Seasonal Dynamics of Leaf Stoichiometry of Phragmites australis: A Case Study From Yangguan Wetland, Dunhuang, China
Abstract
1. Introduction
2. Results
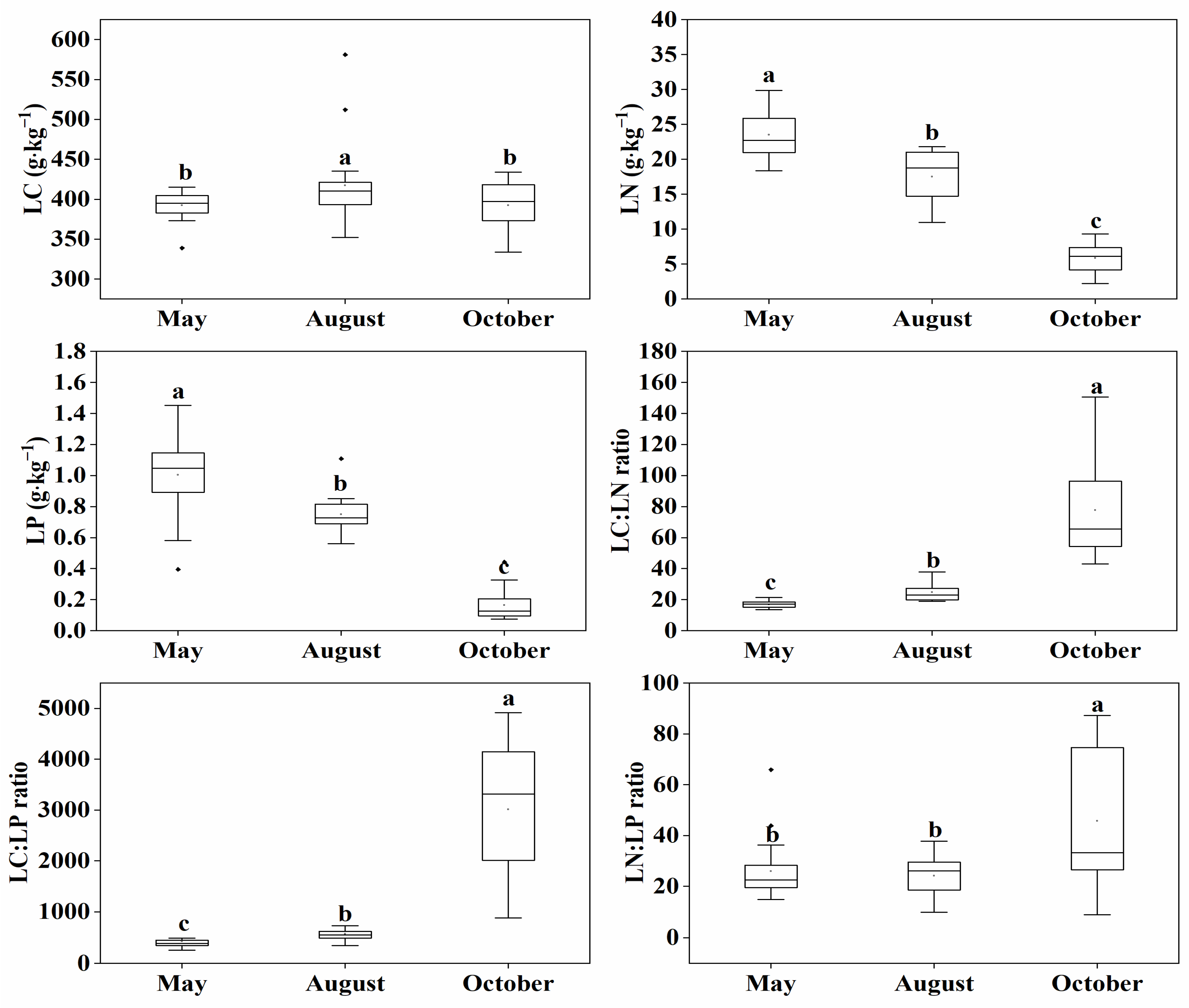
2.1. Variations on Leaf Stoichiometry and Soil Properties with the Seasons
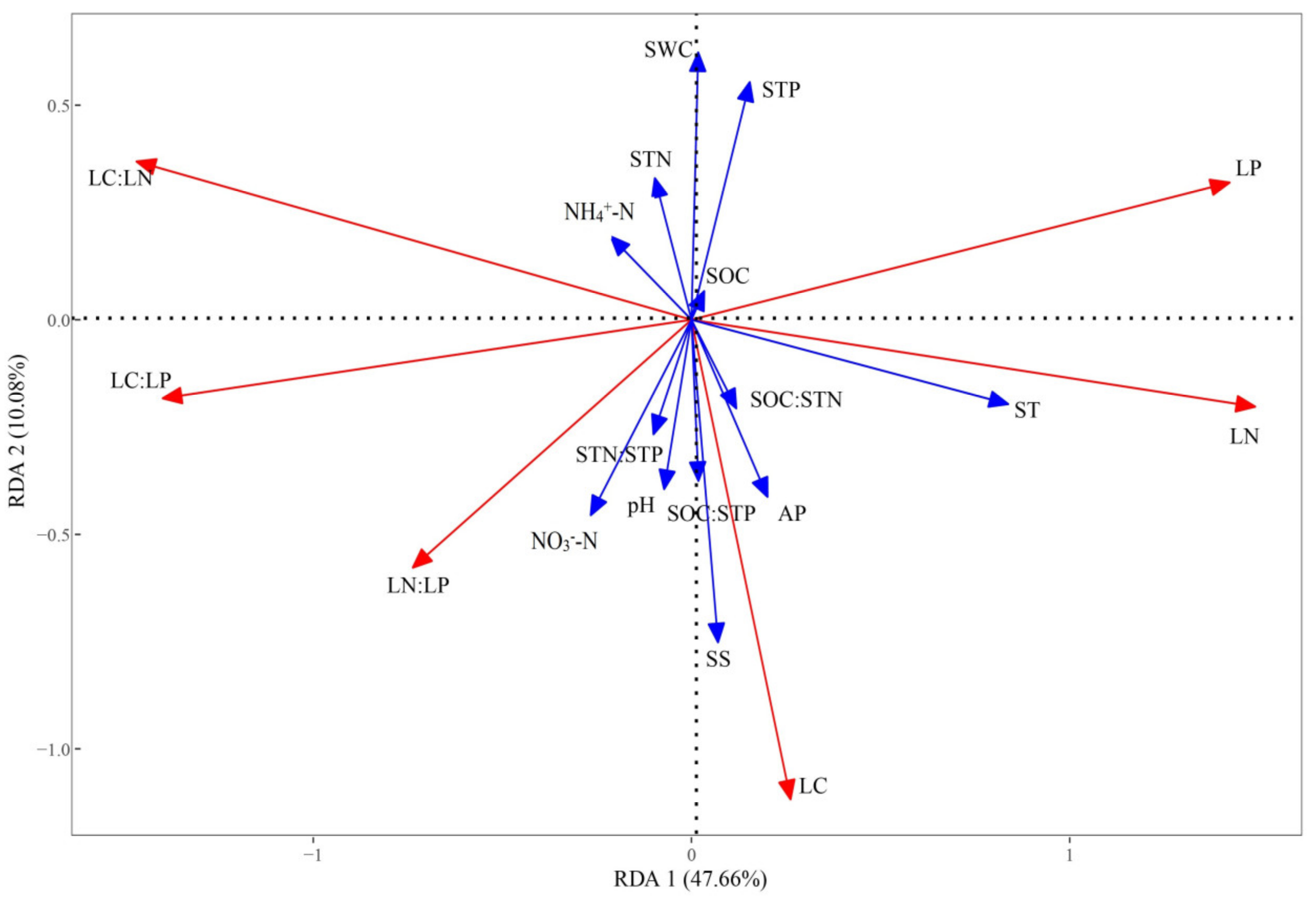
2.2. Relationship Between Leaf Stoichiometry and Soil Properties
3. Discussion
3.1. Seasonal Fluctuations of Leaf Stoichiometry
3.2. The Dominant Environmental Factors Influencing Leaf Stoichiometry
3.3. Stoichiometric Limiting Elements in Leaves
4. Materials and Methods
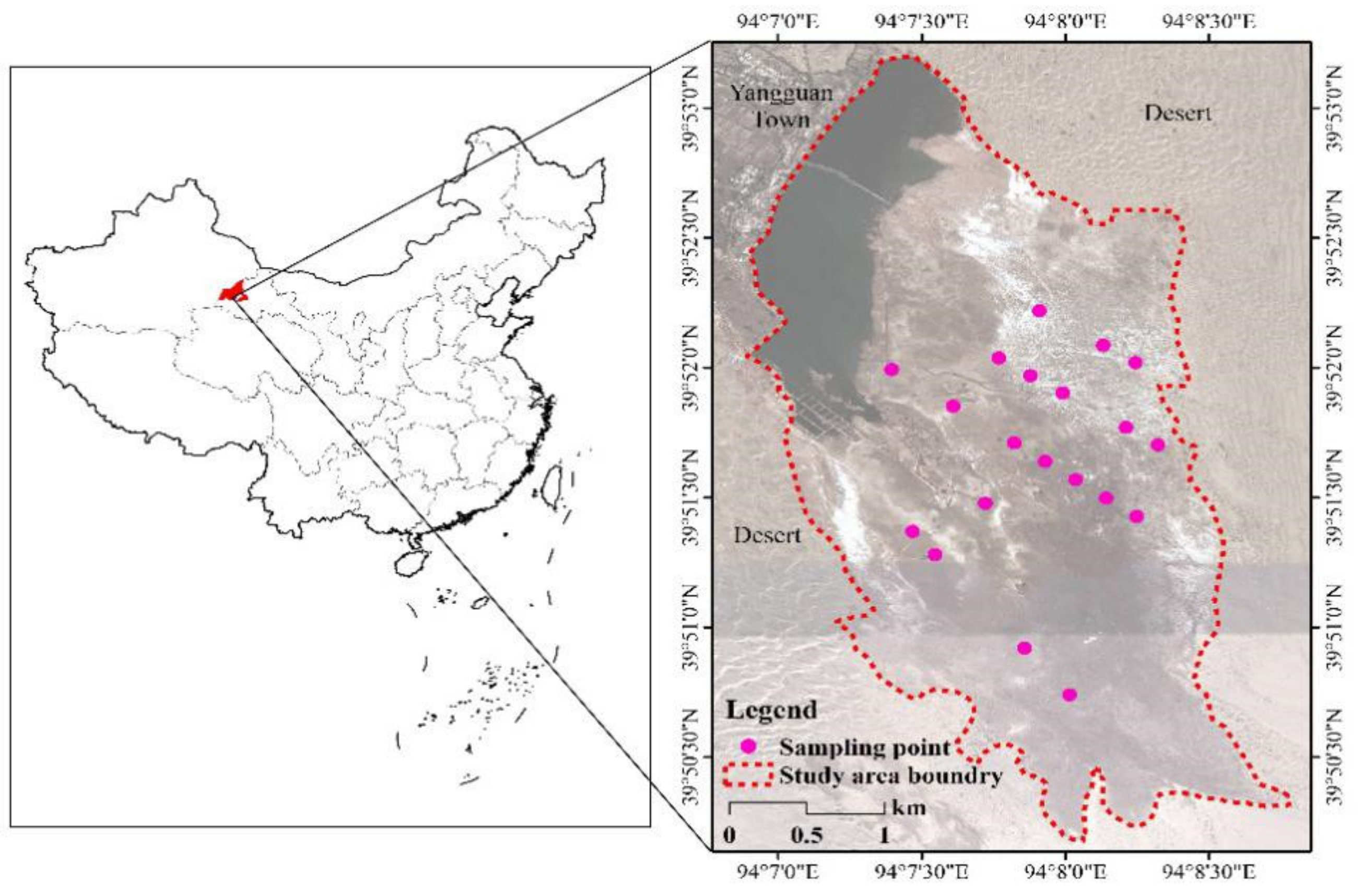
4.1. The Study Area
4.2. Sampling and Measurement
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rong, Q.Q.; Liu, J.T.; Cai, Y.P.; Lu, Z.H.; Yue, W.C.; Xia, J.B. Leaf carbon, nitrogen and phosphorus stoichiometry of Tamarix chinensis Lour. in the Laizhou Bay coastal wetland, China. Ecol. Eng. 2015, 76, 57–65. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, B.R.; An, S.S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Cao, J.J.; Wang, X.Y.; Adamowski, J.F.; Biswas, A.; Liu, C.F.; Chang, Z.Q.; Feng, Q. Response of leaf stoichiometry of Oxytropis ochrocephala to elevation and slope aspect. Catena 2020, 194, 104772. [Google Scholar] [CrossRef]
- Han, W.X.; Fang, J.Y.; Guo, D.L.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.J. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- McGroddy, M.E.; Daufresne, T.; Hedin, L.O. Scaling of C: N: P stoichiometry in forests worldwide: Implications of terrestrial redfield-type ratios. Ecology 2004, 85, 2390–2401. [Google Scholar] [CrossRef]
- Huang, D.; Wang, D.M.; Ren, Y. Using leaf nutrient stoichiometry as an indicator of flood tolerance and eutrophication in the riparian zone of the Lijang River. Ecol. Indic. 2019, 98, 821–829. [Google Scholar] [CrossRef]
- Elser, J.J.; Fagan, W.F.; Kerkhoff, A.J.; Swenson, N.G.; Enquist, B.J. Biological stoichiometry of plant production: Metabolism, scaling and ecological response to global change. New Phytol. 2010, 186, 593–608. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The Vegetation N:P Ratio: A New Tool to Detect the Nature of Nutrient Limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Baxter, I.; Dilkes, B.P. Elemental Profiles Reflect Plant Adaptations to the environment. Sci 2012, 336, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Aerts, R.; Chapin, F.S. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv. Ecol. Res. 1999, 30, 1–67. [Google Scholar]
- Kerkhoff, A.J.; Enquist, B.J.; Elser, J.J.; Fagan, W.F. Plant allometry, stoichiometry and the temperature-dependence of primary productivity. Global Ecol. Biogeogr. 2005, 14, 585–598. [Google Scholar] [CrossRef]
- Liu, F.D.; Liu, Y.H.; Wang, G.M.; Song, Y.; Liu, Q.; Li, D.S.; Mao, P.L.; Zhang, H. Seasonal variations of C:N:P stoichiometry and their trade-offs in different organs of suaeda salsa in coastal wetland of Yellow River Delta, China. PLoS ONE 2015, 10, e0138169. [Google Scholar]
- Li, H.L.; Crabbe, M.J.C.; Xu, F.L.; Wang, W.L.; Ma, L.H.; Niu, R.N.; Gao, X.; Li, X.X.; Zhang, P.; Ma, X.; et al. Seasonal variations in carbon, nitrogen and phosphorus concentrations and C:N:P stoichiometry in different organs of a Larix principis-rupprechtii Mayr. plantation in the Qinling Mountains, China. PLoS ONE 2017, 12, e0185163. [Google Scholar] [CrossRef]
- Fu, X.H.; Li, F.; Zhu, L.L.; Xie, Y.H.; Hu, C.; Chen, X.S.; Deng, Z.M. Change in ecological stoichiometry of Carex brevicuspis in response to sampling time and elevation in Dongting Lake, China. Nord. J. Bot. 2018, 36, e01821. [Google Scholar] [CrossRef]
- Kang, H.Z.; Zhang, H.L.; Wu, L.L.; Liu, Q.L.; Shen, G.R.; Berg, B.; Man, R.Z.; Liu, C.J. Variation in leaf nitrogen and phosphorus stoichiometry in Picea abies across Europe: An analysis based on local observations. Forest Ecol. Manag. 2011, 261, 195–202. [Google Scholar] [CrossRef]
- Güsewell, S. Nutrient resorption of wetland graminoids is related to the type of nutrient limitation. Funct. Ecol. 2005, 19, 344–354. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z.Y.; Liu, Q.; Zhao, Y. Water preservation and the ecological effects of removing leaves from stalks for a reed dominant wetland. Ecol. Eng. 2012, 49, 118–122. [Google Scholar] [CrossRef]
- Wang, W.Q.; Sardans, J.; Wang, C.; Zeng, C.S.; Tong, C.; Asensio, D.; Peñuelas, J. Ecological stoichiometry of C, N, and P of invasive Phragmites australis and native Cyperus malaccensis species in the Minjiang River tidal estuarine wetlands of China. Plant Ecol. 2015, 216, 809–822. [Google Scholar] [CrossRef]
- Bonanno, G.; Giudice, R.L. Heavy metal bioaccumulation by the organs of Phragmites australis (common reed) and their potential use as contamination indicators. Ecol. Indic. 2010, 10, 639–645. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, Z.; Yang, W. Valuation of the loss of plant-related ecosystem services caused by water stress in the wetland of China’s Yellow River Delta. Acta. Ecol. Sinica 2014, 34, 98–105. [Google Scholar] [CrossRef]
- Dong, C.; Huang, G.; Cai, Y.; Xu, Y. An interval-parameter minimax regret programming approach for power management systems planning under uncertainty. Appl. Energy 2011, 88, 2835–2845. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, M.; Lu, X.G.; Zhang, Z.S.; Lou, Y.J. Leaf carbon, nitrogen and phosphorus stoichiometry of Phragmites australis in northeastern China. Fresen. Environ. Bull. 2015, 24, 4711–4719. [Google Scholar]
- Li, L.P.; Zerbe, S.F.; Han, W.X.; Thevs, N.; Li, W.P.; He, P.; O. Schmitt, A.; Liu, Y.N.; Ji, C.J. Nitrogen and phosphorus stoichiometry of common reed (Phragmites australis) and its relationship to nutrient availability in northern China. Aquat Bot. 2014, 112, 84–90. [Google Scholar] [CrossRef]
- Castellanos, A.E.; Llano-Sotelo, J.M.; Machado-Encinas, L.I.; López-Piña, J.E.; Romo-Leon,, J.R.; Sardans, J.; Peñuelas, J. Foliar C, N and P stoichiometry characterize successful plant ecological strategies in the sonoran desert. Plant Ecol. 2018, 219, 775–788. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Z.; Bachofen, C.; Lou, Y.J.; Jiang, M.; Tang, X.G.; Lu, X.G.; Buchmann, N. The effect of saline-alkaline and water stresses on water use efficiency and standing biomass of Phragmites australis and Bolboschoenus planiculmis. Sci. Total Environ. 2018, 644, 207–216. [Google Scholar] [CrossRef]
- Li, W.; Cao, T.; Ni, L.; Zhang, X.; Zhu, G.; Xie, P. Effects of water depth on carbon, nitrogen and phosphorus stoichiometry of five submersed macrophytes in an in situ experiment. Ecol. Eng. 2013, 61, 358–365. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, H.H.; He, N.P.; Lü, X.T.; Wang, Z.P.; Elser, J.J.; Wu, J.G.; Han, X.G. Testing the growth rate hypothesis in vascular plants with above- and below-ground biomass. PLoS ONE 2012, 7, e32162. [Google Scholar] [CrossRef]
- Matzek, V.; Vitousek, P.M. N:P stoichiometry and protein: RNA ratios in vascular plants: An evaluation of the growth-rate hypothesis. Ecol. Lett. 2009, 12, 765–771. [Google Scholar] [CrossRef]
- Gao, W.; Yue, X.J.; Chen, D.Z.; Nie, S.; Chen, M.Y.; Ye, G.F. Seasonal dynamics of leaf and litter stoichiometry of different tree species in a coastal sand dune of southern subtropical China. Vegetos 2018, 31, 37–46. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, J.; Bao, Y.L.; Zhao, H.Y.; Qi, X.X.; Xie, H.J.; Zhang, J.B. Nutrient resorption patterns of Phragmites australis leaves and its respones to soil moisture in Yangguan, Duhuang. Chin. J. Appl. Ecol. 2020, 31, 807–813. [Google Scholar]
- Killingbeck, K.T. Nutrients in senesced leaves: Keys to the search for potential resorption and resorption proficiency. Ecology 1996, 77, 1716–1727. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; CavenderBares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Shipley, B.; Lechowicz, M.J.; Reich, W.P.B. Fundamental Trade-Offs Generating the Worldwide Leaf Economics Spectrum. Ecology 2006, 87, 535–541. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhao, G.X.; Li, M.; Zhang, M.T.; Zhang, L.F.; Zhang, X.F.; An, L.Z.; Xu, S.J. C:N:P Stoichiometry and Leaf Traits of Halophytes in an Arid Saline Environment, Northwest China. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, Y.; Wang, D.E.; Chen, J.H.; Zhang, F.G.; Zhou, J.B.; Li, Y.H. Stoichiometric variation of halophytes in response to changes in soil salinity. Plant Biol. 2017, 19, 360–367. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.; Mittra, B. Effects of salt on growth, ion accumulation, photosynthesis and leaf anatomy of the mangrove, Bruguiera parviflora. Trees 2014, 18, 167–174. [Google Scholar] [CrossRef]
- Finn, D.; Page, K.; Catton, K.; Strounina, E.; Kienzle, M.; Robertson, F.; Armstrong, R.; Dalal, R. Effect of added nitrogen on plant litter decomposition depends on initial soil carbon and nitrogen stoichiometry. Soil Biol. Biochem. 2015, 91, 160–168. [Google Scholar] [CrossRef]
- Lin, Y.M.; Chen, A.; Yan, S.W.; Rafay, L.; Du, K.; Wang, D.J.; Ge, Y.G.; Li, J. Available soil nutrients and water content affect leaf nutrient concentrations and stoichiometry at different ages of Leucaena leucocephala forests in dry-hot valley. J. Soils Sed. 2019, 19, 511–521. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J. Drought changes nutrient sources, content and stoichiometry in the bryophyte Hypnum cupressiforme Hedw. growing in a Mediterranean forest. J. Bryol. 2008, 30, 59–65. [Google Scholar] [CrossRef]
- Ordoñez, J.C.; van Bodegom, P.M.; Witte, J.P.M.; Wright, I.J.; Reich, P.B.; Aerts, R.; Sykes, M. A global study of relationships between leaf traits, climate and soil measures of nutrient fertility. Global Ecol. Biogeogr. 2009, 18, 137–149. [Google Scholar] [CrossRef]
- Ruan, Z.X.; Giordano, M. The use of NH4+ rather than NO3- affects cell stoichiometry, C allocation, photosynthesis and growth in the cyanobacterium Synechococcus sp. UTEX LB 2380, only when energy is limiting. Plant 2017, 40, 227–236. [Google Scholar]
- Li, F.; Hu, J.Y.; Xie, Y.H.; Yang, G.S.; Hu, C.; Chen, X.S.; Deng, Z.M. Foliar stoichiometry of carbon, nitrogen, and phosphorus in wetland sedge Carex brevicuspis along a small-scale elevation gradient. Ecol. Indic. 2018, 92, 322–329. [Google Scholar] [CrossRef]
- Yan, K.; Duan, C.Q.; Fu, D.G.; Li, J.; Michelle, H.G.W.; Qian, L.; Tian, Y.X. Leaf nitrogen and phosphorus stoichiometry of plant communities in geochemically phosphorus-enriched soils in a subtropical mountainous region. SW China. Environ. Earth Sci. 2015, 74, 3867–3876. [Google Scholar] [CrossRef]
- Jaenike, J.; Markow, T.A. Comparative elemental stoichiometry of ecologically diverse Drosophila. Funct. Ecol. 2003, 17, 115–120. [Google Scholar] [CrossRef]
- Zuccarini, P.; Asensio, D.; Ogaya, R.; Sardans, J.; Peñuelas, J. Effects of seasonal and decadal warming on soil enzymatic activity in a P-deficient Mediterranean shrubland. Global Change Biol. 2020, 26, 3698–3714. [Google Scholar] [CrossRef]
- Lu, Q.Q.; Bai, J.H.; Zhang, G.L.; Zhao, Q.Q.; Wu, J.J. Spatial and seasonal distribution of carbon, nitrogen, phosphorus, and sulfur and their ecological stoichiometry in wetland soils along a water and salt gradient in the Yellow River Delta, China. Phys. Chem. Earth 2018, 104, 9–17. [Google Scholar] [CrossRef]
- Tewksbury, C.E.; Miegroet, H.V. Soil organic carbon dynamics along a climatic gradient in a southern appalachian spruce-fir forest. Can. J. For. Res. 2007, 37, 1161–1172. [Google Scholar] [CrossRef]
- Ma, J.; Li, L.H.; Guo, L.P.; Bai, L.; Zhang, J.R.; Chen, Z.H.; Ahmad, S. Variation in soil nutrients in grasslands along the Kunes River in Xinjiang China. Chem. Ecol. 2015, 31, 111–122. [Google Scholar] [CrossRef]
- Li, M.Y.; Huang, W.G.; Yang, J.L.; Li, X.W. Factors influencing leaf nitrogen and phosphorus Stoichiometry in plant species of Ningxia Grassland. Acta Pratacul. Sinica 2019, 28, 23–32. [Google Scholar]
- Zhang, J.; Zhao, N.; Liu, C.; Yang, H.; Li, M.; Yu, G.; Wilcox, K.; Yu, Q.; He, N. C:N:P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 2017, 32, 50–60. [Google Scholar] [CrossRef]
- Bedford, B.L.; Walbridge, M.R.; Aldous, A. Patterns in nutrient availability and plant diversity of temperate north American wetlands. Ecology 1999, 80, 2151–2169. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A. Pattern and variation of C: N: P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhao, X.Y.; Zhang, F.X.; Awada, T.; Wang, S.K.; Zhao, T.H.; Li, Y.L. Accumulation of soil organic carbon during natural restoration of desertified grassland in China’s Horqin Sandy Land. J. Arid Land 2015, 007, 328–340. [Google Scholar] [CrossRef]
- Pan, F.F.; Xie, J.P.; Lin, J.M.; Zhao, T.W.; Ji, Y.Y.; Hu, Q.; Pan, X.B.; Wang, C.; Xi, X.H. Evaluation of Climate Change Impacts on Wetland Vegetation in the Dunhuang Yangguan National Nature Reserve in Northwest China Using Landsat Derived NDVI. Remote Sens. 2018, 10, 735. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, S. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; United States Department of Agriculture: Washington, DC, USA, 1954; USDA Circular 939.
| Factor | Season | |||
|---|---|---|---|---|
| Properties | F | P | df | |
| SOC (g·kg−1) | 19.92 | 0.000 | 2 | |
| STN (g·kg−1) | 2.27 | 0.133 | 2 | |
| STP (g·kg−1) | 1.58 | 0.214 | 2 | |
| SOC:STN | 20.05 | 0.000 | 2 | |
| SOC:STP | 21.65 | 0.000 | 2 | |
| STN:STP | 3.68 | 0.031 | 2 | |
| AP (mg·kg−1) | 0.48 | 0.622 | 2 | |
| NH4+-N (mg·kg−1) | 2.46 | 0.094 | 2 | |
| NO3−-N (mg·kg−1) | 7.47 | 0.001 | 2 | |
| SWC (%) | 0.07 | 0.932 | 2 | |
| ST (°C) | 329.51 | 0.000 | 2 | |
| pH | 0.76 | 0.472 | 2 | |
| SS (g·kg−1) | 10.76 | 0.474 | 2 | |
| Soil Properties Variable | RDA 1 | RDA 2 | R2 | P * | Explains (%) | F | P ** |
|---|---|---|---|---|---|---|---|
| SOC (g·kg−1) | 0.162 | −0.987 | 0.12 | 0.03 | 10 | 12.4 | 0.002 |
| STN (g·kg−1) | 0.355 | −0.935 | 0.09 | 0.088 | 0.5 | 0.7 | 0.56 |
| STP (g·kg−1) | −0.896 | −0.444 | 0.04 | 0.332 | 0.5 | 0.7 | 0.568 |
| SOC:STN | −0.258 | −0.966 | 0.03 | 0.378 | 0.5 | 0.7 | 0.546 |
| SOC:STP | 0.515 | −0.857 | 0.07 | 0.154 | 3.3 | 4.6 | 0.01 |
| STN:STP | 0.763 | −0.647 | 0.05 | 0.279 | 0.4 | 0.6 | 0.664 |
| AP (mg·kg−1) | −0.539 | 0.842 | 0.21 | 0.002 | 3.6 | 4.8 | 0.012 |
| NH4+-N (mg·kg−1) | 0.993 | −0.115 | 0.04 | 0.273 | 0.4 | 0.6 | 0.618 |
| NO3−-N (mg·kg−1) | 0.993 | 0.115 | 0.14 | 0.017 | 0.5 | 0.8 | 0.53 |
| SS (g·kg−1) | −0.307 | 0.952 | 0.24 | 0.001 | 0.9 | 1.3 | 0.244 |
| pH | 0.717 | 0.697 | 0.03 | 0.376 | 1.8 | 2.6 | 0.052 |
| SWC (%) | 0.116 | −0.993 | 0.23 | 0.001 | 8 | 8.3 | 0.002 |
| ST (°C) | −0.983 | 0.181 | 0.64 | 0.001 | 36.6 | 33.4 | 0.002 |
| Variable | Season (Fixed Effects) | Plot (Random Effects) | |||||
|---|---|---|---|---|---|---|---|
| Standard Error | df | t-Value | p-Value | df | L-Ratio | p-Value | |
| LC | 7.9 | 38 | 49.65 | 0.000 | 3 | 3.43 | 0.0641 |
| LN | 0.71 | 38 | 32.9 | 0.000 | 3 | 5.27 | 0.9998 |
| LP | 0.04 | 38 | 25.8 | 0.000 | 3 | 1.46 | 0.9999 |
| LC:LN | 4.25 | 38 | 4.0 | 0.0003 | 3 | 5.28 | 0.9998 |
| LC:LP | 174.58 | 38 | 2.46 | 0.018 | 3 | 1.02 | 0.9997 |
| LN:LP | 4.04 | 38 | 6.44 | 0.000 | 3 | 0.66 | 0.4174 |
| Leaf Stoichiometry | LC (g·kg−1) | LN (g·kg−1) | LP (g·kg−1) | LC:LN | LC:LP | LN:LP |
|---|---|---|---|---|---|---|
| SS (g·kg−1) | 0.501 ** | 0.311 ** | −0.001 | −0.258* | 0.048 | 0.412 ** |
| SWC (%) | −0.234 * | −0.214 | 0.076 | 0.191 | −0.098 | −0.411 ** |
| ST (°C) | 0.310 ** | 0.795 ** | 0.764 ** | −0.781 ** | −0.737 ** | −0.273 * |
| SOC (g·kg−1) | 0.111 | −0.119 | 0.057 | 0.137 | −0.047 | −0.255 * |
| NO3−-N (mg·kg−1) | 0.248 * | −0.242 * | −0.349 ** | 0.281 * | 0.374 ** | 0.285 * |
| AP (mg·kg−1) | 0.225 * | 0.092 | 0.329 ** | −0.311 ** | −0.071 | 0.275 * |
| Parameter | May | August | October |
|---|---|---|---|
| SOC (g·kg−1) | 5.79 ± 0.4b | 10.61 ± 0.57a | 6.89 ± 0.5b |
| STN (g·kg−1) | 0.46 ± 0.03a | 0.55 ± 0.03a | 0.49 ± 0.04a |
| STP (g·kg−1) | 0.43 ± 0.01a | 0.39 ± 0.02a | 0.38 ± 0.02a |
| SOC:STN | 12.96 ± 0.71b | 19.71 ± 0.99a | 14.25 ± 0.55b |
| SOC:STP | 13.71 ± 0.88c | 29.93 ± 3.21a | 18.07 ± 1.18b |
| STN:STP | 1.09 ± 0.07b | 1.55 ± 1.66a | 1.31 ± 0.1ab |
| AP (mg·kg−1) | 4.67 ± 0.57a | 4.95 ± 0.73a | 4.02 ± 0.59a |
| NH4+-N (mg·kg−1) | 9.76 ± 0.95a | 11.24 ± 0.63a | 11.63 ± 0.77a |
| NO3−-N (mg·kg−1) | 6.34 ± 0.94b | 9.53 ± 1.37ab | 11.29 ± 1.17a |
| SWC (%) | 25.31 ± 0.03a | 22.18 ± 0.02a | 22.32 ± 0.02a |
| ST (°C) | 18.09 ± 0.63b | 22.67 ± 0.52a | 12.5 ± 0.4c |
| SS (g·kg−1) | 4.6 ± 0.57a | 6.58 ± 0.95a | 5.5 ± 0.93a |
| pH | 8.38 ± 0.04a | 8.42 ± 0.06a | 8.46 ± 0.04a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhang, J.; Biswas, A.; Cao, J.; Xie, H.; Qi, X. Seasonal Dynamics of Leaf Stoichiometry of Phragmites australis: A Case Study From Yangguan Wetland, Dunhuang, China. Plants 2020, 9, 1323. https://doi.org/10.3390/plants9101323
Liu D, Zhang J, Biswas A, Cao J, Xie H, Qi X. Seasonal Dynamics of Leaf Stoichiometry of Phragmites australis: A Case Study From Yangguan Wetland, Dunhuang, China. Plants. 2020; 9(10):1323. https://doi.org/10.3390/plants9101323
Chicago/Turabian StyleLiu, Dong, Jian Zhang, Asim Biswas, Jianjun Cao, Huanjie Xie, and Xuanxuan Qi. 2020. "Seasonal Dynamics of Leaf Stoichiometry of Phragmites australis: A Case Study From Yangguan Wetland, Dunhuang, China" Plants 9, no. 10: 1323. https://doi.org/10.3390/plants9101323
APA StyleLiu, D., Zhang, J., Biswas, A., Cao, J., Xie, H., & Qi, X. (2020). Seasonal Dynamics of Leaf Stoichiometry of Phragmites australis: A Case Study From Yangguan Wetland, Dunhuang, China. Plants, 9(10), 1323. https://doi.org/10.3390/plants9101323








