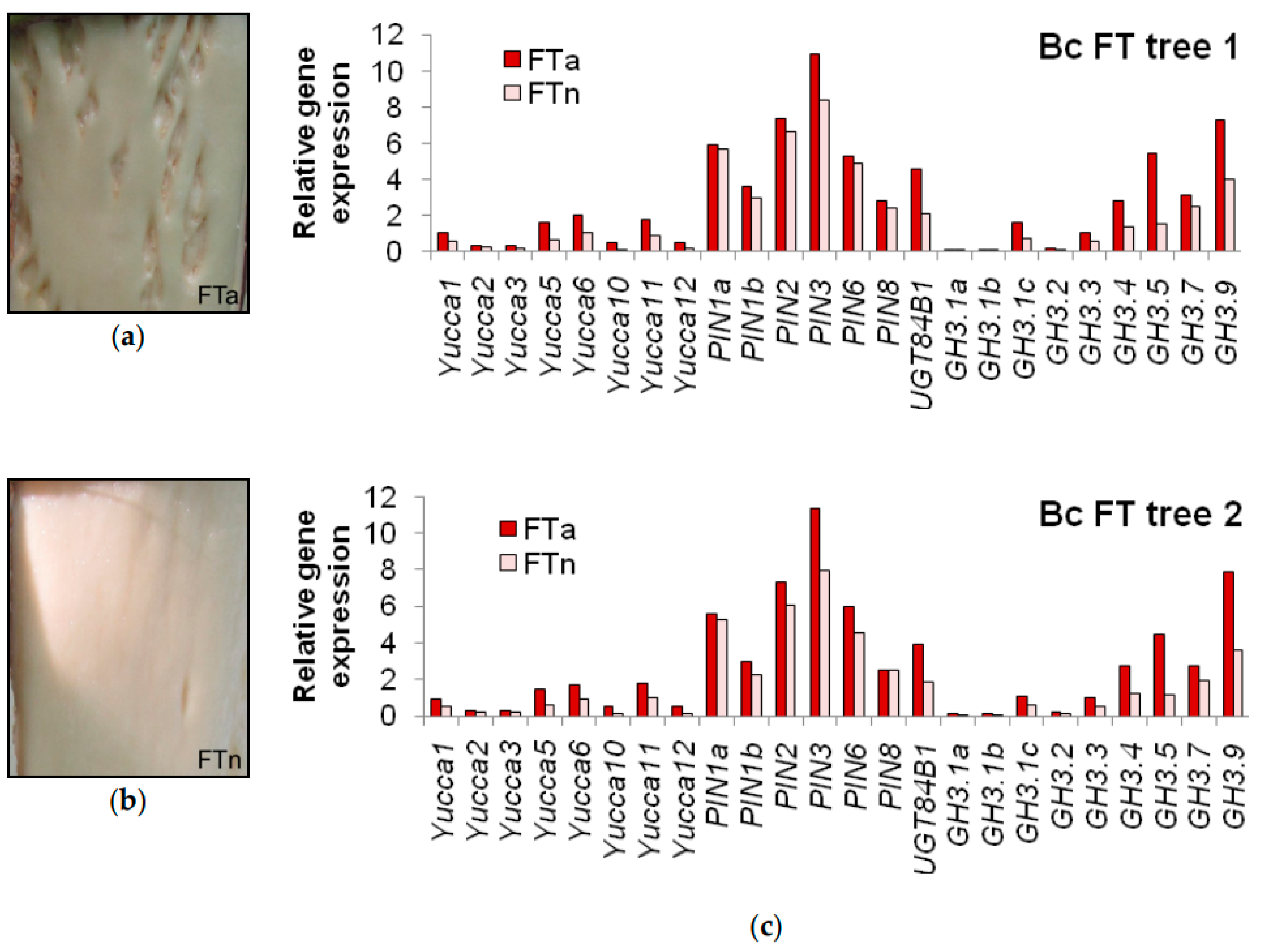
3.4. Specific Traits of Expression of the Genes in Question during Figured Wood Formation in Karelian Birch
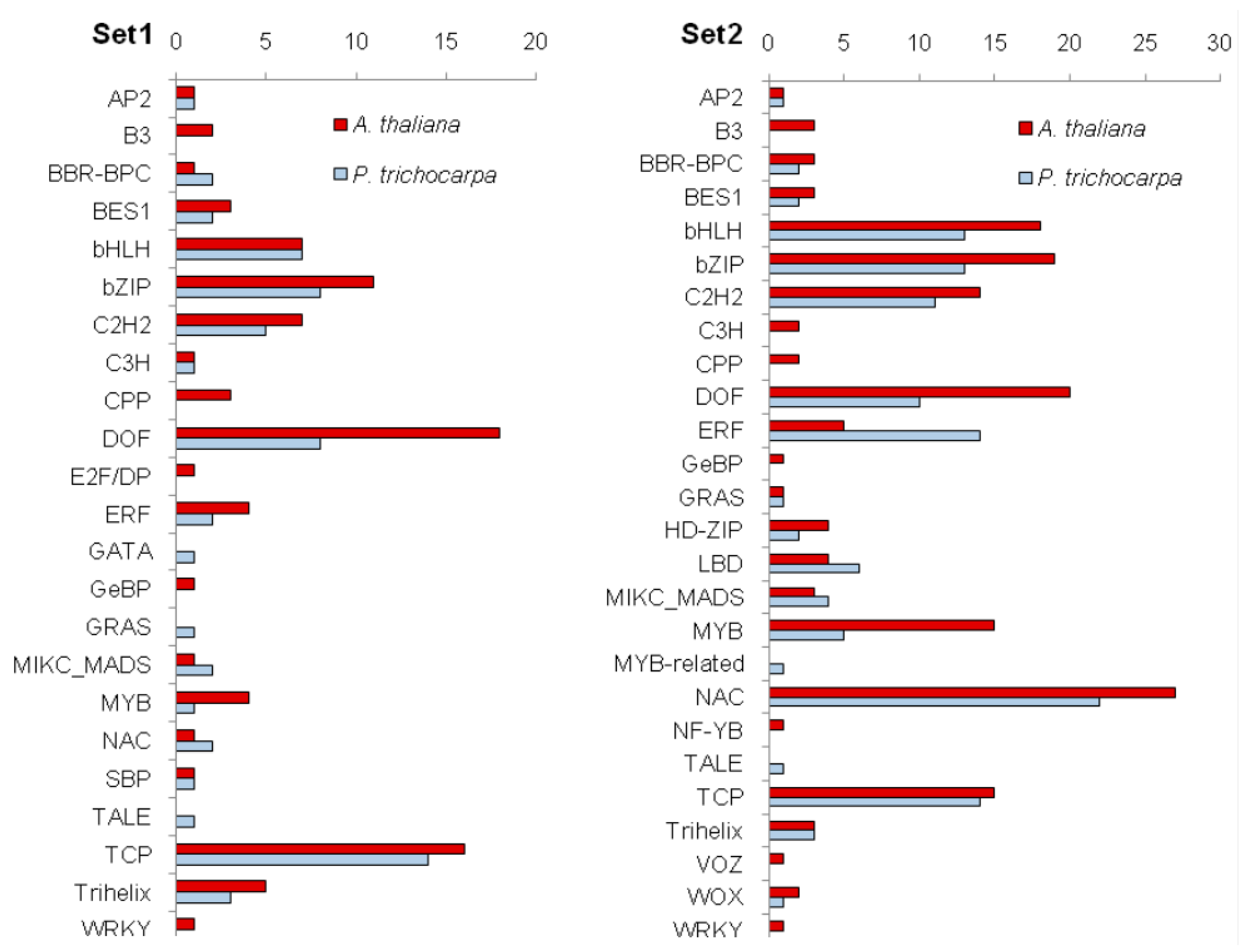
3.5. Identification of Transcription Factors Potentially Involved in Gene Expression Regulation during Figured Wood Formation in Karelian Birch
4. Materials and Methods
4.1. Choice of Trees and Sampling
4.2. Microscopy
4.3. Total RNA Isolation and Complementary DNA Synthesis
4.4. Gene Search in the Silver Birch Genome
4.5. Determination of Gene Expression Levels (Real-Time qPCR)
4.6. Analysis of Promoters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sundberg, B.; Uggla, C.; Tuominen, H. Cambial growth and auxin gradients. In Cell and Molecular Biology of Wood Formation; Savidge, R.A., Barnett, J.R., Napier, R., Eds.; BIOS Scientific Publishers Limited: Oxford, UK, 2000; pp. 169–188. [Google Scholar]
- Savidge, R.A. Intrinsic regulation of cambial growth. J. Plant Growth Regul. 2001, 20, 52–77. [Google Scholar] [CrossRef]
- Uggla, C.; Magel, E.; Moritz, T.; Sundberg, B. Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in scots pine. Plant Physiol. 2001, 125, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.; Karlberg, A.; Antti, H.; Lopez-Vernaza, M.; Mellerowicz, E.; Perrot-Rechenmann, C.; Sandberg, G.; Bhalerao, R.P. Dissecting the molecular basis of the regulation of wood formation by auxin in hybrid aspen. Plant Cell 2008, 20, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R. The induction of vascular tissues by auxin. In Plant Hormones. Biosynthesis, Signal Transduction, Action! Davis, P.J., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 485–518. [Google Scholar]
- Sorce, C.; Giovannelli, A.; Sebastiani, L.; Anfodillo, T. Hormonal signals involved in the regulation of cambial activity, xylogenesis and vessel patterning in trees. Plant Cell Rep. 2013, 32, 885–898. [Google Scholar] [CrossRef]
- Camargo, E.L.O.; Ployet, R.; Cassan-Wang, H.; Mounet, F.; Grima-Pettenati, J. Digging in wood: New insights in the regulation of wood formation in tree species. In Molecular Physiology and Biotechnology of Trees; Cánovas, F.M., Ed.; Academic Press: London, UK, 2019; Volume 89, pp. 201–233. [Google Scholar]
- Buttò, V.; Deslauriers, A.; Rossi, S.; Rozenberg, P.; Shishov, V.; Morin, H. The role of plant hormones in tree-ring formation. Trees 2020, 34, 315–335. [Google Scholar] [CrossRef]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Normanly, J. Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harb. Perspect. Biol. 2010, 2, a001594. [Google Scholar] [CrossRef]
- Ljung, K. Auxin metabolism and homeostasis during plant development. Development 2013, 140, 943–950. [Google Scholar] [CrossRef]
- Ye, X.; Kang, B.; Osburn, L.D.; Li, Y.; Zong-Ming, C. Identification of the flavin-dependent monooxygenase-encoding YUCCA gene family in Populus trichocarpa and their expression in vegetative tissues and in response to hormone and environmental stresses. Plant Cell Tissue Organ Cult. 2009, 97, 271–283. [Google Scholar] [CrossRef]
- Böttcher, C.; Burbidge, C.A.; Boss, P.K.; Davies, C. Interactions between ethylene and auxin are crucial to the control of grape (Vitis vinifera L.) berry ripening. BMC Plant Biol. 2013, 13, 222. [Google Scholar] [CrossRef]
- Pan, L.; Zeng, W.; Niu, L.; Lu, Z.; Liu, H.; Cui, G.; Zhu, Y.; Chu, J.; Li, W.; Fang, W.; et al. PpYUC11, a strong candidate gene for the stony hard phenotype in peach (Prunus persica L. Batsch), participates in IAA biosynthesis during fruit ripening. J. Exp. Bot. 2015, 66, 7031–7044. [Google Scholar] [CrossRef] [PubMed]
- Ruedell, C.M.; de Almeida, M.R.; Fett-Neto, A.G. Concerted transcription of auxin and carbohydrate homeostasis-related genes underlies improved adventitious rooting of microcuttings derived from far-red treated Eucalyptus globulus Labill mother plants. Plant Physiol. Biochem. 2015, 97, 11–19. [Google Scholar] [CrossRef]
- Song, C.; Zhang, D.; Zhang, J.; Zheng, L.; Zhao, C.; Ma, J.; An, N.; Han, M. Expression analysis of key auxin synthesis, transport, and metabolism genes in different young dwarfing apple trees. Acta Physiol. Plant. 2016, 38, 43. [Google Scholar] [CrossRef]
- Song, C.; Zhang, D.; Zheng, L.; Shen, Y.; Zuo, X.; Mao, J.; Meng, Y.; Wu, H.; Zhang, Y.; Liu, X.; et al. Genome-wide identification and expression profiling of the YUCCA gene family in Malus domestica. Sci. Rep. 2020, 10, 10866. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis. Arab. Book 2014, 12, e0173. [Google Scholar] [CrossRef] [PubMed]
- Robert, H.S.; Grones, P.; Stepanova, A.N.; Robles, L.M.; Lokerse, A.S.; Alonso, J.M.; Weijers, D.; Friml, J. Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr. Biol. 2013, 23, 2506–2512. [Google Scholar] [CrossRef]
- Brumos, J.; Robles, L.M.; Yun, J.; Vu, T.C.; Jackson, S.; Alonso, J.M.; Stepanova, A.N. Local auxin biosynthesis is a key regulator of plant development. Dev. Cell 2018, 47, 306–318.e5. [Google Scholar] [CrossRef]
- Sachs, T.; Cohen, D. Circular vessels and the control of vascular differentiation in plants. Differentiation 1982, 21, 22–26. [Google Scholar] [CrossRef]
- Kurczyńska, E.U. Vessel differentiation in isolated stem segments of Fraxinus excelsior L. after treatment with auxin. Acta Soc. Bot. Pol. 1992, 61, 343–357. [Google Scholar] [CrossRef]
- Sachs, T. Integrating cellular and organismic aspects of vascular differentiation. Plant Cell Physiol. 2000, 41, 649–656. [Google Scholar] [CrossRef]
- Junghans, U.; Langenfeld-Heyser, R.; Polle, A.; Teichmann, T. Effect of auxin transport inhibitors and ethylene on the wood anatomy of poplar. Plant Biol. 2004, 6, 22–29. [Google Scholar] [CrossRef]
- Drazeta, L.; Lang, A.; Cappellini, C.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Vessel differentiation in the pedicel of apple and the effects of auxin transport inhibition. Physiol. Plant. 2004, 120, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Aloni, R. Ecophysiological implications of vascular differentiation and plant evolution. Trees 2015, 29, 1–16. [Google Scholar] [CrossRef]
- Mazur, E.; Benková, E.; Friml, J. Vascular cambium regeneration and vessel formation in wounded inflorescence stems of Arabidopsis. Sci. Rep. 2016, 6, 33754. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Eckart, P.; Alsamadisi, N.; Noble, H.; Martin, C.; Spicer, R. Polar auxin transport is implicated in vessel differentiation and spatial patterning during secondary growth in Populus. Am. J. Bot. 2018, 105, 1–11. [Google Scholar] [CrossRef]
- Wisniewska, J.; Xu, J.; Seifertová, D.; Brewer, P.B.; Růžička, K.; Blilou, I.; Rouquié, D.; Benková, E.; Scheres, B.; Friml, J. Polar PIN localization directs auxin flow in plants. Science 2006, 312, 883. [Google Scholar] [CrossRef]
- Křeček, P.; Skůpa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zažímalová, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249. [Google Scholar] [CrossRef]
- Zwiewka, M.; Bilanovičová, V.; Seifu, Y.W.; Nodzyński, T. The nuts and bolts of PIN auxin efflux carriers. Front. Plant Sci. 2019, 10, 985. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Wang, L.; Li, J.; Zheng, H.; Chen, J.; Lu, M. A survey of Populus PIN-FORMED family genes reveals their diversified expression patterns. J. Exp. Bot. 2014, 65, 2437–2448. [Google Scholar] [CrossRef]
- Simon, S.; Skůpa, P.; Viaene, T.; Zwiewka, M.; Tejos, R.; Klíma, P.; Čarná, M.; Rolčík, J.; De Rycke, R.; Moreno, I.; et al. PIN6 auxin transporter at endoplasmic reticulum and plasma membrane mediates auxin homeostasis and organogenesis in Arabidopsis. New Phytol. 2016, 211, 65–74. [Google Scholar] [CrossRef]
- Palovaara, J.; Hallberg, H.; Stasolla, C.; Luit, B.; Hakman, I. Expression of a gymnosperm PIN homologous gene correlates with auxin immunolocalization pattern at cotyledon formation and in demarcation of the procambium during Picea abies somatic embryo development and in seedling tissues. Tree Physiol. 2010, 30, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Carraro, N.; Tisdale-Orr, T.E.; Clouse, R.M.; Knöller, A.S.; Spicer, R. Diversification and expression of the PIN, AUX/LAX, and ABCB families of putative auxin transporters in Populus. Front. Plant Sci. 2012, 3, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kühn, N.; Serrano, A.; Abello, C.; Arce, A.; Espinoza, C.; Gouthu, S.; Deluc, L.; Arce-Johnson, P. Regulation of polar auxin transport in grapevine fruitlets (Vitis vinifera L.) and the proposed role of auxin homeostasis during fruit abscission. BMC Plant Biol. 2016, 16. [Google Scholar] [CrossRef]
- Salazar, R.; Pollmann, S.; Morales-Quintana, L.; Herrera, R.; Caparrós-Ruiz, D.; Ramos, P. In seedlings of Pinus radiata, jasmonic acid and auxin are differentially distributed on opposite sides of tilted stems affecting lignin monomer biosynthesis and composition. Plant Physiol. Biochem. 2019, 135, 215–223. [Google Scholar] [CrossRef]
- Qi, L.; Chen, L.; Wang, C.; Zhang, S.; Yang, Y.; Liu, J.; Li, D.; Song, J.; Wang, R. Characterization of the auxin efflux transporter PIN proteins in pear. Plants 2020, 9, 349. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Okrent, R.A.; Wildermuth, M.C. Evolutionary history of the GH3 family of acyl adenylases in rosids. Plant Mol. Biol. 2011, 76, 489–505. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, K.; Lei, H.; Shen, X.; Liu, Y.; Liao, X.; Li, T. Genome-wide analysis of the GH3 family in apple (Malus × domestica). BMC Genom. 2013, 14, 297. [Google Scholar] [CrossRef]
- Vielba, J.M.; Varas, E.; Rico, S.; Covelo, P.; Sánchez, C. Auxin-mediated expression of a GH3 gene in relation to ontogenic state in chestnut. Trees 2016, 30, 2237–2252. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Wang, C.; Wei, Z.; Xia, D.; Wang, Y.; Liu, G.; Yang, C. Time-course analysis of levels of indole-3-acetic acid and expression of auxin-responsive GH3 genes in Betula platyphylla. Plant Mol. Biol. Report. 2011, 29, 898–905. [Google Scholar] [CrossRef]
- Brunoni, F.; Collani, S.; Casanova-Sáez, R.; Šimura, J.; Karady, M.; Schmid, M.; Ljung, K.; Bellini, C. Conifers exhibit a characteristic inactivation of auxin to maintain tissue homeostasis. New Phytol. 2020, 226, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lan, Q.; Han, S.; Qi, L. A GH3-like gene, LaGH3, isolated from hybrid larch (Larix leptolepis × Larix olgensis) is regulated by auxin and abscisic acid during somatic embryogenesis. Trees 2019, 33, 1723–1732. [Google Scholar] [CrossRef]
- Szerszen, J.; Szczyglowski, K.; Bandurski, R. iaglu, a gene from Zea mays involved in conjugation of growth hormone indole-3-acetic acid. Science 1994, 265, 1699. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.G.; Lim, E.-K.; Li, Y.; Kowalczyk, M.; Sandberg, G.; Hoggett, J.; Ashford, D.A.; Bowles, D.J. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J. Biol. Chem. 2001, 276, 4350–4356. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, T.-T.; Xiao, D.-W.; Zhao, S.-M.; Lin, J.-S.; Wang, T.; Li, Y.-J.; Hou, B.-K. OsIAGT1 is a glucosyltransferase gene involved in the glucose conjugation of auxins in rice. Rice 2019, 12, 92. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska, A. Conjugates of auxin and cytokinin. Phytochemistry 2009, 70, 957–969. [Google Scholar] [CrossRef]
- Nowacki, J.; Bandurski, R.S. Myo-inositol esters of indole-3-acetic acid as seed auxin precursors of Zea mays L. Plant Physiol. 1980, 65, 422–427. [Google Scholar] [CrossRef]
- Staswick, P.E. The tryptophan conjugates of jasmonic and indole-3-acetic acids are endogenous auxin inhibitors. Plant Physiol. 2009, 150, 1310–1321. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, G.; Chen, J.-J.; Taylor, G.; Cui, K.-M.; He, X.-Q. Molecular features of secondary vascular tissue regeneration after bark girdling in Populus. New Phytol. 2011, 192, 869–884. [Google Scholar] [CrossRef]
- Immanen, J.; Nieminen, K.; Smolander, O.-P.; Kojima, M.; Alonso Serra, J.; Koskinen, P.; Zhang, J.; Elo, A.; Mähönen, A.P.; Street, N.; et al. Cytokinin and auxin display distinct but interconnected distribution and signaling profiles to stimulate cambial activity. Curr. Biol. 2016, 26, 1990–1997. [Google Scholar] [CrossRef]
- Environment Directorate. Consensus Document on the Biology of European white Birch (Betula Pendula Roth); Organisation for Economic Co-operation and Development: Paris, France, 2003. [Google Scholar]
- Hynynen, J.; Niemisto, P.; Vihera-Aarnio, A.; Brunner, A.; Hein, S.; Velling, P. Silviculture of birch (Betula pendula Roth and Betula pubescens Ehrh.) in northern Europe. Forestry 2009, 83, 103–119. [Google Scholar] [CrossRef]
- Velling, P.; Vihera-Aarnio, A.; Hagqvist, R.; Lehto, J. Valuable wood as a result of abnormal cambial activity—The case of Betula pendula var. carelica. In Cell and Molecular Biology of Wood Formation; Savidge, R.A., Barnett, J.R., Napier, R., Eds.; BIOS Scientific Publishers Limited: Oxford, UK, 2000; pp. 377–386. [Google Scholar]
- Hagqvist, R.; Mikkola, A. Visakoivun Kasvatus Ja Käyttö; Metsäkustannus & Visaseurary: Hämeenlinna, Finland, 2008. [Google Scholar]
- Novitskaya, L.; Nikolaeva, N.; Tarelkina, T. Endogenous variability of the figured wood of Karelian birch. Wulfenia 2016, 23, 175–188. [Google Scholar]
- Lyubavskaya, A.Y. Karelian Birch; Moscow State Forest University: Moscow, Russia, 1978. [Google Scholar]
- Yermakov, V.I. Mechanisms of Adaptation of Birch in the North; Science: Leningrad, Russia, 1986. [Google Scholar]
- Yevdokimov, A.P. Karelian Birch Biology and Cultivation; Publishing House of the Leningrad University: Leningrad, Russia, 1989. [Google Scholar]
- Paganova, V. Analysis of inheritance and growth of curly birch progenies from controlled hybridisation and possibilities of their utilisation for timber production in agricultural landscape. Czech J. Genet. Plant Breed. 2004, 40, 51–62. [Google Scholar] [CrossRef]
- Novitskaya, L.; Nikolaeva, N.; Galibina, N.; Tarelkina, T.; Semenova, L. The greatest density of parenchyma inclusions in Karelian birch wood occurs at confluences of phloem flows. Silva Fenn. 2016, 50, 1461–1478. [Google Scholar] [CrossRef][Green Version]
- Shchetinkin, S.V. Histogenesis of Figured Wood in Birch (Betula pendula Roth var. carelica Merkl. and Betula pendula Roth). Ph.D. Thesis, Voronezh State University, Voronezh, Russia, 1987. [Google Scholar]
- Novitskaya, L.L.; Kushnir, F.V. The role of sucrose in regulation of trunk tissue development in Betula pendula Roth. J. Plant Growth Regul. 2006, 25, 18–29. [Google Scholar] [CrossRef]
- Galibina, N.A.; Novitskaya, L.L.; Krasavina, M.S.; Moshchenskaya, Y.L. Activity of sucrose synthase in trunk tissues of Karelian birch during cambial growth. Russ. J. Plant Physiol. 2015, 62, 381–389. [Google Scholar] [CrossRef]
- Galibina, N.A.; Novitskaya, L.L.; Nikerova, K.M.; Moshchenskaya, Y.L.; Borodina, M.N.; Sofronova, I.N. Apoplastic invertase activity regulation in the cambial zone of karelian birch. Russ. J. Dev. Biol. 2019, 50, 20–29. [Google Scholar] [CrossRef]
- Galibina, N.A.; Novitskaya, L.L.; Krasavina, M.S.; Moshchenskaya, J.L. Invertase activity in trunk tissues of Karelian birch. Russ. J. Plant Physiol. 2015, 62, 753–760. [Google Scholar] [CrossRef]
- Novitskaya, L.L.; Tarelkina, T.V.; Galibina, N.A.; Moshchenskaya, Y.L.; Nikolaeva, N.N.; Nikerova, K.M.; Podgornaya, M.N.; Sofronova, I.N.; Semenova, L.I. The formation of structural abnormalities in Karelian birch wood is associated with auxin inactivation and disrupted basipetal auxin transport. J. Plant Growth Regul. 2020, 39, 378–394. [Google Scholar] [CrossRef]
- Zajączkowski, S.; Wodzicki, T.J. Auxin and plant morphogenesis—A model of regulation. Acta Soc. Bot. Pol. 1978, 47, 233–243. [Google Scholar] [CrossRef]
- Zajączkowski, S.; Wodzicki, T.J. On the question of stem polarity with respect to auxin transport. Physiol. Plant. 1978, 44, 122–126. [Google Scholar] [CrossRef]
- Savidge, R.A. The role of plant hormones in higher plant cellular differentiation. II. Experiments with the vascular cambium, and sclereid and tracheid differentiation in the pine, Pinus contorta. Histochem. J. 1983, 15, 447–466. [Google Scholar] [CrossRef]
- Zagórska-Marek, B.; Little, C.H.A. Control of fusiform initial orientation in the vascular cambium of Abies balsamea stems by indol-3-ylacetic acid. Can. J. Bot. 1986, 64, 1120–1128. [Google Scholar] [CrossRef]
- Kramer, E.M. Wood grain pattern formation: A brief review. J. Plant Growth Regul. 2006, 25, 290–301. [Google Scholar] [CrossRef]
- Nikerova, K.M.; Galibina, N.A.; Sinkevich, S.M.; Borodina, M.N.; Sofronova, I.N. The alternative ways of sucrose utilization in Scots pine (Pinus sylvestris L.) spiral grain trees. Unpublished; Manuscript in Preparation.
- Eveland, A.L.; Jackson, D.P. Sugars, signalling, and plant development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ruan, Y.-L. Regulation of cell division and expansion by sugar and auxin signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Dang, T.V.T.; Hwang, I. Emergence of plant vascular system: Roles of hormonal and non-hormonal regulatory networks. Curr. Opin. Plant Biol. 2017, 35, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Serra, J.; Safronov, O.; Lim, K.; Fraser-Miller, S.J.; Blokhina, O.B.; Campilho, A.; Chong, S.; Fagerstedt, K.; Haavikko, R.; Helariutta, Y.; et al. Tissue-specific study across the stem reveals the chemistry and transcriptome dynamics of birch bark. New Phytol. 2019, 222, 1816–1831. [Google Scholar] [CrossRef]
- Staswick, P.E.; Serban, B.; Rowe, M.; Tiryaki, I.; Maldonado, M.T.; Maldonado, M.C.; Suza, W. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 2005, 17, 616. [Google Scholar] [CrossRef]
- Staswick, P.E.; Tiryaki, I.; Rowe, M.L. Jasmonate Response Locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 2002, 14, 1405–1415. [Google Scholar] [CrossRef]
- Vielba, J.M. Identification and initial characterization of a new subgroup in the GH3 gene family in woody plants. J. Plant Biochem. Biotechnol. 2019, 28, 280–290. [Google Scholar] [CrossRef]
- Mazur, E.; Friml, J. Vascular tissue development and regeneration in the model plant Arabidopsis. In Plant Engineering; Jurić, S., Ed.; InTech: Rijeka, Croatia, 2017; ISBN 978-953-51-3607-1. [Google Scholar]
- Doley, D.; Leyton, L. Effects of growth regulating substances and water potential on the development of secondary xylem in Fraxinus. New Phytol. 1968, 67, 579–594. [Google Scholar] [CrossRef]
- Aloni, R.; Zimmermann, M.H. The control of vessel size and density along the plant axis—A new hypothesis. Differentiation 1983, 24, 203–208. [Google Scholar] [CrossRef]
- Zakrzewski, J. Hormonal control of cambial activity and vessel differentiation in Quercus robur. Physiol. Plant. 1983, 57, 537–542. [Google Scholar] [CrossRef]
- Zakrzewski, J. Effect of indole-3-acetic acid (IAA) and sucrose on vessel size and density in isolated stem segments of oak (Quercus robur). Physiol. Plant. 1991, 81, 234–238. [Google Scholar] [CrossRef]
- Rinne, P.; Tuominen, H.; Sundberg, B. Growth patterns and endogenous indole-3-acetic acid concentrations in current-year coppice shoots and seedlings of two Betula species. Physiol. Plant. 1993, 88, 403–412. [Google Scholar] [CrossRef]
- Tuominen, H.; Sitbon, F.; Jacobsson, C.; Sandberg, G.; Olsson, O.; Sundberg, B. Altered growth and wood characteristics in transgenic hybrid aspen expressing Agrobacterium tumefaciens T-DNA indoleacetic acid-biosynthetic genes. Plant Physiol. 1995, 109, 1179–1189. [Google Scholar] [CrossRef]
- Lovisolo, C.; Schubert, A.; Sorce, C. Are xylem radial development and hydraulic conductivity in downwardly-growing grapevine shoots influenced by perturbed auxin metabolism? New Phytol. 2002, 156, 65–74. [Google Scholar] [CrossRef]
- Lachaud, S.; Bonnemain, J.L. Seasonal variations in the polar-transport pathways and retention sites of [3H]indole-3-acetic acid in young branches of Fagus sylvatica L. Planta 1984, 161, 207–215. [Google Scholar] [CrossRef]
- Spicer, R.; Tisdale-Orr, T.; Talavera, C. Auxin-responsive DR5 promoter coupled with transport assays suggest separate but linked routes of auxin transport during woody stem development in Populus. PLoS ONE 2013, 8, e72499. [Google Scholar] [CrossRef]
- Schrader, J.; Baba, K.; May, S.T.; Palme, K.; Bennett, M.; Bhalerao, R.P.; Sandberg, G. Polar auxin transport in the wood-forming tissues of hybrid aspen is under simultaneous control of developmental and environmental signals. Proc. Natl. Acad. Sci. USA 2003, 100, 10096–10101. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Moyle, R.; Bhalerao, R.; Hertzberg, M.; Lundeberg, J.; Nilsson, P.; Bhalerao, R.P. Cambial meristem dormancy in trees involves extensive remodelling of the transcriptome. Plant J. 2004, 40, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Novitskaya, L.L. About the possible cause of the formation of structural anomalies in the Karelian birch trunk. Bot. Zhurnal 1997, 82, 61–66. [Google Scholar]
- Fu, J.; Yu, H.; Li, X.; Xiao, J.; Wang, S. Rice GH3 gene family: Regulators of growth and development. Plant Signal. Behav. 2011, 6, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Moshchenskaya, Y.; Galibina, N.; Nikerova, K.; Novitskaya, L. Activity of sucrose dissimilating enzymes in early ontogeny in different forms of silver birch. Proc. Karelian Res. Cent. Russ. Acad. Sci. 2016, 78–87. [Google Scholar] [CrossRef]
- Galibina, N.A.; Novitskaya, L.L.; Nikerova, K.M. Source–sink relations in the organs and tissues of silver birch during different scenarios of xylogenesis. Russ. J. Plant Physiol. 2019, 66, 308–315. [Google Scholar] [CrossRef]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef]
- LeClere, S.; Schmelz, E.A.; Chourey, P.S. Sugar levels regulate tryptophan-dependent auxin biosynthesis in developing maize kernels. Plant Physiol. 2010, 153, 306–318. [Google Scholar] [CrossRef]
- Lilley, J.L.S.; Gee, C.W.; Sairanen, I.; Ljung, K.; Nemhauser, J.L. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. 2012, 160, 2261–2270. [Google Scholar] [CrossRef]
- Sairanen, I.; Novák, O.; Pěnčík, A.; Ikeda, Y.; Jones, B.; Sandberg, G.; Ljung, K. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell 2012, 24, 4907–4916. [Google Scholar] [CrossRef]
- Han, L.; Li, J.L.; Jin, M.; Su, Y.H. Transcriptome analysis of Arabidopsis seedlings responses to high concentrations of glucose. Genet. Mol. Res. 2015, 14, 4784–4801. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Peron, T.; Lecerf, M.; Perez-Garcia, M.-D.; Barriere, Q.; Rolcik, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef]
- Mao, J.; Li, W.; Mi, B.; Ma, Z.; Dawuda, M.M.; Zuo, C.; Zhang, Y.; Jiang, X.; Chen, B. Transcriptome analysis revealed glucose application affects plant hormone signal transduction pathway in “Red Globe” grape plantlets. Plant Growth Regul. 2018, 84, 45–56. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, Z.; Ji, C.Y.; Jeong, J.C.; Lee, H.-S.; Li, H.; Xu, B.; Deng, X.; Kwak, S.-S. Transgenic poplar expressing Arabidopsis YUCCA6 exhibits auxin-overproduction phenotypes and increased tolerance to abiotic stress. Plant Physiol. Biochem. 2015, 94, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-C.; Kim, H.S.; Lee, H.-U.; Kim, Y.-H.; Kwak, S.-S. Overexpression of Arabidopsis YUCCA6 enhances environment stress tolerance and inhibits storage root formation in sweet potato. Plant Biotechnol. Rep. 2019, 13, 345–352. [Google Scholar] [CrossRef]
- Jackson, R.G.; Kowalczyk, M.; Li, Y.; Higgins, G.; Ross, J.; Sandberg, G.; Bowles, D.J. Over-expression of an Arabidopsis gene encoding a glucosyltransferase of indole-3-acetic acid: Phenotypic characterisation of transgenic lines. Plant J. 2002, 32, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J.; Walz, A.; Slovin, J.P.; Epstein, E.; Cohen, J.D.; Dong, W.; Town, C.D. Overexpression of maize IAGLU in Arabidopsis thaliana alters plant growth and sensitivity to IAA but not IBA and 2,4-D. J. Plant Growth Regul. 2005, 24, 127–141. [Google Scholar] [CrossRef]
- Dai, X.; Mashiguchi, K.; Chen, Q.; Kasahara, H.; Kamiya, Y.; Ojha, S.; DuBois, J.; Ballou, D.; Zhao, Y. The biochemical mechanism of auxin biosynthesis by an Arabidopsis YUCCA flavin-containing monooxygenase. J. Biol. Chem. 2013, 288, 1448–1457. [Google Scholar] [CrossRef]
- Nishimura, T.; Hayashi, K.; Suzuki, H.; Gyohda, A.; Takaoka, C.; Sakaguchi, Y.; Matsumoto, S.; Kasahara, H.; Sakai, T.; Kato, J.; et al. Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J. 2014, 77, 352–366. [Google Scholar] [CrossRef]
- Chen, Q.; Westfall, C.S.; Hicks, L.M.; Wang, S.; Jez, J.M. Kinetic basis for the conjugation of auxin by a GH3 family indole-acetic acid-amido synthetase. J. Biol. Chem. 2010, 285, 29780–29786. [Google Scholar] [CrossRef]
- Kramer, E.M.; Ackelsberg, E.M. Auxin metabolism rates and implications for plant development. Front. Plant Sci. 2015, 6, 150. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, M.; Mierek-Adamska, A.; Porowińska, D.; Goc, A.; Jakubowska, A. Cloning and biochemical characterization of indole-3-acetic acid-amino acid synthetase PsGH3 from pea. Plant Physiol. Biochem. 2016, 107, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Friml, J.; Jones, A.R. Endoplasmic reticulum: The rising compartment in auxin biology. Plant Physiol. 2010, 154, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Enders, T.A.; Strader, L.C. Auxin activity: Past, present, and future. Am. J. Bot. 2015, 102, 180–196. [Google Scholar] [CrossRef]
- Middleton, A.M.; Dal Bosco, C.; Chlap, P.; Bensch, R.; Harz, H.; Ren, F.; Bergmann, S.; Wend, S.; Weber, W.; Hayashi, K.; et al. Data-driven modeling of intracellular auxin fluxes indicates a dominant role of the ER in controlling nuclear auxin uptake. Cell Rep. 2018, 22, 3044–3057. [Google Scholar] [CrossRef]
- Dal Bosco, C.; Dovzhenko, A.; Liu, X.; Woerner, N.; Rensch, T.; Eismann, M.; Eimer, S.; Hegermann, J.; Paponov, I.A.; Ruperti, B.; et al. The endoplasmic reticulum localized PIN8 is a pollen-specific auxin carrier involved in intracellular auxin homeostasis. Plant J. 2012, 71, 860–870. [Google Scholar] [CrossRef]
- Mravec, J.; Skůpa, P.; Bailly, A.; Hoyerová, K.; Křeček, P.; Bielach, A.; Petrášek, J.; Zhang, J.; Gaykova, V.; Stierhof, Y.-D.; et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER-localized PIN5 transporter. Nature 2009, 459, 1136–1140. [Google Scholar] [CrossRef]
- Gerttula, S.; Zinkgraf, M.; Muday, G.K.; Lewis, D.R.; Ibatullin, F.M.; Brumer, H.; Hart, F.; Mansfield, S.D.; Filkov, V.; Groover, A. Transcriptional and hormonal regulation of gravitropism of woody stems in Populus. Plant Cell 2015. [Google Scholar] [CrossRef]
- Zamski, E.; Warwing, P.F. Vertical and radial movement of auxin in young sycamore plants. New Phytol. 1974, 73, 61–69. [Google Scholar] [CrossRef]
- Novitskaya, L.L. Karelian Birch: Mechanisms of Growth and Development of Structural Abnormalities; Verso: Petrozavodsk, Russia, 2008. [Google Scholar]
- Nikolaeva, N.N. Formation of Leaf Apparatus in Silver Birch (Betula pendula Roth.) with Different Wood Grain. Ph.D. Thesis, Komarov Botanical Institute of the Russian Academy of Sciences, Saint Petersburg, Russia, 2004. [Google Scholar]
- Price, J.; Laxmi, A.; St. Martin, S.K.; Jang, J.-C. Global transcription profiling reveals multiple sugar signal transduction mechanisms in Arabidopsis. Plant Cell 2004, 16, 2128–2150. [Google Scholar] [CrossRef]
- Smeekens, S.; Ma, J.; Hanson, J.; Rolland, F. Sugar signals and molecular networks controlling plant growth. Curr. Opin. Plant Biol. 2010, 13, 273–278. [Google Scholar] [CrossRef]
- Sakr, S.; Wang, M.; Dédaldéchamp, F.; Perez-Garcia, M.-D.; Ogé, L.; Hamama, L.; Atanassova, R. The sugar-signaling hub: Overview of regulators and interaction with the hormonal and metabolic network. Int. J. Mol. Sci. 2018, 19, 2506. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Demura, T. Transcriptional regulation of secondary wall formation controlled by NAC domain proteins. Plant Biotechnol. 2010, 27, 237–242. [Google Scholar] [CrossRef]
- Le Hir, R.; Bellini, C. The plant-specific Dof transcription factors family: New players involved in vascular system development and functioning in Arabidopsis. Front. Plant Sci. 2013, 4, 164. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S. Structure, function, and evolution of the Dof transcription factor family. In Plant Transcription Factors; Gonzalez, D.H., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 183–197. [Google Scholar]
- Kubo, M. Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 2005, 19, 1855–1860. [Google Scholar] [CrossRef]
- Mitsuda, N.; Iwase, A.; Yamamoto, H.; Yoshida, M.; Seki, M.; Shinozaki, K.; Ohme-Takagi, M. NAC transcription factors, NST1 and NST3, are key regulators of the formation of secondary walls in woody tissues of Arabidopsis. Plant Cell 2007, 19, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Wang, J.P.; Lin, Y.-C.; Li, Q.; Sun, Y.-H.; Chen, H.; Sederoff, R.R.; Chiang, V.L. Tissue and cell-type co-expression networks of transcription factors and wood component genes in Populus trichocarpa. Planta 2017, 245, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Laubscher, M.; Brown, K.; Tonfack, L.B.; Myburg, A.A.; Mizrachi, E.; Hussey, S.G. Temporal analysis of Arabidopsis genes activated by Eucalyptus grandis NAC transcription factors associated with xylem fibre and vessel development. Sci. Rep. 2018, 8, 10983. [Google Scholar] [CrossRef]
- Miyashima, S.; Roszak, P.; Sevilem, I.; Toyokura, K.; Blob, B.; Heo, J.; Mellor, N.; Help-Rinta-Rahko, H.; Otero, S.; Smet, W.; et al. Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature 2019, 565, 490–494. [Google Scholar] [CrossRef]
- Tarelkina, T.V.; Galibina, N.A.; Moshchenskaya, Y.L.; Novitskaya, L.L. In silico analysis of regulatory cis-elements in the promoters of genes encoding apoplastic invertase and sucrose synthase in silver birch. Russ. J. Dev. Biol. 2020, 51, 323–335. [Google Scholar] [CrossRef]
- Manassero, N.G.U.; Viola, I.L.; Welchen, E.; Gonzalez, D.H. TCP transcription factors: Architectures of plant form. Biomol. Concepts 2013, 4, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Danisman, S. TCP transcription factors at the interface between environmental challenges and the plant’s growth responses. Front. Plant Sci. 2016, 7, 1930. [Google Scholar] [CrossRef] [PubMed]
- Dröge-Laser, W.; Snoek, B.L.; Snel, B.; Weiste, C. The Arabidopsis bZIP transcription factor family—An update. Curr. Opin. Plant Biol. 2018, 45, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, C.; Jin, X.; Xue, W.; Dubreuil, C.; Lezhneva, L.; Fischer, U. The plant hormone auxin directs timing of xylem development by inhibition of secondary cell wall deposition through repression of secondary wall NAC-domain transcription factors. Physiol. Plant. 2019, 165, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Kim, S.J.; Abbasi, N.; Bressan, R.A.; Yun, D.-J.; Yoo, S.-D.; Kwon, S.-Y.; Choi, S.-B. The DOF transcription factor Dof5.1 influences leaf axial patterning by promoting Revoluta transcription in Arabidopsis. Plant J. 2010, 64, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Cubas, P. TCP factors: New kids on the signaling block. Curr. Opin. Plant Biol. 2016, 33, 33–41. [Google Scholar] [CrossRef]
- Heinekamp, T.; Strathmann, A.; Kuhlmann, M.; Froissard, M.; Müller, A.; Perrot-Rechenmann, C.; Dröge-Laser, W. The tobacco bZIP transcription factor BZI-1 binds the GH3 promoter in vivo and modulates auxin-induced transcription. Plant J. 2004, 38, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Berendzen, K.W.; Weiste, C.; Wanke, D.; Kilian, J.; Harter, K.; Dröge-Laser, W. Bioinformatic cis-element analyses performed in Arabidopsis and rice disclose bZIP- and MYB-related binding sites as potential AuxRE-coupling elements in auxin-mediated transcription. BMC Plant Biol. 2012, 12, 125. [Google Scholar] [CrossRef]
- Weiste, C.; Pedrotti, L.; Selvanayagam, J.; Muralidhara, P.; Fröschel, C.; Novák, O.; Ljung, K.; Hanson, J.; Dröge-Laser, W. The Arabidopsis bZIP11 transcription factor links low-energy signalling to auxin-mediated control of primary root growth. PLoS Genet. 2017, 13, e1006607. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef]
- IAWA Committee. IAWA list of microscopic features for hardwood identification. IAWA Bull. 1989, 10, 219–332. [Google Scholar]
- Scholz, A.; Klepsch, M.; Karimi, Z.; Jansen, S. How to quantify conduits in wood? Front. Plant Sci. 2013, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Angyalossy, V.; Pace, M.R.; Evert, R.F.; Marcati, C.R.; Oskolski, A.A.; Terrazas, T.; Kotina, E.; Lens, F.; Mazzoni-Viveiros, S.C.; Angeles, G.; et al. IAWA list of microscopic bark features. IAWA J. 2016, 37, 517–615. [Google Scholar] [CrossRef]
- Xu, M.; Zang, B.; Yao, H.S.; Huang, M.R. Isolation of high quality RNA and molecular manipulations with various tissues of Populus. Russ. J. Plant Physiol. 2009, 56, 716–719. [Google Scholar] [CrossRef]
- Salojärvi, J.; Smolander, O.-P.; Nieminen, K.; Rajaraman, S.; Safronov, O.; Safdari, P.; Lamminmäki, A.; Immanen, J.; Lan, T.; Tanskanen, J.; et al. Genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat. Genet. 2017, 49, 904–912. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bryant, S.H. CD-Search: Protein domain annotations on the fly. Nucleic Acids Res. 2004, 32, W327–W331. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK; New York, NY, USA, 2000. [Google Scholar]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.-Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [PubMed]
- Moshchenskaya, Y.L.; Galibina, N.A.; Tarelkina, T.V.; Nikerova, K.M.; Chirva, O.V.; Novitskaya, L.L. Selection of reference genes for normalization of quantitative PCR data in real time in two forms of silver birch. Russ. J. Plant Physiol. in press.
- Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Jin, J.; Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2019, 48, D1104–D1113. [Google Scholar] [CrossRef] [PubMed]




| Gene ID | B. pendula Protein Name | Protein Length (aa) | Motif Sequences | Closest Homolog in A. thaliana (% Identity) | Closest Homolog in P. trichocarpa (% Identity) | |
|---|---|---|---|---|---|---|
| ATG-Containing Motif 1 | ATG-Containing Motif 2 | |||||
| Bpev01.c0000.g0045.m0001 | BpYucca5 | 414 | WLVVATGENAE | LATGY | AtYucca9 (74.3%) | PtYucca8 (86%) |
| Bpev01.c0039.g0010.m0001 | BpYucca10 | 330 | FLIAATGENSE | FATGY | AtYucca10 (49.1%) | PtYucca9 (47.9%) |
| Bpev01.c0118.g0037.m0001 | BpYucca1 | 391 | WFIVATGENAE | LATGY | AtYucca4 (65.9%) | PtYucca1 (71.2%) |
| Bpev01.c0171.g0019.m0001 | BpYucca12 | 381 | FLVVATGEATD | FCTGF | AtYucca10 (48.1%) | PtYucca12 (68.5%) |
| Bpev01.c0363.g0023.m0001 | BpYucca11 | 802 | FLVVATGENSE | FATGY | AtYucca10 (27.4%) | PtYucca12 (27.2%) |
| Bpev01.c0389.g0021.m0001 | BpYucca2 | 421 | WLIVATGENAE | LATGY | AtYucca2 (67.9%) | PtYucca6 (70%) |
| Bpev01.c0547.g0012.m0001 | BpYucca3 | 426 | WLVVATGENAE | LATGY | AtYucca7 (71.4%) | PtYucca3 (79.1%) |
| Bpev01.c1498.g0001.m0001 | BpYucca6 | 424 | WLVVATGENAE | LATGY | AtYucca6 (68.2%) | PtYucca2 (73.5%) |
| Gene ID | B. pendula Protein Name | Protein Length (aa) | Closest Homolog in A. thaliana (% identity) | Closest Homolog in P. trichocarpa (% identity) |
|---|---|---|---|---|
| Bpev01.c0015.g0076.m0001 | BpGH3.1a | 806 | AtGH3.1 (55.2%) | PtGH3.1 (59.9%) |
| Bpev01.c0015.g0077.m0001 | BpGH3.1b | 612 | AtGH3.1 (73.0%) | PtGH3.1 (79.1%) |
| Bpev01.c0015.g0078.m0001 | BpGH3.1c | 612 | AtGH3.1 (72.0%) | PtGH3.1 (78.1%) |
| Bpev01.c0052.g0011.m0001 | BpGH3.7 | 753 | AtGH3.17 (57.4%) | PtGH3.7 (66.2%) |
| Bpev01.c0092.g0010.m0001 | BpGH3.5 | 612 | AtGH3.6 (84.3%) | PtGH3.5 (87.7%) |
| Bpev01.c0579.g0028.m0001 | BpGH3.9 | 595 | AtGH3.9 (72.1%) | PtGH3.9 (81.5%) |
| Bpev01.c1074.g0008.m0001 | BpGH3.4 | 749 | AtGH3.6 (64.2%) | PtGH3.4 (67.5%) |
| Bpev01.c1398.g0014.m0001 | BpGH3.2 | 598 | AtGH3.1 (81.5%) | PtGH3.3 (86.8%) |
| Bpev01.c1522.g0002.m0001 | BpGH3.3 | 495 | AtGH3.1 (50.0%) | PtGH3.3 (53.9%) |
| Process | Gene family | Set 1 | Set 2 |
|---|---|---|---|
| IAA biosynthesis | Yucca | BpYucca5 | BpYucca1 |
| BpYucca10 | BpYucca5 | ||
| BpYucca11 | BpYucca6 | ||
| BpYucca12 | BpYucca10 | ||
| BpYucca12 | |||
| IAA transport | PIN | BpPIN1b | |
| BpPIN3 | |||
| BpPIN6 | |||
| BpPIN8 | |||
| IAA conjugation | GH3 | GH3.1a | GH3.1c |
| GH3.1b | GH3.2 | ||
| GH3.4 | GH3.4 | ||
| GH3.5 | GH3.5 | ||
| GH3.9 | |||
| UGT | UGT84B1 | UGT84B1 |
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| BpYucca1 | ATTCAATAATCTTAGCAACTG | TGTATAAGTCCGTTCTCT |
| BpYucca2 | GTTAGAGATTCGGTTCAT | TGACATTAGAAGCAAGAA |
| BpYucca3 | TATTAGTTGTTGGTTGTG | ATATCAGCAATATCTTATCG |
| BpYucca5 | GGAGTACAAGAACACCAT | GCAAGAACAACTGAATCAA |
| BpYucca6 | TATACTCAGTTGGGTTTA | AAATGAAATGAAGAATAAGG |
| BpYucca10 | AGGTGGAAGTATATGTTG | TGTAATGGTTGGAATGTA |
| BpYucca11 | TAGAAGGAAGTATGGAAG | GTTGGAATGGAGAATATC |
| BpYucca12 | GCTTCAAGAGGTCCACAA | TAGTCCAACGCAGTATAATCC |
| BpPIN1a | AAGGCTTATTCTCATCAT | GTATGGAAATGGACTTTG |
| BpPIN1b | CTCTCCTTTCCTTTCACTTC | ACCACCAAGACGATTACT |
| BpPIN2 | AATAGAGGAAGGATTGAAG | GACTTGAGTAGGTGTTAG |
| BpPIN3 | GTTCTCGTCCACTATTACTAA | GCAACTCCTTAGCATCAT |
| BpPIN6 | TCATATCTCAGAACAATCC | AAGAGTGATAAGCCAATC |
| BpPIN8 | CATTATATGTTGTGATGATAGTAG | AGCAGAGGAATTGAGTAT |
| BpGH3.1a | GAAGCAATGGATATGATT | CAATTAGTGTCTAGGATG |
| BpGH3.1b | AGTGTTCAGAAGAGAATT | GAGTGTTGGTATGTATTG |
| BpGH3.1c | ATTCCTACAACTCAACTG | ATTCGTGTGATTATACCTT |
| BpGH3.2 | TTTATTGCCAAAGAATGC | AGATTGACTCCGAAATAG |
| BpGH3.3 | CAGACTCTGGATCACTAT | CATTATGGTGTACGAGAC |
| BpGH3.4 | TTATAGTAACAGGAACAATG | GCAAGAACTCATAATAACA |
| BpGH3.5 | AGTATGTGGATGTTATTGT | AAGAGGATTAAGGTTGAC |
| BpGH3.7 | CTGTTGTGAATTATGAAGA | AAGGATGTTGTAGAAGAA |
| BpGH3.9 | TATCTCAACAACTATCTCAA | TCACCATTAGCAATTCTT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarelkina, T.V.; Novitskaya, L.L.; Galibina, N.A.; Moshchenskaya, Y.L.; Nikerova, K.M.; Nikolaeva, N.N.; Sofronova, I.N.; Ivanova, D.S.; Semenova, L.I. Expression Analysis of Key Auxin Biosynthesis, Transport, and Metabolism Genes of Betula pendula with Special Emphasis on Figured Wood Formation in Karelian Birch. Plants 2020, 9, 1406. https://doi.org/10.3390/plants9111406
Tarelkina TV, Novitskaya LL, Galibina NA, Moshchenskaya YL, Nikerova KM, Nikolaeva NN, Sofronova IN, Ivanova DS, Semenova LI. Expression Analysis of Key Auxin Biosynthesis, Transport, and Metabolism Genes of Betula pendula with Special Emphasis on Figured Wood Formation in Karelian Birch. Plants. 2020; 9(11):1406. https://doi.org/10.3390/plants9111406
Chicago/Turabian StyleTarelkina, Tatiana V., Ludmila L. Novitskaya, Natalia A. Galibina, Yulia L. Moshchenskaya, Kseniya M. Nikerova, Nadezhda N. Nikolaeva, Irina N. Sofronova, Diana S. Ivanova, and Ludmila I. Semenova. 2020. "Expression Analysis of Key Auxin Biosynthesis, Transport, and Metabolism Genes of Betula pendula with Special Emphasis on Figured Wood Formation in Karelian Birch" Plants 9, no. 11: 1406. https://doi.org/10.3390/plants9111406
APA StyleTarelkina, T. V., Novitskaya, L. L., Galibina, N. A., Moshchenskaya, Y. L., Nikerova, K. M., Nikolaeva, N. N., Sofronova, I. N., Ivanova, D. S., & Semenova, L. I. (2020). Expression Analysis of Key Auxin Biosynthesis, Transport, and Metabolism Genes of Betula pendula with Special Emphasis on Figured Wood Formation in Karelian Birch. Plants, 9(11), 1406. https://doi.org/10.3390/plants9111406






