Reaching Natural Growth: The Significance of Light and Temperature Fluctuations in Plant Performance in Indoor Growth Facilities
Abstract
:1. Introduction
2. Results
2.1. Environmental Conditions
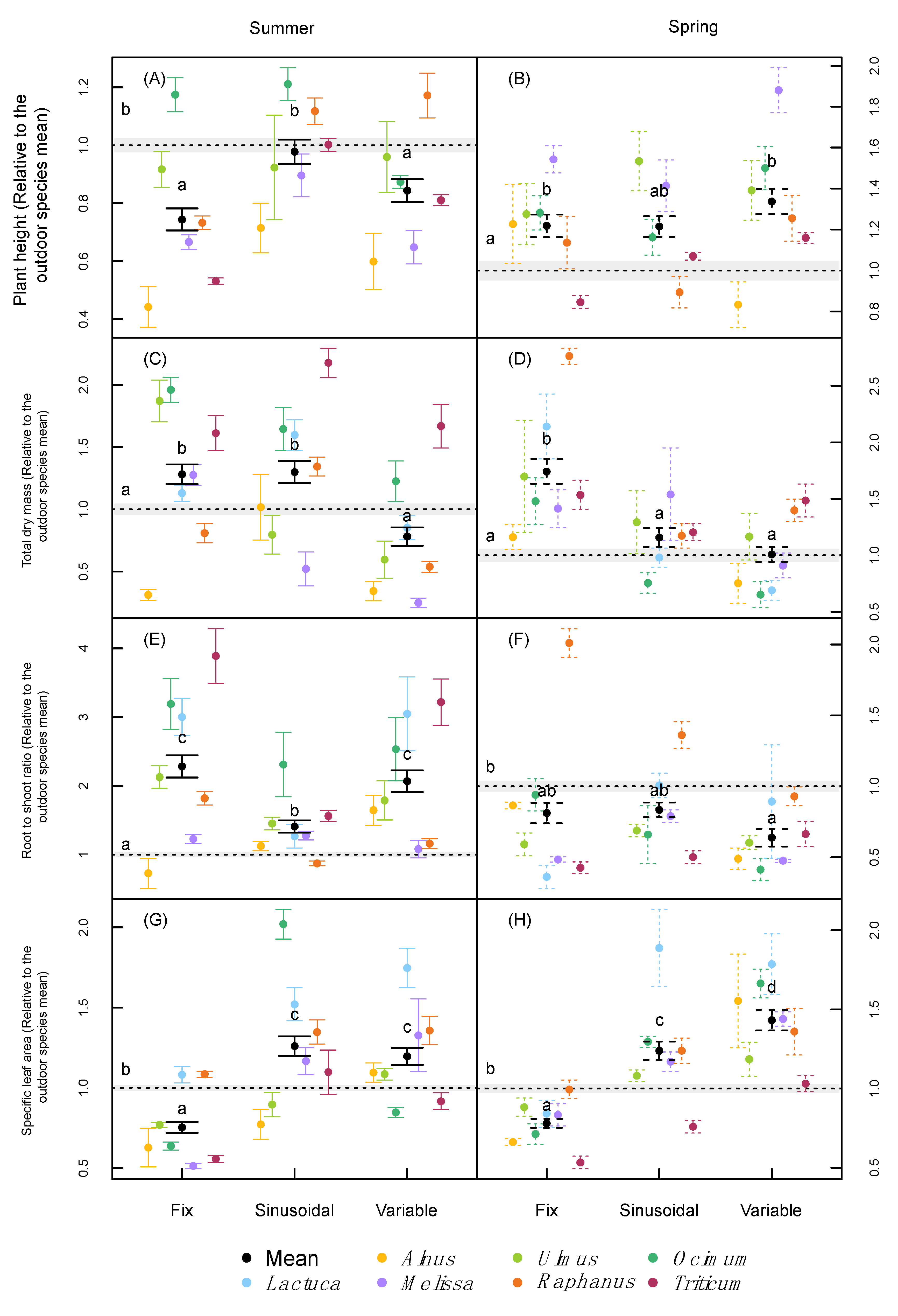
2.2. Plant Growth and Morphology
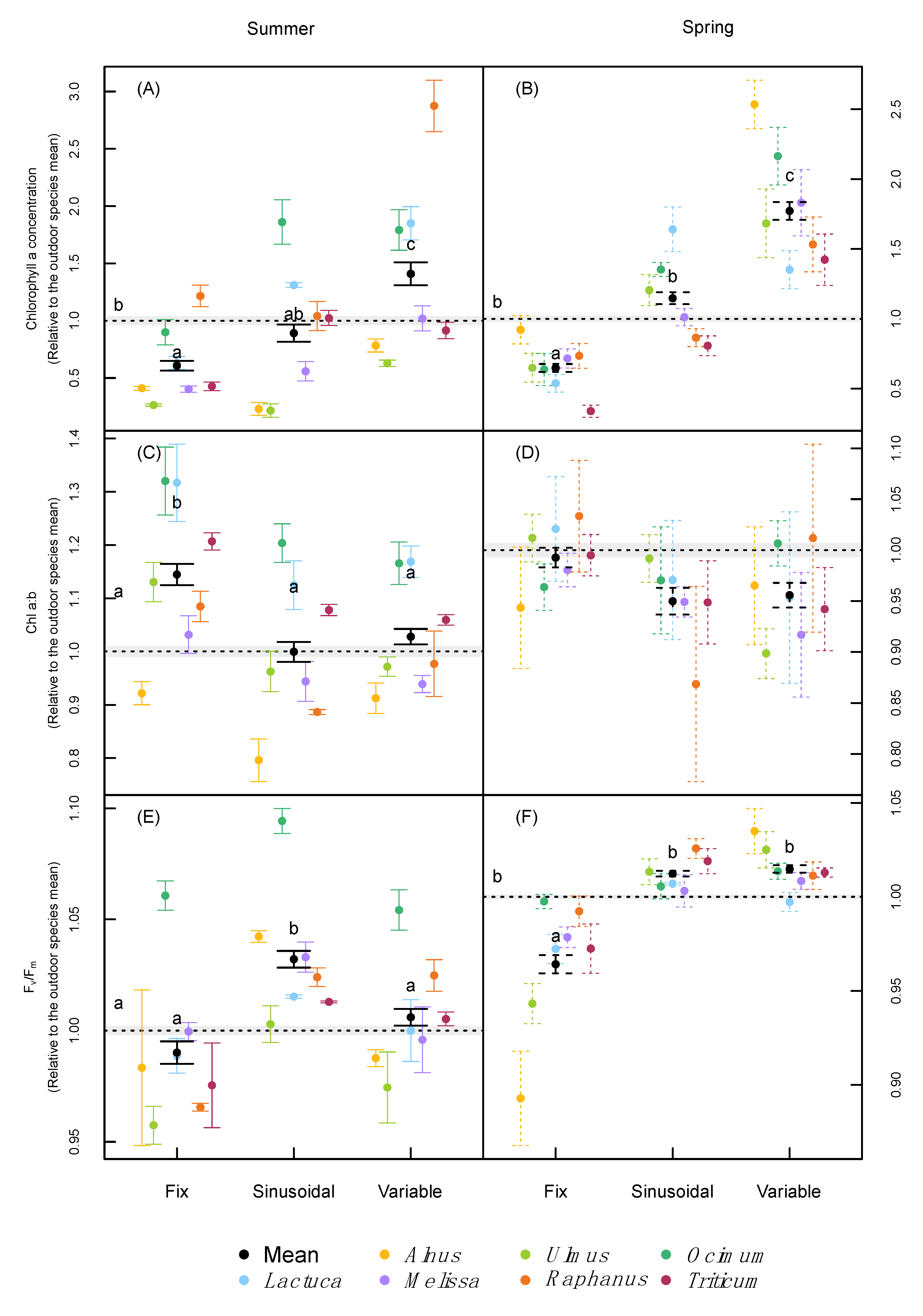
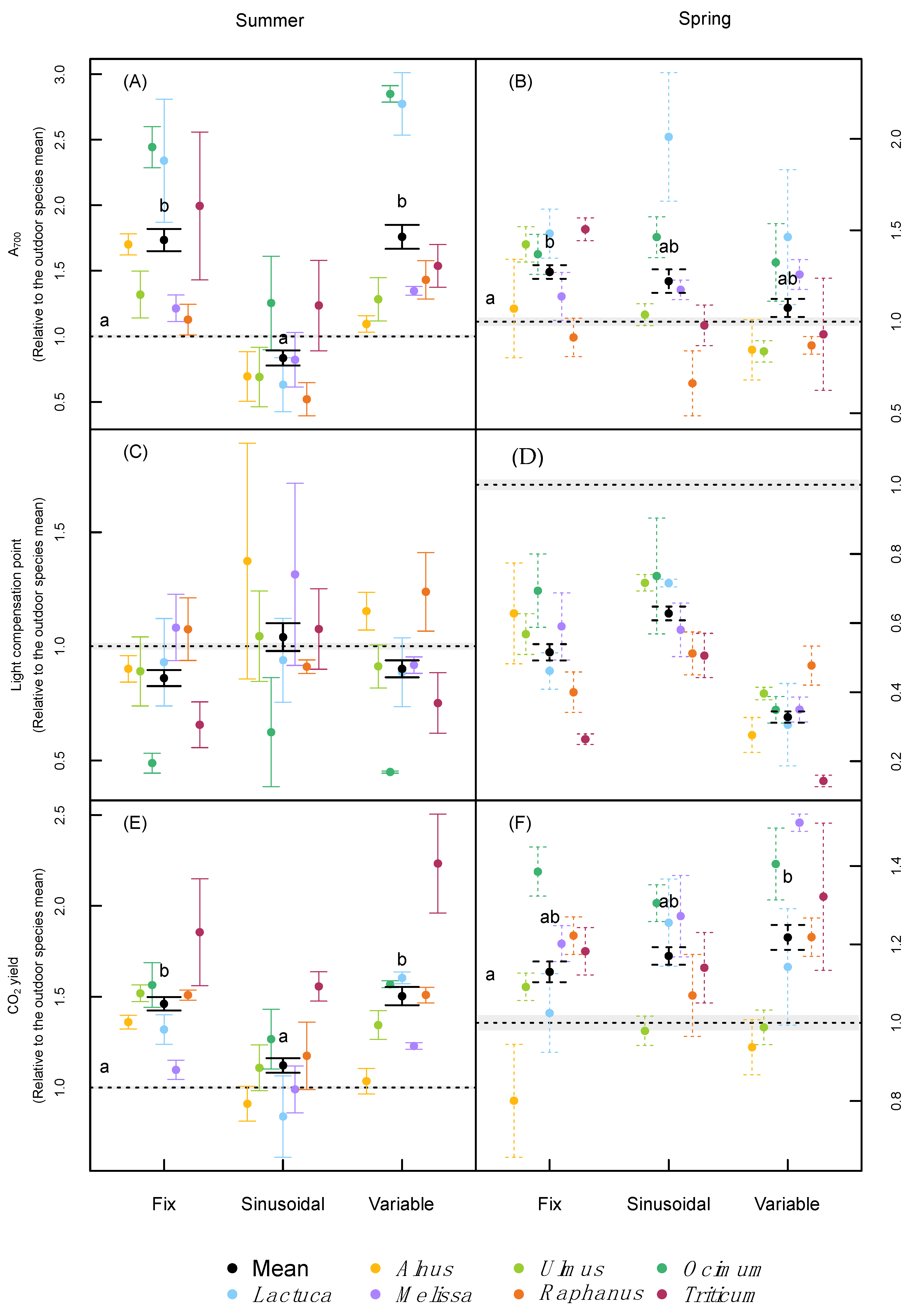
2.3. Leaf Pigmentation and Leaf Gas-Exchange
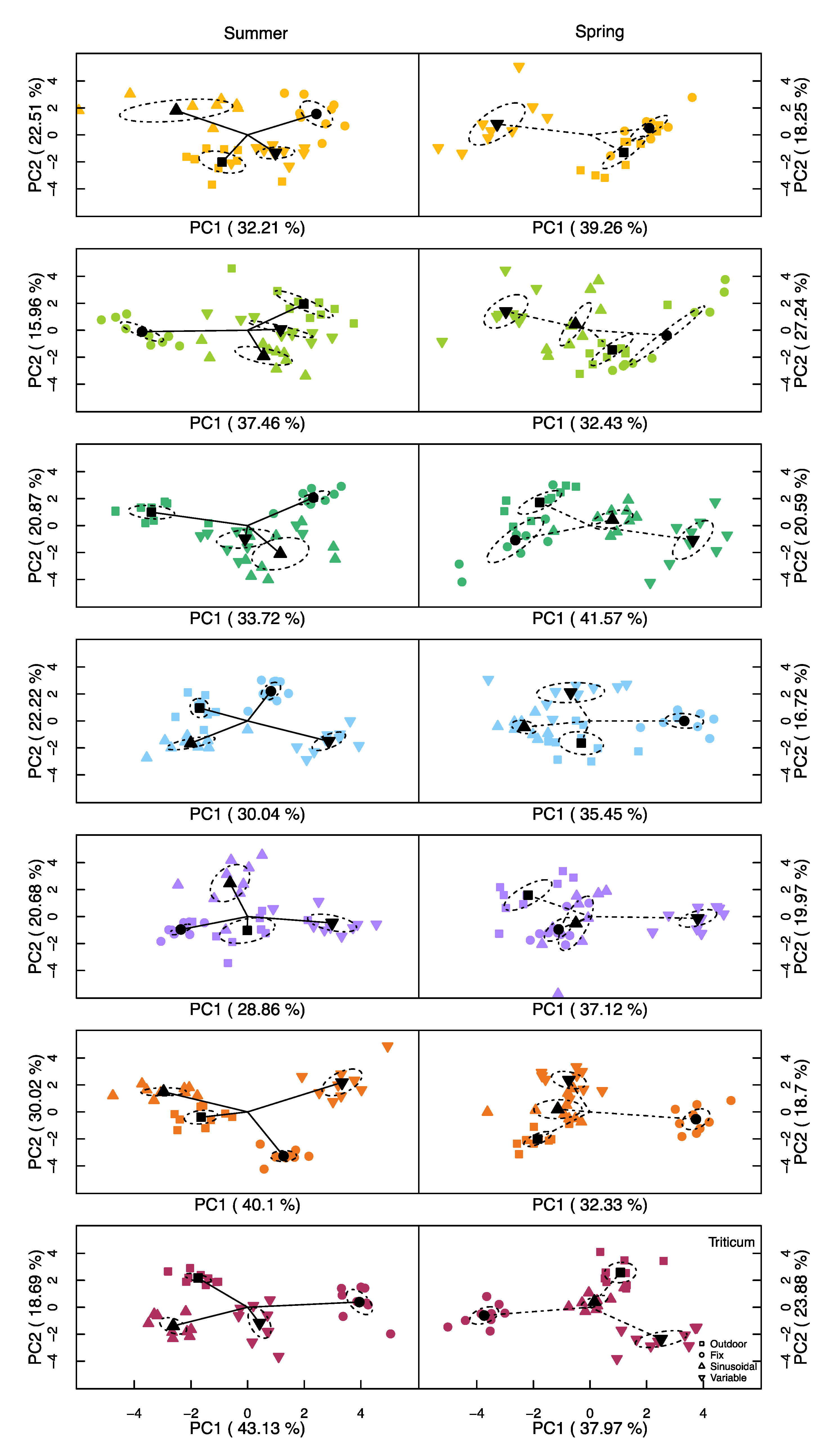
Principal Component Analysis (PCA)
3. Discussion
3.1. Plant Growth and Morphology
3.2. Leaf Pigmentation and Photosynthesis
3.3. Principal Component Analysis
4. Materials and Methods
4.1. Plant Material and Pre-Growing Conditions
4.2. Outdoor Trial and Environmental Conditions
4.3. Experimental Phytotron Runs
4.4. Plant Growth and Morphology
4.5. Chlorophyll Fluorescence and Leaf Pigment Content
4.6. Photosynthesis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsubara, S. Growing plants in fluctuating environments: Why bother? J. Exp. Bot. 2018, 69, 4651–4654. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plant growing in controlled conditions and in the field. New Phytol. 2016, 213, 383–855. [Google Scholar] [CrossRef] [PubMed]
- Myster, J.; Moe, R. Effect of diurnal temperature alternations on plants morphology in some greenhouse crops—A mini review. Sci. Hortic. 1995, 62, 205–215. [Google Scholar] [CrossRef]
- Kaiser, E.; Morales, A.; Harbinson, J.; Kromdijk, J.; Heuvelink, E.; Marcelis, L. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 2015, 66, 2415–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vialet-Chabrand, S.; Matthews, J.; Simkin, A.; Raines, C.; Lawson, T. Importance of fluctuations in light on plant photosynthetic acclimation. Plant Physiol. 2017, 173, 2163–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, E.; Morales, A.; Harbinson, J. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 2018, 176, 977–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamori, W. Photosynthetic response to fluctuating environments and photoprotective strategies under abiotic stress. J. Plant Res. 2016, 129, 379–395. [Google Scholar] [CrossRef]
- Kromdijk, J.; Glowacka, K.; Leonelli, L.; Gabilly, S.; Iwai, M.; Niyogi, K.; Long, S. Improving photosynthesis and crop productivity by accelerating recovey from photoprotection. Science 2016, 364, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Matsubara, S.; Harbinson, J.; Heuvelink, E.; Marcelis, L. Acclimation of photosynthesis to lightflecks in tomato leaves: Interaction with progressive shading in a growing canopy. Physiol. Plant. 2017, 162, 506–517. [Google Scholar] [CrossRef] [Green Version]
- Strømme, C.; Julkunen-Tiitto, R.; Krishna, U.; Lavola, A.; Olsen, J.E.; Nybakken, L. UV-B and temperature enhancement affect spring and autumn phenology in Populus tremula. Plant Cell Environ. 2015, 38, 867–877. [Google Scholar] [CrossRef]
- Søgaard, G.; Johnsen, Ø.; Nilsen, J.; Juntilla, O. Climatic control of bud burst in young seedlings of nine provenances of Norway spruce. Tree Physiol. 2008, 28, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Kalcits, L.; Silim, S.; Tanino, K. Warm temperatures accelerates short-photoperiod induced growth cessation and dormancy induction in hybrid poplar (Populus x spp). Trees 2009, 23, 971–979. [Google Scholar] [CrossRef]
- Junker, A.; Muraya, M.; Weigelt-Fischer, K.; Arana-Ceballos, F.; Klukas, C.; Melchinger, A.; Meyer, R.; Riewe, D.; Altmann, T. Optimizing experimental procedures for quantitative evaluation of crop plant performance in high throughput phenotyping systems. Front. Plant Sci. 2015, 5, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohmann, M.; Stahl, A.; Rudloff, J.; Wittkop, B.; Showdon, R. Not a load of rubbish: Simulated field trials in large-scale containers. Plant Cell Environ. 2016, 39, 2064–2073. [Google Scholar] [CrossRef]
- Hogewoning, S.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Harbison, J. An artificial solar spectrum substantially alters plants development compared with usual climate room irradiance spectra. J. Exp. Bot. 2010, 61, 1267–1276. [Google Scholar] [CrossRef] [Green Version]
- Hernandez, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Alpelt, F.; Carillo, P.; Krause, U.; Feil, R.; Mengin, V.; Lauxmann, M.; Nikoloski, Z.; Stitt, M.; Lunn, J. Getting back to nature: A reality check for experiments in controlled environments. J. Exp. Bot. 2017, 68, 4463–4477. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Apelt, F.; Carillo, P.; Krause, U.; Feil, R.; Koehl, K.; Lunn, J.; Slitt, M. Response of Arabidopsis primary metabolism and circadian clock to low night temperature in a natural light environment. J. Exp. Bot. 2018, 69, 4881–4895. [Google Scholar] [CrossRef]
- Jensen, E.; Eilertsen, S.; Ernsten, A.; Juntilla, O.; Moe, R. Thermoperiodic control of steam elongation and endogenous gibberellins in Campanula isophylla. J. Plant Growth Regul. 1996, 15, 167–171. [Google Scholar] [CrossRef]
- Thingnaes, E.; Torre, S.; Ernsten, A.; Moe, R. Day and night temperature response in Arabidopsis: Effects on gibberellin and auxin content, cell size, morphology and flowering time. Ann. Bot. 2003, 92, 601–612. [Google Scholar] [CrossRef] [Green Version]
- Sitt, M.; Zemma, S. Starch turnover: Pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012, 15, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Pilkingston, S.; Encke, B.; Krohn, N.; Höhne, M.; Stitt, M.; Pyil, E.T. Relationship between starch degradation and carbon demand for maintenance and growth in Arabidopsis thaliana in different irradiance and temperature regimes. Plant Cell Environ. 2015, 38, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Randeni, G.; Caesar, K. Effect on soil temperature on the carbohydrate status in the potato plant (S. tuberosum L.). J. Agron. Crop Sci. 1986, 156, 217–224. [Google Scholar] [CrossRef]
- Domish, T.; Finér, L.; Lehto, T. Effects of soil temperature on biomass and carbohydrate allocation in Scot pine (Pinus sylvestris) seedlings at the beginning of the growing season. Tree Physiol. 2001, 21, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, J.; Vialet-Chabrand, S.; Lawson, T. Acclimation to fluctuating light impacts the rapidity of response and diurnal rhythm of stomata conductance. Plant Physiol. 2018, 176, 1939–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terashima, I.; Hanba, Y.; Tazoe, Y.; Vyas, P.; Yano, S. Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 2006, 57, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, G.I. The UV-B photoreceptor UVR8: From structure to physiology. Plant Cell. 2014, 26, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; He, N.; Hou, J.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors influencing leaf chlorophyll content in natural forests at the biome scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Erwin, J.; Heins, R. Thermomorphogenic response in steam and leaf development. Hortscience 1995, 30, 940–949. [Google Scholar] [CrossRef] [Green Version]
- Abu-gosh, S.; Fixler, D.; Dubinsky, Z.; Iluz, D. Flashing light in microalgae biotechnology. Bioresour. Technol. 2016, 203, 357–363. [Google Scholar] [CrossRef]
- Sager, J.; Smith, W.; Edwards, J.; Cyr, K. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASAE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Kalaji, H.; Schansker, G.; Ladle, R.; Goltsev, V.; Bosa, K.; Allakhverdiev, S.; Brestic, M.; Bussoti, F.; Calatayud, A.; Dabrowski, O.; et al. Frequently asked questions about in vivo chlorophyll fluorescence: Practical issues. Photosynth. Res. 2014, 122, 121–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellburn, A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Lobo, F.; Barros, M.; Dalmagro, H.; Dalmolin, A.; Pereira, W.; Souza, E.; Vourlitis, G.; Rodriguez, C. Fitting net photosynthetic light-response curves with Microsoft Excel- a critical look at the models. Photosynthetica 2013, 51, 445–456. [Google Scholar] [CrossRef]
- Eilers, P.; Peeters, J. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol. Model. 1988, 42, 199–215. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 3 August 2020).
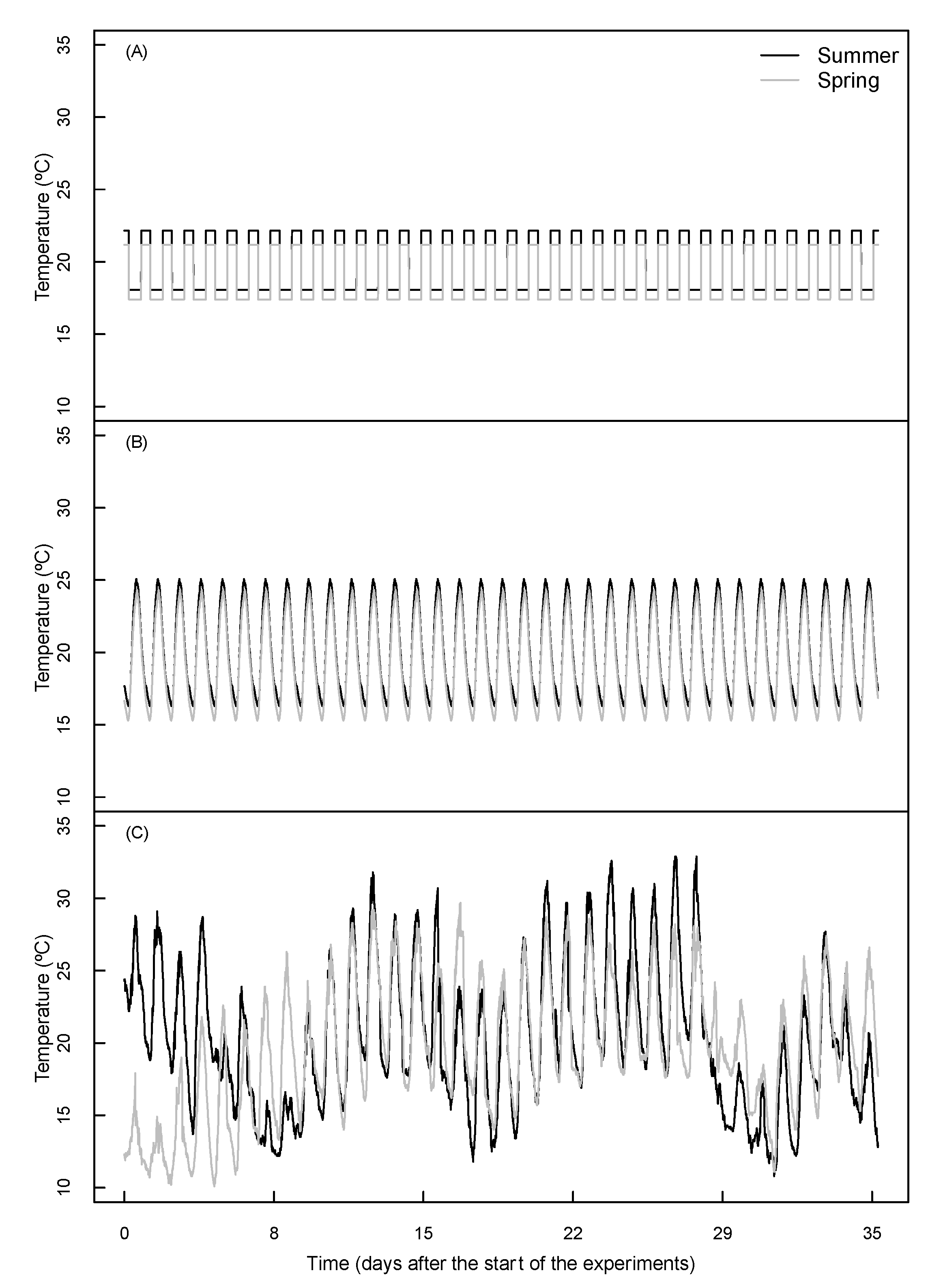
| Summer Run | Spring Run | |||
|---|---|---|---|---|
| Night | Day | Night | Day | |
| Air temperature (°C) | 18.1 | 22.2 | 17.4 | 21.2 |
| Relative humidity (%) | 79.2 | 64.9 | 81.7 | 67.4 |
| PPFD (μmol m−2 s−1) | 0 | 575.5 | 0 | 609 |
| Duration per day (h) | 11.05 | 12.95 | 9.92 | 14.08 |
| Summer | Spring | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Treatment | Species | Treatment × Species | Treatment | Species | Treatment × Species | |
| Biomass and Morphology | Height ** | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Dry weight shoot | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Dry weight roots | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Total dry weight | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Root to shoot ratio | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| SLA | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Chlorophyll | Chlorophyll α (mg g−1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Chlorophyll β (mg g−1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Chl α:β ratio | <0.001 | <0.001 | <0.001 | 0.095 | <0.001 | - | |
| Carotenoids (mg g−1) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Fv/Fm | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Pi | <0.001 | <0.001 | <0.001 | 0.445 | <0.001 | - | |
| Photosynthesis | Max. photosynthesis | <0.001 | <0.001 | - | 0.0567 | <0.001 | - |
| Light compensation point | 0.060 | <0.001 | - | <0.001 | 0.005 | - | |
| Quantum yield for CO2 fixation | <0.001 | <0.001 | 0.006 | 0.003 | <0.001 | - | |
| Dark respiration | 0.5223 | <0.001 | - | <0.001 | <0.001 | <0.001 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, C.; Bånkestad, D.; Hoch, G. Reaching Natural Growth: The Significance of Light and Temperature Fluctuations in Plant Performance in Indoor Growth Facilities. Plants 2020, 9, 1312. https://doi.org/10.3390/plants9101312
Chiang C, Bånkestad D, Hoch G. Reaching Natural Growth: The Significance of Light and Temperature Fluctuations in Plant Performance in Indoor Growth Facilities. Plants. 2020; 9(10):1312. https://doi.org/10.3390/plants9101312
Chicago/Turabian StyleChiang, Camilo, Daniel Bånkestad, and Günter Hoch. 2020. "Reaching Natural Growth: The Significance of Light and Temperature Fluctuations in Plant Performance in Indoor Growth Facilities" Plants 9, no. 10: 1312. https://doi.org/10.3390/plants9101312
APA StyleChiang, C., Bånkestad, D., & Hoch, G. (2020). Reaching Natural Growth: The Significance of Light and Temperature Fluctuations in Plant Performance in Indoor Growth Facilities. Plants, 9(10), 1312. https://doi.org/10.3390/plants9101312






