Morpho-Colorimetric Characterization of the Sardinian Endemic Taxa of the Genus Anchusa L. by Seed Image Analysis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Diagnostic Characteristics of Mericarps of the Studied Taxa
4.2. Plant Material
4.3. Seed Image Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Akcin, T.A.; Ulu, S.; Akcin, A. Morphological, anatomical and numerical studies on some Anchusa L. (Boraginaceae) taxa from Turkey. Pak. J. Bot. 2010, 42, 2231–2247. [Google Scholar]
- Al-Shehbaz, I.A. The genera of Boraginaceae in the southeastern United States. J. Arnold Arbor. Suppl. Ser. 1991, 1, 1–169. [Google Scholar]
- Bigazzi, M.; Selvi, F. Pollen morphology in the Boragineae (Boraginaceae) in relation to the taxonomy of the tribe. Plant Syst. Evol. 1998, 213, 121–151. [Google Scholar] [CrossRef]
- Selvi, F.; Bigazzi, M. Anchusa L. and allied genera (Boraginaceae) in Italy. Plant Biosyst. 2013, 132, 113–142. [Google Scholar] [CrossRef]
- Selvi, F.; Bigazzi, M. Leaf surface and anatomy in Boraginaceae tribe Boragineae with respect to ecology and taxonomy. Flora 2001, 196, 269–285. [Google Scholar] [CrossRef]
- Coppi, A.; Mengoni, A.; Selvi, F. AFLP fingerprinting of Anchusa (Boraginaceae) in the Corso-Sardinian system: Genetic diversity, population differentiation and conservation priorities in an insular endemic group threatened with extinction. Biol. Conserv. 2008, 141, 2000–2011. [Google Scholar] [CrossRef]
- Selvi, F.; Bigazzi, M. Revision of genus Anchusa (Boraginaceae-Boragineae) in Greece. Bot. J. Linn. Soc. 2003, 142, 431–454. [Google Scholar] [CrossRef]
- Cañadas, E.M.; Fenu, G.; Peñas, J.; Lorite, J.; Mattana, E.; Bacchetta, G. Hotspots within hotspots: Endemic plant richness, environmental drivers, and implications for conservation. Biol. Conserv. 2014, 170, 282–291. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112. [Google Scholar] [CrossRef]
- Thompson, J. Plant Evolution in the Mediterranean; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Paradis, G.; Piazza, C.; Quilichini, Y. Anchusa crispa subsp. valincoana (Boraginaceae), une nouvelle sous-espèce endémique de Corse. Candollea 2018, 73, 201–207. [Google Scholar] [CrossRef]
- Mansion, G.; Selvi, F.; Guggisberg, A.; Conti, E. Origin of Mediterranean insular endemics in the Boraginales: Integrative evidence from molecular dating and ancestral area reconstruction. J. Biogeogr. 2009, 36, 1282–1296. [Google Scholar] [CrossRef]
- Bacchetta, G.; Coppi, A.; Pontecorvo, C.; Selvi, F. Systematics, phylogenetic relationships and conservation of the taxa of Anchusa (Boraginaceae) endemic to Sardinia (Italy). Syst. Biodivers. 2008, 6, 161–174. [Google Scholar] [CrossRef]
- Fenu, G.; Cogoni, D.; Ulian, T.; Bacchetta, G. The impact of human trampling on a threatened coastal Mediterranean plant: The case of Anchusa littorea Moris (Boraginaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 104–110. [Google Scholar] [CrossRef]
- Fenu, G.; Bacchetta, G. Anchusa littorea Moris. Informatore Botanico Italiano 2008, 40, 53–55. [Google Scholar]
- Budroni, M.A.; Farris, E.; Zirulia, A.; Pisanu, S.; Filigheddu, R.; Rustici, M. Evidence for age-structured depensation effect in fragmented plant populations: The case of the Mediterranean endemic Anchusa sardoa (Boraginaceae). Ecol. Complex. 2014, 20, 142–150. [Google Scholar] [CrossRef]
- Farris, E.; Filigheddu, R.S. Anchusa sardoa (Illario) Selvi et Bigazzi. Informatore Botanico Italiano 2008, 40, 56–57. [Google Scholar]
- Farris, E.; Pisanu, S.; Ceccherelli, G.; Filigheddu, R. Human trampling effects on Mediterranean coastal dune plants. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2013, 147, 1043–1051. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination, 2nd ed.; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Mattana, E.; Grillo, O.; Venora, G.; Bacchetta, G. Germplasm image analysis of Astragalus maritimus and A. verrucosus of Sardinia (subgen. Trimeniaeus, Fabaceae). Anales del Jardin Botanico de Madrid 2008, 65, 149–155. [Google Scholar] [CrossRef]
- Bacchetta, G.; Fenu, G.; Grillo, O.; Mattana, E.; Venora, G. Identification of Sardinian species of Astragalus section Melanocercis (Fabaceae) by seed image analysis. Annales Botanici Fennici 2011, 48, 449–454. [Google Scholar] [CrossRef]
- Bacchetta, G.; García, P.E.; Grillo, O.; Mascia, F.; Venora, G. Seed image analysis provides evidence of taxonomical differentiation within the Lavatera triloba aggregate (Malvaceae). Flora Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 468–472. [Google Scholar] [CrossRef]
- Grillo, O.; Mattana, E.; Fenu, G.; Venora, G.; Bacchetta, G. Geographic isolation affects inter- and intra-specific seed variability in the Astragalus tragacantha complex, as assessed by morpho-colorimetric analysis. Comptes Rendus Biologies 2013, 336, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, M.; Grillo, O.; Cañadas, E.; Venora, G.; Bacchetta, G. Inter- and intraspecific diversity in Cistus L. (Cistaceae) seeds, analysed with computer vision techniques. Plant Biol. 2017, 19, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, M.; Grillo, O.; Escobar Garcia, P.; Mascia, F.; Venora, G.; Bacchetta, G. Morpho-colorimetric characterisation of Malva alliance taxa by seed image analysis. Plant Biol. 2017, 19, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Sarigu, M.; Porceddu, M.; Schmitt, E.; Camarda, I.; Bacchetta, G. Taxonomic discrimination of the Paeonia mascula group in the Tyrrhenian Islands by seed image analysis. Syst. Biodivers. 2019, 17, 801–810. [Google Scholar] [CrossRef]
- de Medeiros, A.D.; Pinheiro, D.T.; Xavier, W.A.; da Silva, L.J.; dos Santos Dias, D.C.F. Quality classification of Jatropha curcas seeds using radiographic images and machine learning. Ind. Crops Prod. 2020, 146, 112162. [Google Scholar] [CrossRef]
- Pinna, M.S.; Grillo, O.; Mattana, E.; Cañadas, E.M.; Bacchetta, G. Inter- and intraspecific morphometric variability in Juniperus L. seeds (Cupressaceae). Syst. Biodivers. 2014, 12, 211–223. [Google Scholar] [CrossRef]
- Lo Bianco, M.; Ferrer-Gallego, P.; Grillo, O.; Laguna, E.; Venora, G.; Bacchetta, G. Seed image analysis provides evidence of taxonomic differentiation within the Medicago L. sect. Dendrotelis (Fabaceae). Syst. Biodivers. 2015, 13, 484–495. [Google Scholar] [CrossRef]
- Orrù, M.; Grillo, O.; Venora, G.; Bacchetta, G. Seed morpho-colorimetric analysis by computer vision: A helpful tool to identify grapevine (Vitis vinifera L.) cultivars. Aust. J. Grape Wine Res. 2015, 21, 508–519. [Google Scholar] [CrossRef]
- Orrù, M.; Grillo, O.; Venora, G.; Bacchetta, G. Computer vision as a method complementary to molecular analysis: Grapevine cultivar seeds case study. Comptes Rendus Biologies 2012, 335, 602–615. [Google Scholar] [CrossRef]
- Piras, F.; Grillo, O.; Venora, G.; Lovicu, G.; Campus, M.; Bacchetta, G. Effectiveness of a computer vision technique in the characterization of wild and farmed olives. Comput. Electron. Agric. 2016, 122, 86–93. [Google Scholar] [CrossRef]
- Sau, S.; Ucchesu, M.; Dondini, L.; de Franceschi, P.; D’hallewin, G.; Bacchetta, G. Seed morphometry is suitable for apple-germplasm diversity-analyses. Comput. Electron. Agric. 2018, 151, 118–125. [Google Scholar] [CrossRef]
- Sau, S.; Ucchesu, M.; D’hallewin, G.; Bacchetta, G. Potential use of seed morpho-colourimetric analysis for Sardinian apple cultivar characterisation. Comput. Electron. Agric. 2019, 162, 373–379. [Google Scholar] [CrossRef]
- Ucchesu, M.; Sarigu, M.; del Vais, C.; Sanna, I.; d’Hallewin, G.; Grillo, O.; Bacchetta, G. First finds of Prunus domestica L. in Italy from the Phoenician and Punic periods (6th–2nd centuries bc). Veg. Hist. Archaeobotany 2017, 26, 539–549. [Google Scholar] [CrossRef]
- Sarigu, M.; Grillo, O.; Lo Bianco, M.; Ucchesu, M.; d’Hallewin, G.; Loi, M.C.; Venora, G.; Bacchetta, G. Phenotypic identification of plum varieties (Prunus domestica L.) by endocarps morpho-colorimetric and textural descriptors. Comput. Electron. Agric. 2017, 136, 25–30. [Google Scholar] [CrossRef]
- Sabato, D.; Esteras, C.; Grillo, O.; Picó, B.; Bacchetta, G. Seeds morpho-colourimetric analysis as complementary method to molecular characterization of melon diversity. Scientia Horticulturae 2015, 192, 441–452. [Google Scholar] [CrossRef]
- Quilichini, A.; Debussche, M. Seed dispersal and germination patterns in a rare Mediterranean island endemic (Anchusa crispa Viv., Boraginaceae). Acta Oecologica 2000, 21, 303–313. [Google Scholar] [CrossRef]
- Nathan, R.; Katul, G.G.; Horn, H.S.; Thomas, S.M.; Oren, R.; Avissar, R.; Pacala, S.W.; Levin, S.A. Mechanisms of long-distance dispersal of seeds by wind. Nature 2002, 418, 409–413. [Google Scholar] [CrossRef]
- Consiglio Nazionale Delle Ricerche. Available online: http://www.seaforecast.cnr.it/forecast/index.php/it/previsioni/mediterraneo-occidentale/sardegna202 no/. (accessed on 10 September 2020).
- Kamm, U.; Rotach, P.; Gugerli, F.; Siroky, M.; Edwards, P.; Holderegger, R. Frequent long-distance gene flow in a rare temperate forest tree (Sorbus domestica) at the landscape scale. Heredity 2009, 103, 476–482. [Google Scholar] [CrossRef]
- Bacchetta, G.; Fenu, G.; Mattana, E.; Piotto, B.; Virevaire, M. Manuale per la Raccolta, Studio, Conservazione e Gestione ex situ del Germoplasma; APAT: Roma, Italy, 2006; Volume 37. [Google Scholar]
- Bacchetta, G.; Bueno Sanchez, A.; Fenu, G.; Jiménez Alfaro, B.; Mattana, E.; Piotto, B.; Virevaire, M. Conservación ex situ de Plantas Silvestres; Principado de Asturias. Obra Social La Caixa y Gobierno del Principado de Asturias: Principado de Asturias, Spain, 2008. [Google Scholar]
- Porceddu, M.; Santo, A.; Orrù, M.; Meloni, F.; Ucchesu, M.; Picciau, R.; Sarigu, M.; Cuena Lombrana, A.; Podda, L.; Sau, S.; et al. Seed conservation actions for the preservation of plant diversity: The case of the Sardinian Germplasm Bank (BG-SAR). Plant Sociol. 2017, 54, 111–117. [Google Scholar]
- Bacchetta, G.; Grillo, O.; Mattana, E.; Venora, G. Morpho-colorimetric characterization by image analysis to identify diaspores of wild plant species. Flora Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 669–682. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Landini, G. Novel Context-Based Segmentation Algorithms for Intelligent Microscopy. Available online: https://blog.bham.ac.uk/intellimic/g-landini-software/ (accessed on 10 September 2020).
- Box, G.E.P. A general distribution theory for a class of likelihood criteria. Biometrika 1949, 36, 317. [Google Scholar] [CrossRef] [PubMed]
- Levene, H. Robust Tests for Equality of Variances. Contributions to Probability and Statistics. Essays in Honor of Harold Hotelling; Stanford University Press: Redwood City, CA, USA, 1961; pp. 279–292. [Google Scholar]
- Sugiyama, M. Dimensionality reduction of multimodal labeled data by local fisher discriminant analysis. J. Mach. Learn. Res. 2007, 8, 1027–1061. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: New York, NY, USA, 2009. [Google Scholar]
- Holden, J.E.; Finch, W.H.; Kelley, K. A Comparison of two-group classification methods. Educ. Psychol. Meas. 2011, 71, 870–901. [Google Scholar] [CrossRef]
- Kuhn, M.; Johnson, K.; Kuhn, M.; Johnson, K. Discriminant analysis and other linear classification models. In Applied Predictive Modeling; Springer: New York, NY, USA, 2013; pp. 275–328. [Google Scholar]
- Rencher, A.C.; Christensen, W.F. Methods of Multivariate Analysis, 3rd ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
- Venora, G.; Grillo, O.; Saccone, R. Quality assessment of durum wheat storage centres in Sicily: Evaluation of vitreous, starchy and shrunken kernels using an image analysis system. J. Cereal Sci. 2009, 49, 429–440. [Google Scholar] [CrossRef]
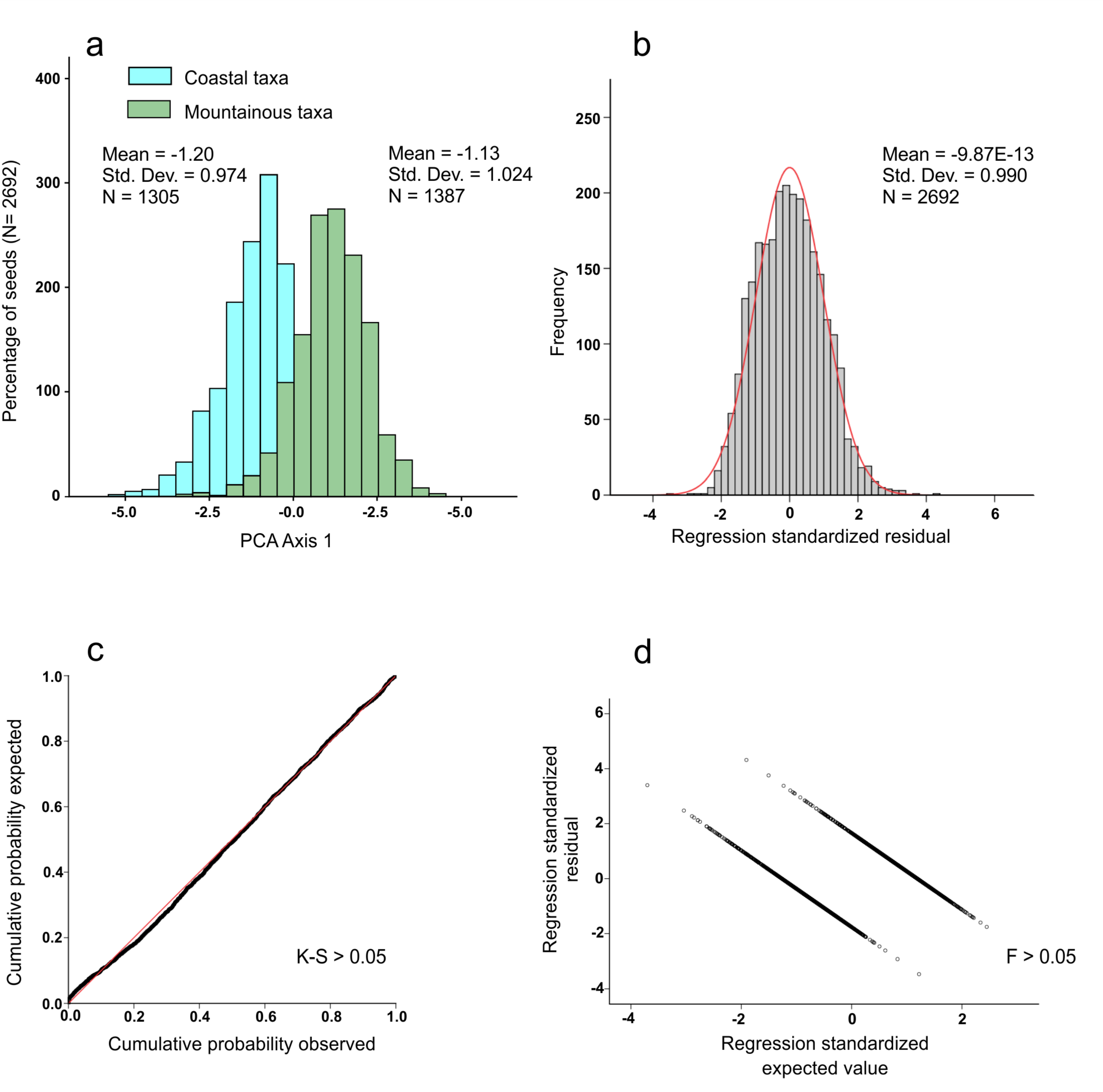


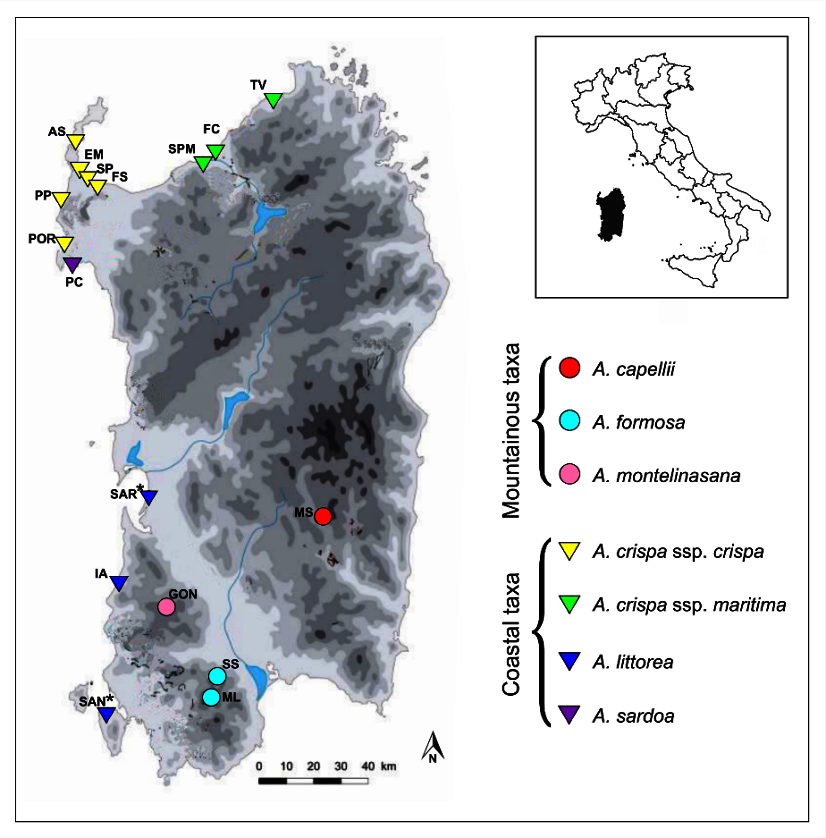

| Ecological Group | Coastal Taxa | Mountainous Taxa | Total |
|---|---|---|---|
| Coastal taxa | 90.8 (1185) | 9.2 (120) | 100 (1305) |
| Mountainous taxa | 12.6 (175) | 87.4 (1212) | 100 (1387) |
| Cross-validated | 89.0% (2692) |
| Ecological Conditions | Coastal Taxa | Mountainous Taxa | ||||||
|---|---|---|---|---|---|---|---|---|
| Taxa | A. crispa ssp. crispa | A. crispa ssp. maritima | A. littorea | A. sardoa | A. capellii | A. formosa | A. montelinasana | Total |
| A. crispa ssp. crispa | 70.7 (191) | 14.1 (38) | 1.1 (3) | 8.1 (22) | 5.2 (14) | 0.4 (1) | 0.4 (1) | 100 (270) |
| A. crispa ssp. maritima | 21.1 (111) | 34.9 (183) | 10.3 (54) | 19.0 (100) | 9.5 (50) | 1.7 (9) | 3.4 (18) | 100 (525) |
| A. littorea | 3.5 (14) | 5.5 (22) | 81.2 (324) | 4.0 (16) | - | 2.8 (11) | 3.0 (12) | 100 (399) |
| A. sardoa | 3.6 (4) | 7.2 (8) | - | 82.9 (92) | 4.5 (5) | 1.8 (2) | - | 100 (111) |
| A. capellii | 4.5 (13) | 8.0 (23) | 2.4 (7) | 4.2 (12) | 71.3 (204) | 4.2 (12) | 5.2 (15) | 100 (286) |
| A. formosa | 2.2 (17) | 3.6 (28) | 2.4 (19) | 2.3 (18) | 9.4 (74) | 53.9 (424) | 26.2 (206) | 100 (786) |
| A. montelinasana | 2.5 (8) | 3.2 (10) | 0.3 (1) | 2.5 (8) | 8.9 (28) | 26.0 (82) | 56.5 (178) | 100 (315) |
| Cross-validated (%) | 59.3% (2692) | |||||||
| Morphometric Parameters | Description | Colorimetric Parameters | Description |
|---|---|---|---|
| Perim | Perimeter, calculated from the centres of the boundary pixels | GrIntDen | Greyscale integrated density (the sum of the greyscale values in the particle) |
| Area | Area inside the polygon defined by the perimeter | GrMin | Minimum greyscale |
| Pixels | Number of pixels forming the endocarp image | GrMax | Maximum greyscale |
| MinR | Radius of the inscribed circle centred at the middle of mass | GrMode | Modal greyscale |
| MaxR | Radius of the enclosing circle centred at the middle of mass | GrMedian | Median greyscale |
| Feret | Largest axis length | GrAverage | Average greyscale |
| Breadth | Largest axis perpendicular to the Feret | GrAvDeve | Average deviation of greyscale |
| CHull | Convex hull or convex polygon calculated from pixel centres | GrStDev | Standard deviation of the greyscale |
| CArea | Area of the convex hull polygon | GrVa | Variance of the greyscale values |
| MBCRadius | Radius of the minimal bounding circle | GrSkew | Skewness of the greyscale |
| AspRatio | Aspect ratio = Feret/Breadth | GrKurt | Kurtosis of the greyscale |
| Circ | Circularity = 4·π·Area/Perimeter2 | GrEntr | Entropy of the greyscale |
| Roundness | Roundness = 4·Area/(π·Feret2) | RedIntDen | Redscale integrated density |
| ArEquivD | Area equivalent diameter = √((4/π)·Area) | RedMin | Minimum redscale |
| PerEquivD | Perimeter equivalent diameter = Area/π | RedMax | Maximum redscale |
| EquivEllAr | Equivalent ellipse area = (π·Feret·Breadth)/4 | RedMode | Modal redscale |
| Compactness | Compactness = √((4/π)·Area)/Feret | RedMedian | Median redscale |
| Solidity | Solidity = Area/Convex_Area | RedAverage | Average redscale |
| Concavity | Concavity = Convex_Area-Area | RedAvDev | Average deviation of redscale |
| Convexity | Convexity = Convex_hull/Perimeter | RedStDev | Standard deviation of the redscale |
| Shape | Shape = Perimeter2/Area | RedVar | Variance of the redscale |
| RFactor | RFactor = Convex_Hull /(Feret·π) | RedSkew | Skewness of the redscale |
| ModRatio | Modification ratio = (2·MinR)/Feret | RedKurt | Kurtosis of the redscale |
| Sphericity | Sphericity = MinR/MaxR | RedEntr | Entropy of the redscale |
| ArBBox | Area of the bounding box along the feret diameter = Feret·Breadth | GreenIntDen | Greenscale integrated density |
| Rectang | Rectangularity = Area/ArBBox | GreenMin | Minimum greenscale |
| GreenMax | Maximum greenscale | ||
| GreenMode | Modal greenscale | ||
| GreenMedian | Median greenscale | ||
| GreenAverage | Average greenscale | ||
| GreenAvDev | Average deviation of greenscale | ||
| GreenStDev | Standard deviation of the greenscale | ||
| GreenVar | Variance of the greenscale | ||
| GreenSkew | Skewness of the greenscale | ||
| GreenKurt | Kurtosis of the greenscale | ||
| GreenEntr | Entropy of the greenscale | ||
| BlueIntDen | Bluescale integrated density | ||
| BlueMin | Minimum bluescale | ||
| BlueMax | Maximum bluescale | ||
| BlueMode | Modal bluescale | ||
| BlueMedian | Median bluescale | ||
| BlueAverage | Average bluescale | ||
| BlueAvDev | Average deviation of bluescale | ||
| BlueStDev | Standard deviation of the bluescale | ||
| BlueVar | Variance of the bluescale | ||
| BlueSkew | Skewness of the bluescale | ||
| BlueKurt | Kurtosis of the bluescale | ||
| BlueEntr | Entropy of the bluescale |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farris, E.; Orrù, M.; Ucchesu, M.; Amadori, A.; Porceddu, M.; Bacchetta, G. Morpho-Colorimetric Characterization of the Sardinian Endemic Taxa of the Genus Anchusa L. by Seed Image Analysis. Plants 2020, 9, 1321. https://doi.org/10.3390/plants9101321
Farris E, Orrù M, Ucchesu M, Amadori A, Porceddu M, Bacchetta G. Morpho-Colorimetric Characterization of the Sardinian Endemic Taxa of the Genus Anchusa L. by Seed Image Analysis. Plants. 2020; 9(10):1321. https://doi.org/10.3390/plants9101321
Chicago/Turabian StyleFarris, Emmanuele, Martino Orrù, Mariano Ucchesu, Arianna Amadori, Marco Porceddu, and Gianluigi Bacchetta. 2020. "Morpho-Colorimetric Characterization of the Sardinian Endemic Taxa of the Genus Anchusa L. by Seed Image Analysis" Plants 9, no. 10: 1321. https://doi.org/10.3390/plants9101321
APA StyleFarris, E., Orrù, M., Ucchesu, M., Amadori, A., Porceddu, M., & Bacchetta, G. (2020). Morpho-Colorimetric Characterization of the Sardinian Endemic Taxa of the Genus Anchusa L. by Seed Image Analysis. Plants, 9(10), 1321. https://doi.org/10.3390/plants9101321








