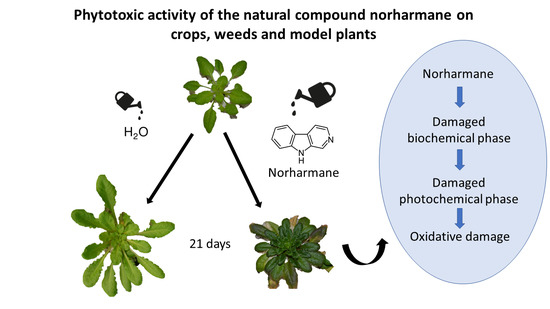
Phytotoxic Activity of the Natural Compound Norharmane on Crops, Weeds and Model Plants
Abstract
:1. Introduction
2. Results
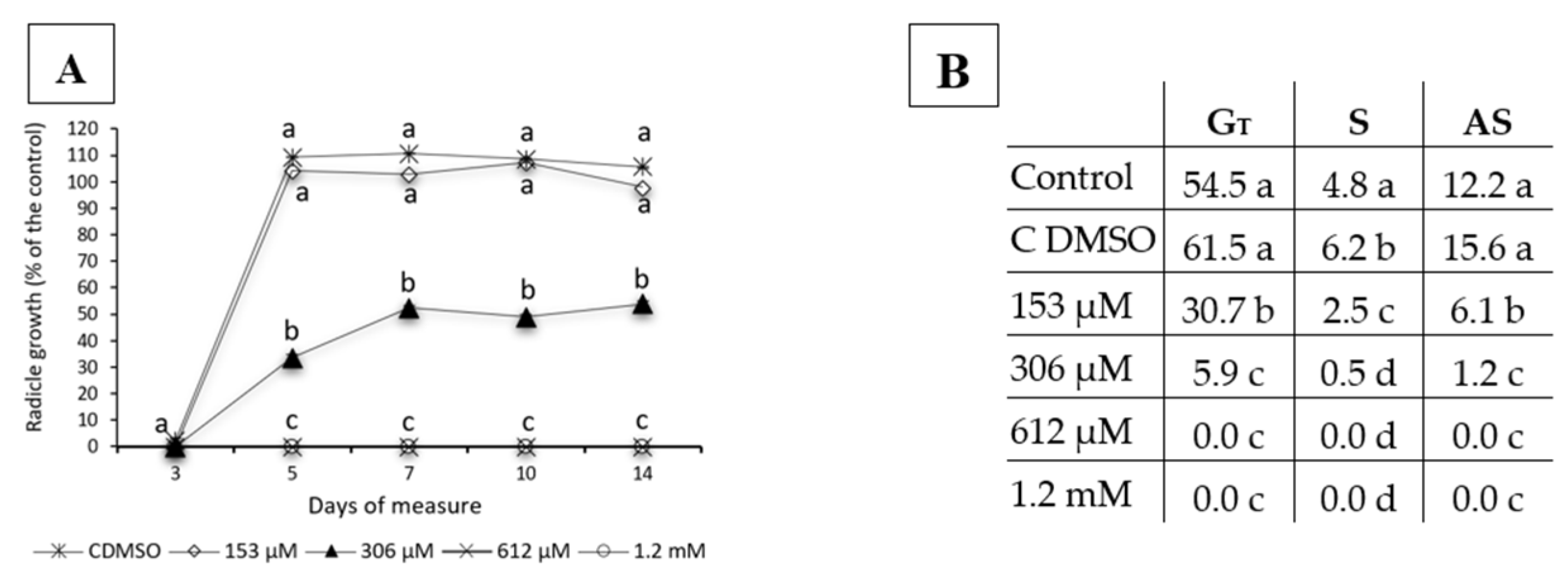
2.1. Effects of Norharmane on the Germination and Growth of Weeds and Crops
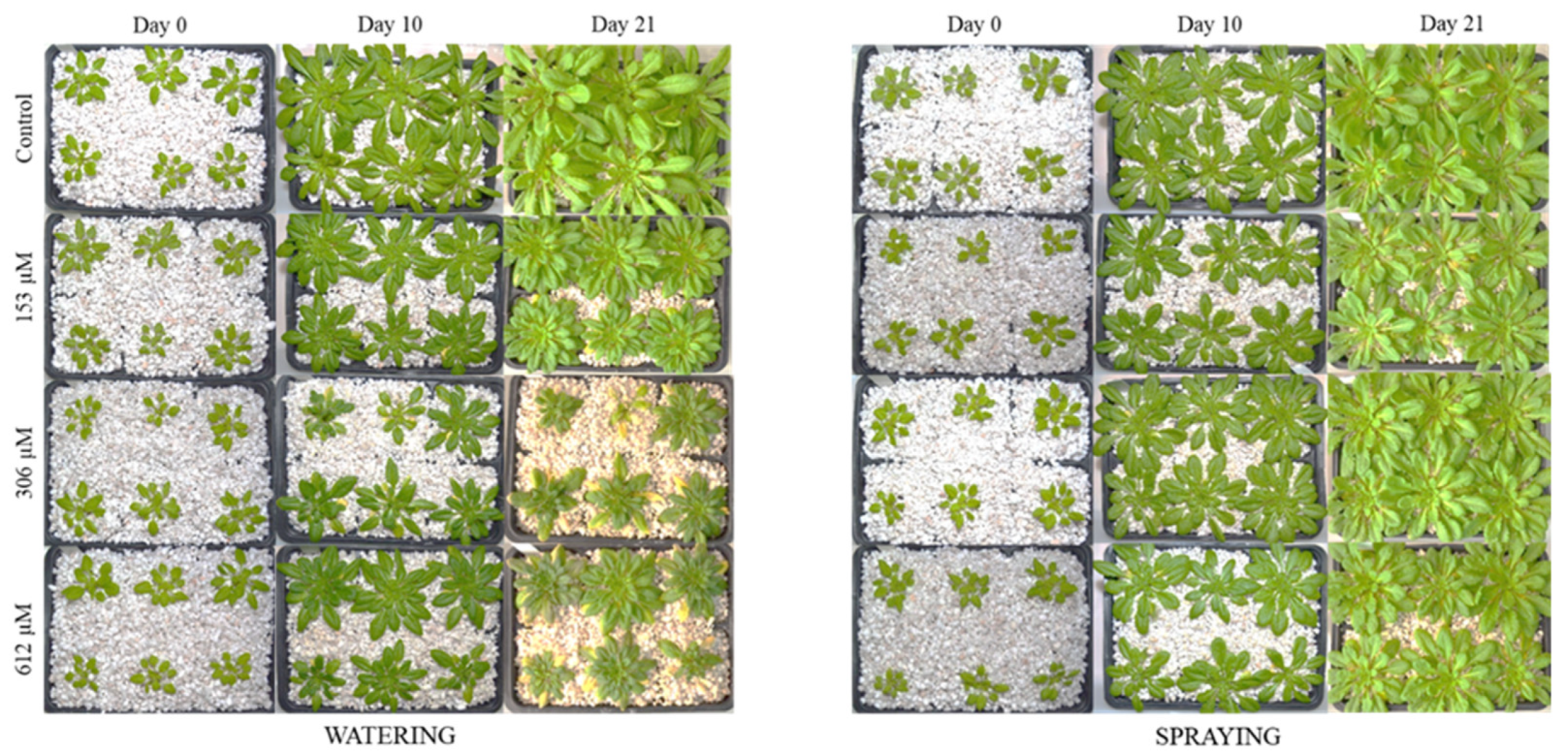
2.2. Effect of Norharmane on A. thaliana Adult Plants
2.2.1. Morphological Study
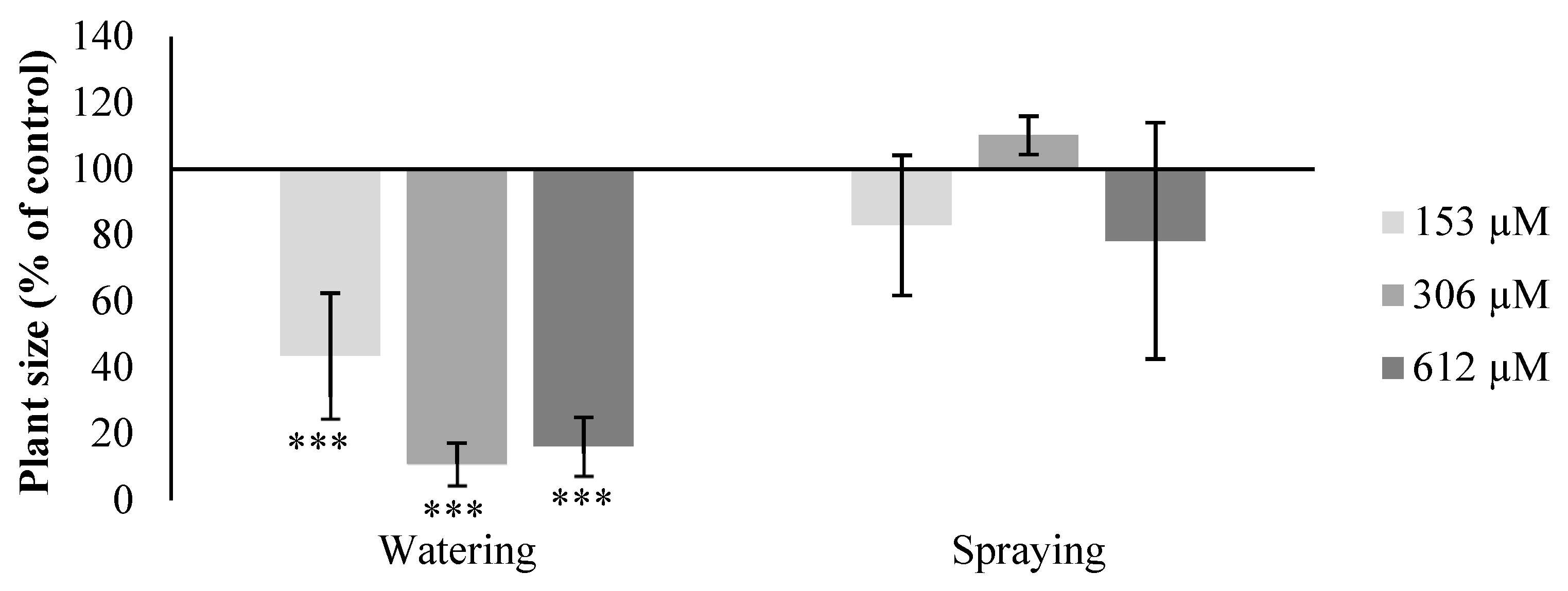
2.2.2. Leaf Area
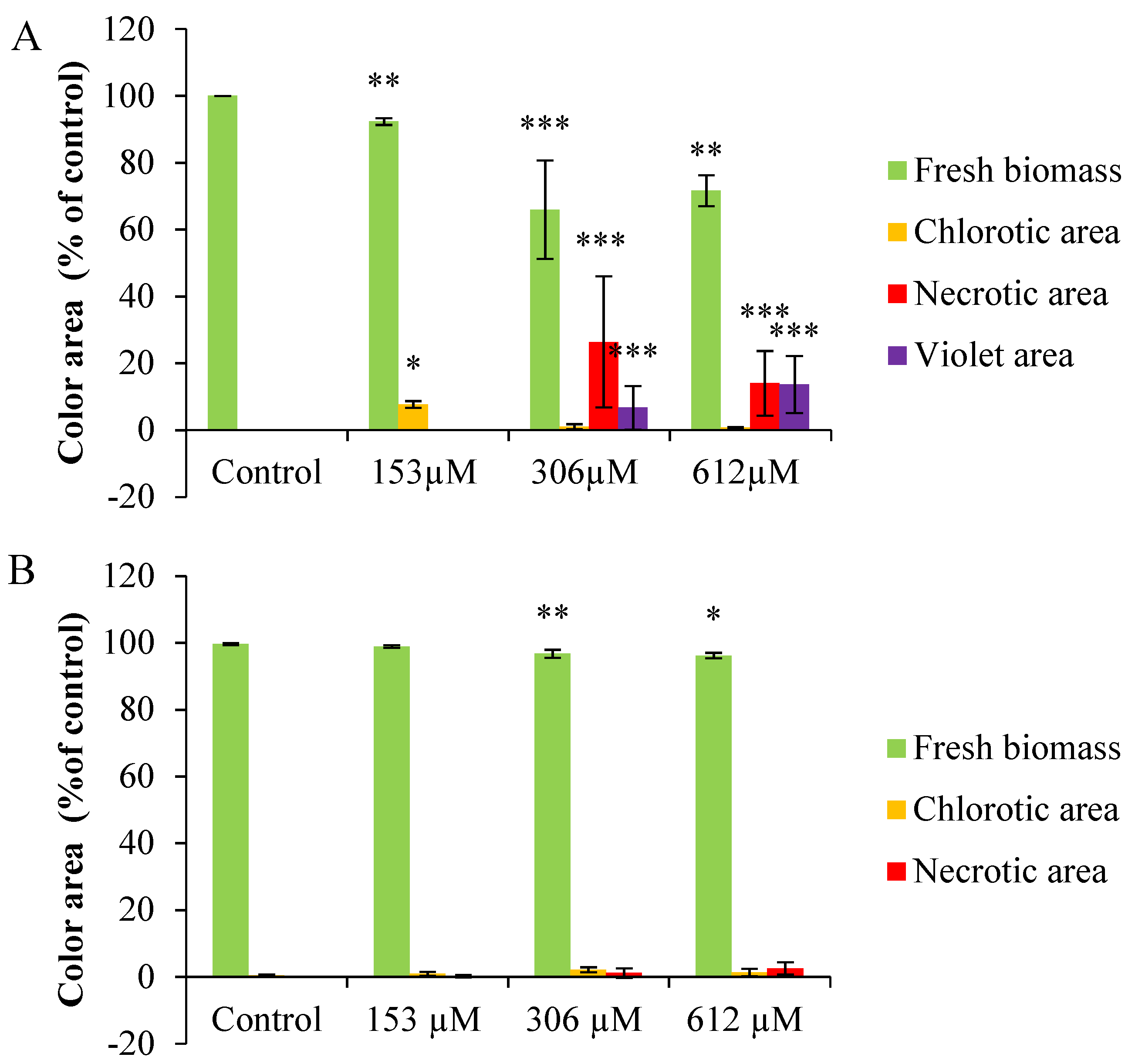
2.2.3. Colorimetric Analysis
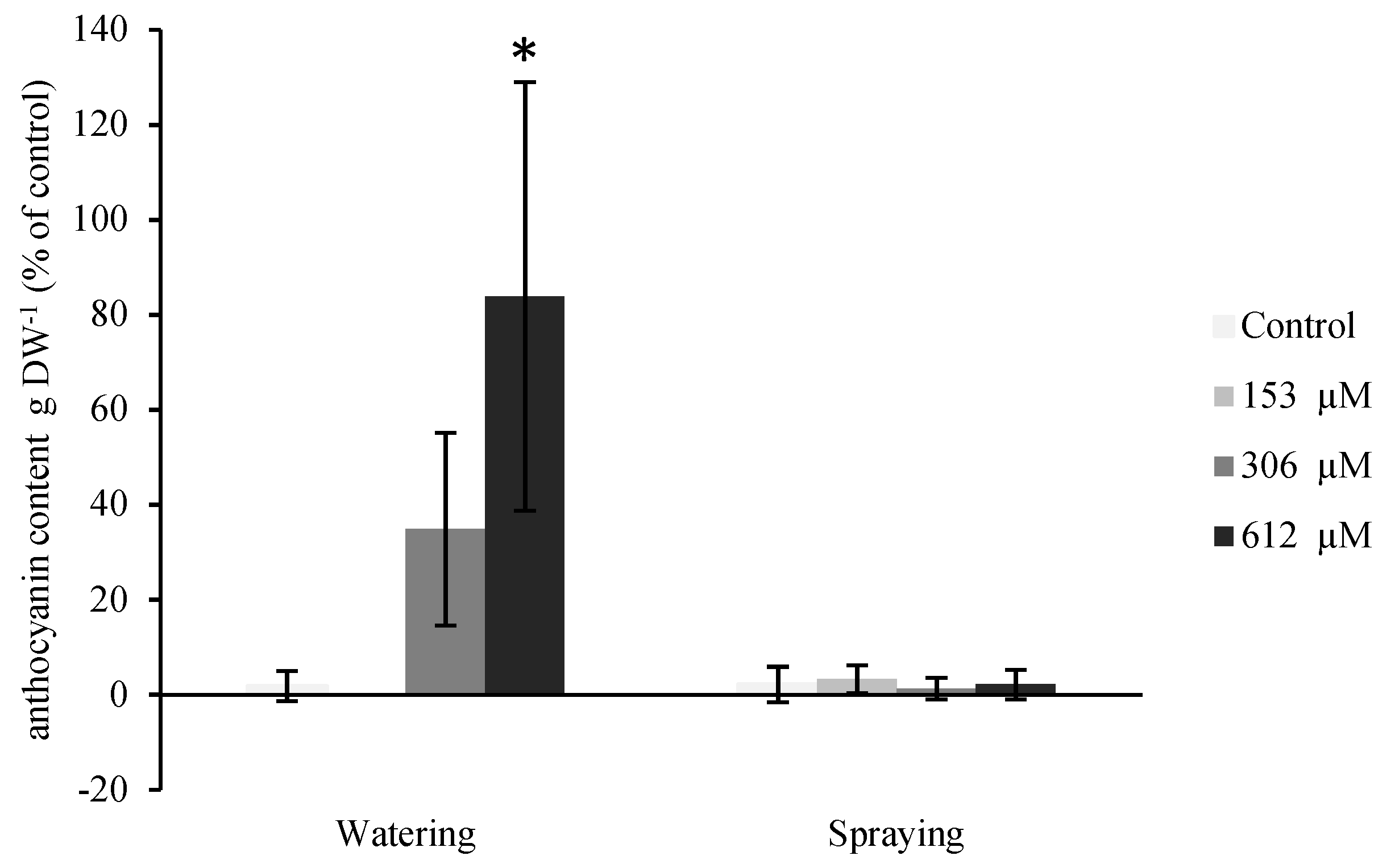
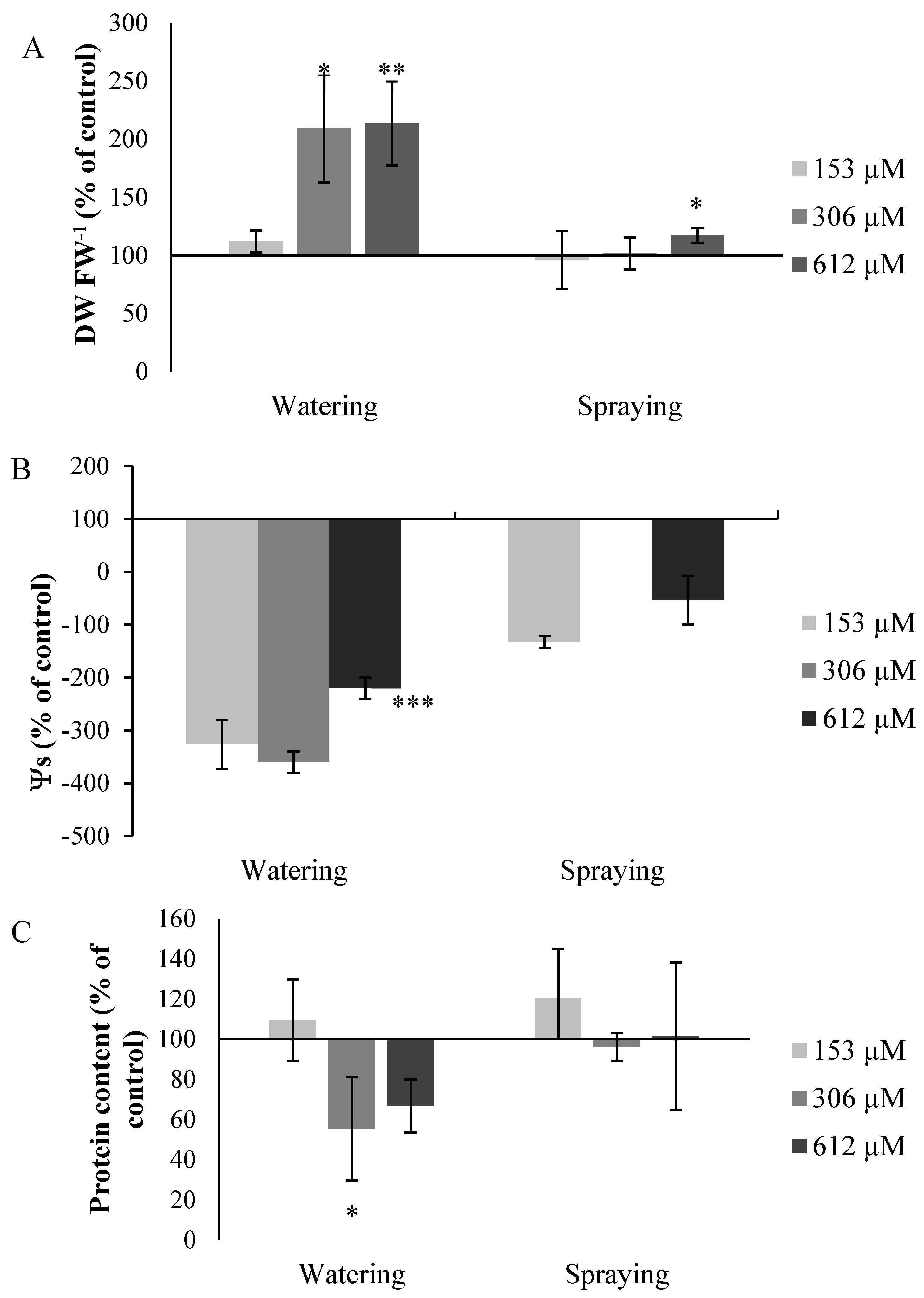
2.2.4. Anthocyanin Content
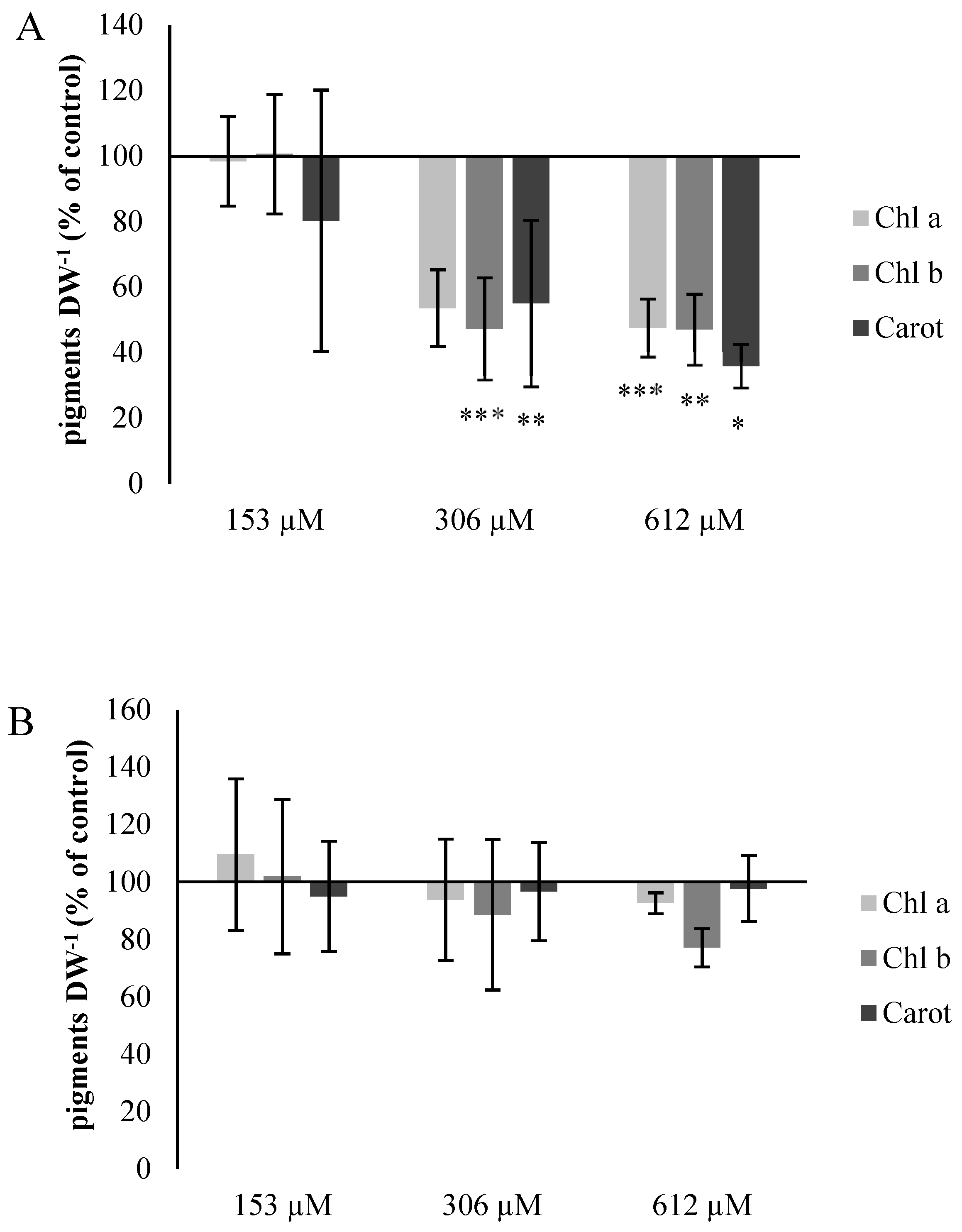
2.2.5. Chlorophyll and Carotene Content
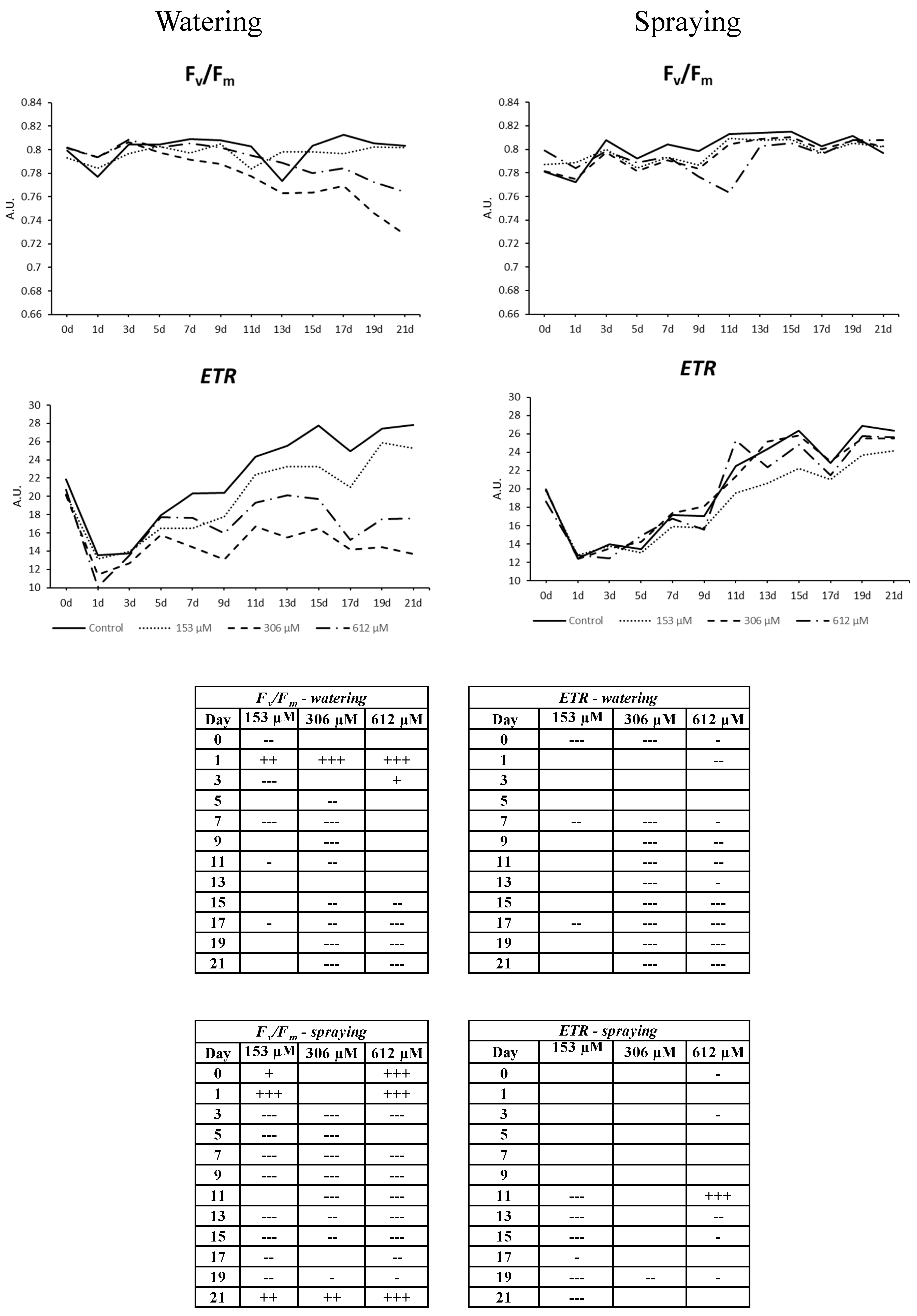
2.2.6. Chlorophyll a Fluorescence Measurement
2.2.7. Other Measures
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Germination and Growth Tests
5.1.1. Screening of Crops and Weeds
- Total germination (GT): NT/N × 100, where T indicates the time of the latest observation and N represents the total number of seeds sown.
- Germination speed (S): the number of seeds that germinated between two measurement time points, and it was calculated as follows: n1 + (1/2) × n2 + (1/3) × n3 +…+(1/T) × nT, where nt = (Nt − Nt−1)/NT (with N0 = 0) is the number of seeds that germinated between times t−1 and t; it is expressed as a proportion of all the seeds that germinated. This parameter indicates the number of germinated seeds between two measurement times.
- Accumulative germination speed (AS): the number of cumulative germinated seeds for each measurement time point, and it was calculated as follows: N1+ (1/2) × N2 + (1/3) × N3 +…+ (1/T) × NT, which may be proportional to a weighted average of the speed, in which the weight of each element of the equation increases with the measurement time point. This index describes the accumulated number of seed germinated in each measurement period.
5.1.2. Monitoring of E. bonariensis Pre-Emergence
5.2. Phytotoxicity Bioassay on the Metabolism of Adult Plants
5.2.1. Measurement of Chlorophyll a Fluorescence
- ΦII is the the effective photochemical quantum yield of Photosystem II (PSII), which measures the proportion of light absorbed by the chlorophyll associated to PSII that is used in the quantum yield. It can provide insight into the speed of the linear electron flow and, therefore, can be an indicator of the overall photosynthetic capacity in vivo [51]. ΦII + ΦNPQ + ΦNO = 1.
- ΦNPQ is the measurement of regulated non-photochemical non-fluorescent energy dissipation (as heat). This parameter is linearly related to heat dissipation and ranges from 0 to infinity. The common values in a healthy plant are in the range of 0.5–3.5 at saturating light intensities, although this varies between different species [51]. High values of this parameter indicate an excessive photon flux density, so that plant protects itself dissipating the energy as heat. An increase of this parameter indicates that excess energy is being produced in the photosynthetic apparatus, but the plant is able to regulate such excess avoiding damages; ΦNPQ = 1 − ΦII − ΦNO.
- ΦNO is the measurement of non-regulated emission of energy (as fluorescence). It determines the non-regulated energy loss as fluorescence [52]. High values in this parameter indicate that photochemical energy conversion and protective regulatory mechanisms are inefficient, which means that the plant is struggling to deal with incident radiation. Very high values indicate that the PSII reaction centers are blocked, preventing the build-up of a trans-thylakoid proton gradient, and suggest that the plant has been damaged or is about to be; ΦNO = Fs/Fm.
- Fv/Fm is the maximum efficiency of PSII. After being exposed to darkness, PSII reaction centers are normally open (F = F0), non-photochemical quenching is minimal (NPQ = 0) and the fluorescence yield is at its maximum. In this state, the increase of fluorescence is induced by saturation pulses (Fv) and the quantum yield of PSII is the maximum. A reduction of this parameter usually indicates physical damages in the antenna system of PSII; Fv/Fm = (Fm − F0)/Fm.
- ETR is the apparent electron transport rate. Estimate of the electron flow rate, calculated with the following equation. ETR = 0.5 × Yield × PAR × 0.84 μequiv m−2 s−1, where PAR is the distance between the sample and the LED lighting unit (Cond. Est. = 18.5 cm).
5.2.2. Post-Harvest Measurements
Measurement of Dry Weight/Fresh Weight Ratio (DW/FW)
Measurement of Osmotic Potential (Ψs)
Measurement of Photosynthetic Pigments
Measurement of Anthocyanins
Measurement of Total Proteins
Colorimetric Analysis and Leaf Surface
5.3. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Manag. Sci. 2011, 68, 505–512. [Google Scholar] [CrossRef] [Green Version]
- Dayan, F.E.; Owens, D.K.; Duke, S.O. Rationale for a natural products approach to herbicide discovery. Pest Manag. Sci. 2012, 68, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Soltys, D.; Krasuska, U.; Bogatek, R.; Gniazdowsk, A. Allelochemicals as bioherbicides—Present and perspectives. In Herbicides—Current Research and Case Studies in Use; Price, A., Kelto, J., Eds.; IntechOpen: London, UK, 2013; pp. 517–542. [Google Scholar]
- Bailey, K.L. The Bioherbicide Approach to Weed Control Using Plant Pathogens. In Integrated Pest Management: Current Concepts and Ecological Perspective, 1st ed.; Abrol, D., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 245–266. [Google Scholar]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar]
- Radhakrishnan, R.; Alqarawi, A.A.; Abd Allah, E.F. Bioherbicides: Current knowledge on weed control mechanism. Ecotoxicol. Environ. Safe. 2018, 158, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.J.; Wilson, H.P.; Hines, T.E. Preemergence herbicides followed by trifloxysulfuron postemergence in cotton. Weed Technol. 2007, 21, 1–6. [Google Scholar] [CrossRef]
- Ahmed, S.; Chauhan, B.S. Performance of different herbicides in dry-seeded rice in Bangladesh. Sci. World J. 2014, 2014, 729418. [Google Scholar] [CrossRef] [Green Version]
- Kelley, K.B.; Wax, L.M.; Hager, A.G.; Riechers, D.E. Soybean response to plant growth regulator herbicides is affected by other postemergence herbicides. Weed Sci. 2005, 53, 101–112. [Google Scholar] [CrossRef]
- Volk, R.B. Screening of microalgal culture media for the presence of algicidal compounds and isolation and identification of two bioactive metabolites, excreted by the cyanobacteria Nostoc insulare and Nodularia harveyana. J. Appl. Phycol. 2005, 17, 339–347. [Google Scholar] [CrossRef]
- Poindexter, E.H.; Carpenter, R.D. The isolation of harmane and norharmane from tobacco and cigarette smoke. Phytochemistry 1962, 1, 215–221. [Google Scholar] [CrossRef]
- Volk, R.B. Antialgal activity of several cyanobacterial exometabolites. J. Appl. Phycol. 2006, 18, 145–151. [Google Scholar] [CrossRef]
- Wojtowicz, E.; Zawirska-Wojtasiak, R.; Przygoński, K.; Mildner-Szkudlarz, S. Bioactive β-carbolines norharman and harman in traditional and novel raw materials for chicory coffee. Food Chem. 2015, 175, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase enzyme inhibition by coffee and β-carboniles norharman and harman isolated from coffee. Life Sci. 2006, 78, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Herraiz, T. Analysis of the bioactive alkaloids tetrahydro-β-carboline and β- carboline in food. J. Chromatogr. A 2000, 881, 483–499. [Google Scholar] [CrossRef]
- Cooper, E.J.; Hudson, A.L.; Parker, C.A.; Morgan, N.G. Effects of the β-carbolines, harmane and pinoline, on insulin secretion from isolated human islets of Langerhans. Eur. J. Pharmacol. 2003, 482, 189–196. [Google Scholar] [CrossRef]
- Nii, H. Possibility of the involvement of 9H-pyrido[3,4-b] indole (norharman) in carcinogenesis via inhibition of cytochrome P450-related activities and intercalation to DNA. Mutat. Res. 2003, 541, 123–136. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Allelopathic activity of the norharmane-producing cyanobacterium Synechocystis aquatilis against cyanobacteria and microalgae. Oceanol. Hydrobiol. Stud. 2013, 42, 1–7. [Google Scholar] [CrossRef]
- López-González, D.; Costas-Gil, A.; Reigosa, M.J.; Araniti, F.; Sánchez-Moreiras, A.M. A natural indole alkaloid, norharmane, affects PIN expression patterns and compromises root growth in Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 151, 378–390. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Valera-Burgos, J.; Díaz-Barradas, M.C.; Zunzunegui, M. Effects of Pinus pinea litter on seed germination and seedling performance of three Mediterranean shrub species. Plant Growth Regul. 2012, 66, 285–292. [Google Scholar] [CrossRef]
- Begna, S.H.; Hamilton, R.I.; Dwyer, L.M.; Stewart, D.W.; Cloutier, D.; Assemat, L.; Foroutan-Pour, K.; Smith, D.L. Weed biomass production response to plant spacing and corn (Zea mays) hybrids differing in canopy architecture. Weed Technol. 2001, 15, 647–653. [Google Scholar] [CrossRef]
- Gaba, S.; Reboud, X.; Fried, G. Agroecology and conservation of weed diversity in agricultural lands. Bot. Lett. 2016, 163, 351–354. [Google Scholar] [CrossRef] [Green Version]
- Le, D.; Nguyen, C.M.; Mann, R.K.; Yerkes, C.N.; Kumar, B.V.N. Genetic diversity and herbicide resistance of 15 Echinochloa crus-galli populations to quinclorac in Mekong Delta of Vietnam and Arkansas of United States. J. Plant Biotechnol. 2017, 44, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Khanh, T.D.; Trung, K.H.; Anh, L.H.; Xuan, T.D. Allelopathy of barnyardgrass (Echinochloa crus-galli) weed: An allelopathic Interaction with rice (Oryza sativa). Vietnam J. Agric. Sci 2018, 1, 97–116. [Google Scholar]
- Graña, E.; Sotelo, T.; Díaz-Tielas, C.; Reigosa, M.J.; Sánchez-Moreiras, A.M. The phytotoxic potential of the terpenoid citral on seedlings and adult plants. Weed Sci. 2013, 61, 469–481. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Tielas, C.; Sotelo, T.; Graña, E.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Phytotoxic potential of trans-chalcone on crop plants and model species. J. Plant Growth Regul. 2014, 33, 181–194. [Google Scholar] [CrossRef]
- Pellissier, F. Improved germination bioassays for allelopathy research. Acta Physiol. Plant. 2013, 35, 23–30. [Google Scholar] [CrossRef]
- Liebman, M.; Davis, A.S. Integration of soil, crop and weed management in low-external-input farming systems. Weed Res. 2000, 40, 27–47. [Google Scholar] [CrossRef] [Green Version]
- Mall, U.; Singh, G.S. Soil Seed Bank Dynamics: History and Ecological Significance in Sustainability of Different Ecosystems. In Environment and Sustainable Development; Fulekar, M., Pathak, B., Kale, R., Eds.; Springer: New Delhi, India, 2014; pp. 31–46. [Google Scholar]
- Chaves, N.L.; Alías, J.C.G.; Sosa, T.D.; Escudero, J.C.G. Allelopathic potential of Cistus ladanifer chemicals in response to variations of light and temperature. Chemoecology 2002, 12, 139–145. [Google Scholar] [CrossRef]
- Chen, S.; Xiao, S.; Callaway, R.M. Light intensity alters the allelopathic effects of an exotic invader. Plant Ecol. Divers. 2012, 5, 521–526. [Google Scholar] [CrossRef]
- Hashoum, H.; Saatkamp, A.; Gauquelin, T.; Ruffault, J.; Fernandez, C.; Bousquet-Mélou, A. Mediterranean woody plant specialized metabolites affect germination of Linum perenne at its dry and upper thermal limits. Plant Soil 2020, 446, 291–305. [Google Scholar] [CrossRef]
- Cronquist, A. 1976. Conyza Less. In Flora Europaea, Plantaginaceae to Compositae (and Rubiaceae), 4th ed.; Tutin, T.G., Heywood, V.H., Burges, N.A., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1976. [Google Scholar]
- Bajwa, A.A.; Sadia, S.; Ali, H.H.; Jabran, K.; Peerzada, A.M.; Chaudan, B.S. Biology and management of two important Conyza weeds: A global review. Environ. Sci. Pollut. Res. 2016, 23, 24694–24710. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Walker, S.; Robinson, G.; Coombers, N. Control of Flaxleaf Fleabane (Conyza bonariensis) in Wheat and Sorghum. Weed Technol. 2010, 24, 102–107. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Alvino, A.; Delfine, S.; Mori, M. Foliar senescence in maize plants grown under different water regimes. Agronomie 1999, 19, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.I.; Reigosa, M.J.; Al-Dakheel, A.J. Biochemical, physiological and isotopic responses to natural product p-hydroxybenzoic acid in Cocksfoot (Dactylis glomerata L.). Plant Growth Regul. 2015, 75, 783–792. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Interaction of proline, sugars, and anthocyanins during photosynthetic acclimation of Arabidopsis thaliana to drought stress. J. Plant Physiol. 2012, 169, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Ozfidan, C.; Turkan, I.; Sekmen, A.H.; Seckin, B. Time course analysis of ABA and non-ionic osmotic stress-induced changes in water status, chlorophyll fluorescence and osmotic adjustment in Arabidopsis thaliana wild-type (Columbia) and ABA-deficient mutant (aba2). Environ. Exp. Bot. 2013, 86, 44–51. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Bresson, J.; Vasseur, F.; Dauzat, M.; Koch, G.; Granier, C.; Vile, D. Quantifying spatial heterogeneity of chlorophyll fluorescence during plant growth and in response to water stress. Plant Methods 2015, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Beninger, C.W.; Abou-Zaid, M.M.; Kistner, A.L.E.; Hallett, R.H.; Iqbal, M.J.; Grodzinski, B.; Hall, J.C. A flavanone and two phenolic acids from Chrysanthemum morifolium with phytotoxic and insect growth regulating activity. J. Chem. Ecol. 2004, 30, 589–606. [Google Scholar] [CrossRef]
- Oikonomou, A.; Ladikou, E.V.; Chatziperou, G.; Margaritopoulou, T.; Landi, M.; Sotiropoulos, T.; Araniti, F.; Papadakis, I.E. Boron excess imbalances root/shoot allometry, photosynthetic and chlorophyll fluorescence parameters and sugar metabolism in apple plants. Agronomy 2019, 9, 731. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Chen, S.; Fan, X.; Song, H.; Li, X.; Xu, J.; Qian, H. Diuron treatment reveals the different roles of two cyclic electron transfer pathways in photosystem II in Arabidopsis thaliana. Pestic. Biochem. Physiol. 2017, 137, 15–20. [Google Scholar] [CrossRef]
- Sánchez-Moreiras, A.M.; Martínez-Peñalver, A.; Reigosa, M.J. Early senescence induced by 2-3H-benzoxazolinone (BOA) in Arabidopsis thaliana. J. Plant Physiol. 2011, 168, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Sade, N.; del Mar Rubio-Wilhelmi, M.; Umnajkitikorn, K.; Blumwald, E. Stress-induced senescence and plant tolerance to abiotic stress. J. Exp. Bot. 2018, 69, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Chiapusio, G.; Sánchez, A.M.; Reigosa, M.J.; González, L.; Pellissier, F. Do germination indices adequately reflect allelochemical effects on the germination process? J. Chem. Ecol. 1997, 23, 2445–2453. [Google Scholar] [CrossRef]
- Martínez-Peñalver, A.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Imaging chlorophyll a fluorescence reveals specific spatial distributions under different stress conditions. Flora Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 836–844. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Wellburn, R.W. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L.; Brown, P.H.; Holz, G.K. Cold-induced photoinhibition affects establishment of Eucalyptus nitens (Deane and Maiden) Maiden and Eucalyptus globulus Labill. Trees Struct. Funct. 2000, 15, 32–41. [Google Scholar] [CrossRef]
- Bonjoch, N.P.; Tamayo, P.R. Protein content quantification by Bradford method. In Handbook of Plant Ecophysiology Techniques, 1st ed.; Reigosa, M.J.R., Ed.; Springer: Dordrecht, The Netherlands, 2001; pp. 283–295. [Google Scholar]
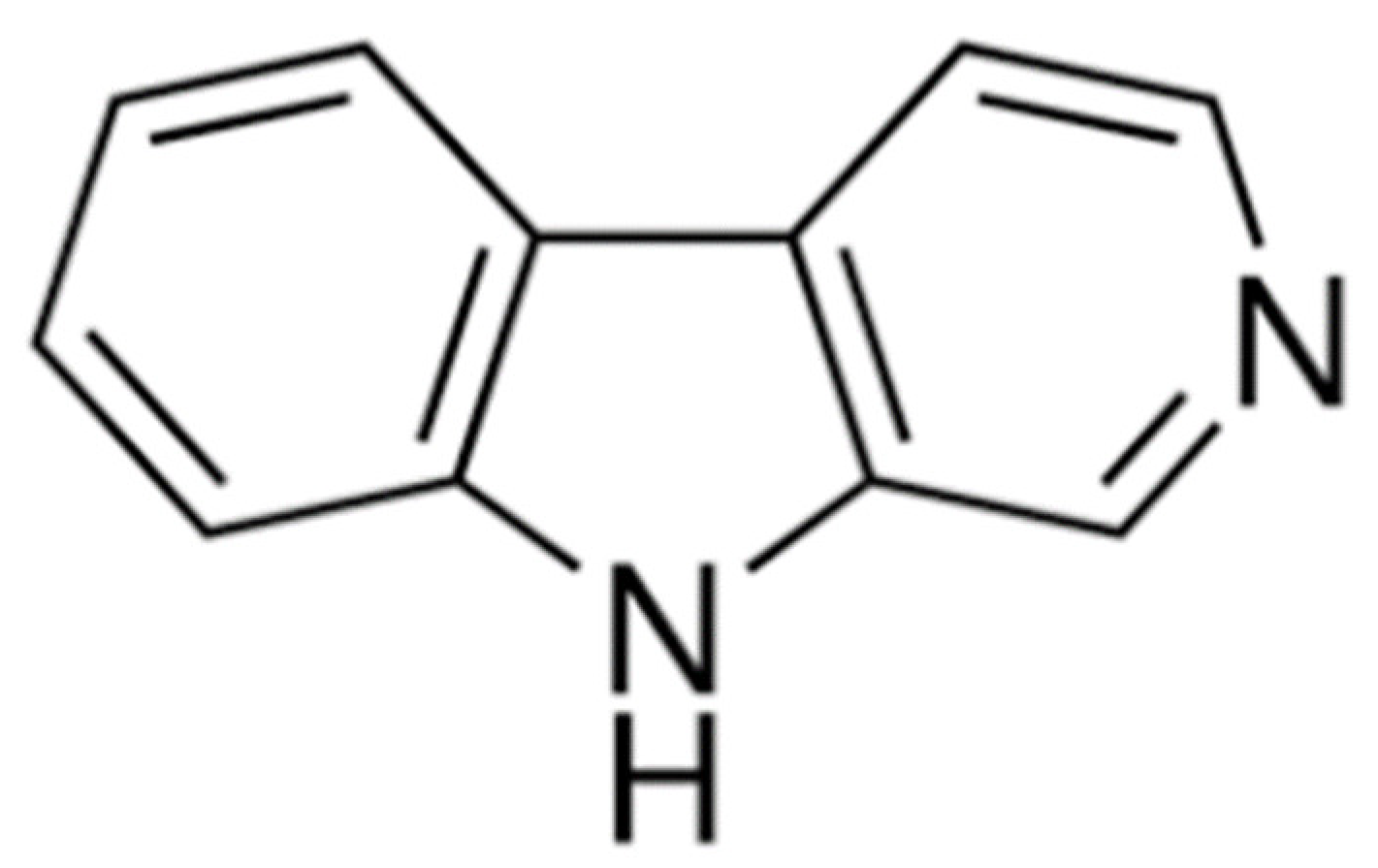

| P. lanceolata | GT (%) | S | AS | E. crus-galli | GT (%) | S | AS | P. oleracea | GT (%) | S | AS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 100 ab | 2.6 ab | 7.9 a | Control | 100 a | 3.48 a | 12.55 a | Control | 100 ab | 3.10 ab | 9.63 a |
| 50 µM | 115 a | 2.9 a | 9.2 a | 50 µM | 101 a | 3.62 a | 12.56 ab | 50 µM | 95.4 ab | 3.09 ab | 9.52 a |
| 100 µM | 111 ab | 3.4 a | 10.2 a | 100 µM | 98.7 a | 3.4o a | 10.94 ab | 100 µM | 136 a | 3.80 ab | 13.5 a |
| 200 µM | 94.5 b | 2.6 a | 7.8 a | 200 µM | 90.2 a | 3.1o a | 9.45 ab | 200 µM | 131 ab | 4.20 a | 12.9 a |
| 400 µM | 97.9 ab | 1.8 b | 5.1 b | 400 µM | 91.4 a | 3.15 a | 9.52 ab | 400 µM | 76.7 b | 2.57 b | 8.00 a |
| 800 µM | 27.1 b | 1 c | 1.6 c | 800 µM | 90.6 a | 3.20 a | 9.57 b | 800 µM | 99.5 ab | 2.73 b | 8.43 a |
| A. retroflexus | GT (%) | S | AS | A. fatua | GT (%) | S | AS | T. aestivum | GT (%) | S | AS |
| Control | 100 a | 5.90 ab | 25.9 ab | Control | 100 ab | 3.74 a | 13.0 a | Control | 100 a | 11.9 a | 28.4 a |
| 50 µM | 102 a | 6.00 ab | 26.5 ab | 50 µM | 104 ab | 3.96 a | 13.9 a | 50 µM | 101 a | 11.2 ab | 28.5 a |
| 100 µM | 102 a | 6.40 a | 27.7 ab | 100 µM | 92.8 a | 3.87 a | 13.8 a | 100 µM | 121 a | 10.3 ab | 23.5 ab |
| 200 µM | 103 a | 6.40 ab | 28.1 a | 200 µM | 76.7 bc | 3.35 ab | 12.0 ab | 200 µM | 128 a | 11.0 ab | 25.7 ab |
| 400 µM | 99.2 a | 6.80 ab | 29.2 ab | 400 µM | 81.4 abc | 3.10 ab | 10.8 ab | 400 µM | 119 a | 10.2 ab | 23.7 ab |
| 800 µM | 101 a | 7.10 b | 30.4 b | 800 µM | 57.0 c | 1.88 b | 6.33 b | 800 µM | 90.3 a | 9.36 b | 21.6 b |
| O. sativa | GT (%) | S | AS | Z. mays | GT (%) | S | AS | L. sativa | GT (%) | S | AS |
| Control | 100 ab | 4.86 abc | 11.4 abc | Control | 100 ab | 7.96 a | 18.6 a | Control | 100 a | 2.33 a | 5.79 a |
| 50 µM | 118 ab | 6.53 a | 15.0 a | 50 µM | 91.7 ab | 6.64 ab | 14.3 bc | 50 µM | 84.8 ab | 1.96 ab | 4.79 ab |
| 100 µM | 101 ab | 4.90 b | 12.0 b | 100 µM | 81.5 ab | 6.45 ab | 14.1 bc | 100 µM | 88.4 ab | 2.04 ab | 5.36 ab |
| 200 µM | 115 a | 5.40 b | 12.3 ab | 200 µM | 95.7 a | 7.93 a | 17.1 ab | 200 µM | 71.6 b | 1.64 b | 4.90 b |
| 400 µM | 80.8 b | 3.64 c | 8.74 c | 400 µM | 85.2 ab | 5.91 b | 12.3 c | 400 µM | 41.8 c | 0.96 c | 2.29 c |
| 800 µM | 79.6 ab | 4.33 abc | 10.7 abc | 800 µM | 82.4 b | 6.00 b | 14.0 bc | 800 µM | 47.8 c | 1.10 c | 2.64 c |
| Root Length (%) | P. lanceolata | A. retroflexus | A. fatua | E. crus-galli | P. oleracea | Z. mays | L. sativa | T. aestivum | O. sativa |
|---|---|---|---|---|---|---|---|---|---|
| Control | 100 a | 100 a | 100 a | 100 a | 100 a | 100 a | 100 a | 100 a | 100 a |
| 50 µM | 75.0 b | 88.9 b | 91.7 a | 85.9 b | 154 b | 95.5 a | 120 b | 90.3 ab | 93.6 b |
| 100 µM | 78.5 b | 96.0 ac | 105 a | 81.9 b | 142 b | 106 ab | 108 ac | 86.6 ab | 96.8 ab |
| 200 µM | 68.8 b | 90.6 bc | 94.7 a | 73.5 b | 102 a | 110 b | 114 bc | 91.6 ab | 101 ab |
| 400 µM | 39.5 c | 74.0 d | 59.4 b | 75.1 b | 57.1 c | 111 b | 67.3 d | 74.9 b | 99.0 ab |
| 800 µM | 8.46 d | 56.6 e | 19.8 c | 49.4 c | 9.85 d | 98.0 ab | 38.8 e | 51.3 c | 87.7 c |
| IC50 | 353 µM | 890 µM | 525 µM | 767 µM | 511 µM | Tolerant | 893 µM | Out of range | Tolerant |
| IC80 | 661 µM | Out of range | 809 µM | Out of range | 713 µM | Tolerant | Out of range | Out of range | Tolerant |
| Crop | Associated Weed |
|---|---|
| Zea mays (maize) | Echinochloa crus-galli (barnyard grass), |
| Amaranthus retroflexus (redroot pigweed) | |
| Portulaca oleracea (common purslane) | |
| Erigeron bonariensis (flax-leaf fleabane) | |
| Triticum aestivum (wheat) | Avena fatua (wild oat) |
| Plantago lanceolata (ribwort) | |
| Erigeron bonariensis (flax-leaf fleabane) | |
| Oryza sativa (rice) | Echinochloa crus-galli (barnyard grass) |
| Portulaca oleracea (common purslane) | |
| Lactuca sativa (lettuce) | Portulaca oleracea (common purslane) |
| Plantago lanceolata (ribwort) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-González, D.; Ledo, D.; Cabeiras-Freijanes, L.; Verdeguer, M.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Phytotoxic Activity of the Natural Compound Norharmane on Crops, Weeds and Model Plants. Plants 2020, 9, 1328. https://doi.org/10.3390/plants9101328
López-González D, Ledo D, Cabeiras-Freijanes L, Verdeguer M, Reigosa MJ, Sánchez-Moreiras AM. Phytotoxic Activity of the Natural Compound Norharmane on Crops, Weeds and Model Plants. Plants. 2020; 9(10):1328. https://doi.org/10.3390/plants9101328
Chicago/Turabian StyleLópez-González, David, David Ledo, Luz Cabeiras-Freijanes, Mercedes Verdeguer, Manuel J. Reigosa, and Adela M. Sánchez-Moreiras. 2020. "Phytotoxic Activity of the Natural Compound Norharmane on Crops, Weeds and Model Plants" Plants 9, no. 10: 1328. https://doi.org/10.3390/plants9101328
APA StyleLópez-González, D., Ledo, D., Cabeiras-Freijanes, L., Verdeguer, M., Reigosa, M. J., & Sánchez-Moreiras, A. M. (2020). Phytotoxic Activity of the Natural Compound Norharmane on Crops, Weeds and Model Plants. Plants, 9(10), 1328. https://doi.org/10.3390/plants9101328