The Influence of Environmental Features on the Morphometric Variation in Mauritia flexuosa L.f. Fruits and Seeds
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Data Collection and Samples
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ratter, J.A. Transitions between cerrado and forest vegetation in Brazil. In Nature and Dynamics of Forest–Savanna Boundaries; Furley, P.A., Proctor, J., Ratter, J.A., Eds.; Chapman & Hall: London, UK, 1992; pp. 417–429. [Google Scholar]
- Assis, A.C.C.; Coelho, R.M.; Pinheiro, E.S.; Durigan, G. Water availability determines physiognomic gradient in an area of low-fertility soils under Cerrado vegetation. Plant Ecol. 2011, 212, 1135–1147. [Google Scholar] [CrossRef]
- Delgado, C.; Couturier, G.; Mejia, K. Mauritia flexuosa L.f. (Arecaceae: Calamoideae), an Amazonian palm with cultivation purposes in Peru. Fruits 2007, 62, 157–169. [Google Scholar] [CrossRef]
- Rull, V.; Montoya, E. Mauritia flexuosa palm swamp communities: Natural or human-made? A palynological study of the Gran Sabana region (northern South America) within a neotropical context. Quat. Sci. Rev. 2014, 99, 17–33. [Google Scholar] [CrossRef]
- Virapongse, A.; Endress, B.A.; Gilmore, M.P.; Horn, C.; Romulo, C. Ecology, livelihoods, and management of the Mauritia flexuosa palm in South America. Glob. Ecol. Conserv. 2017, 10, 70–92. [Google Scholar] [CrossRef]
- Barros, F.B.; Da Silva, D. Os mingauleiros de miriti: Trabalho, sociabilidade e consumo na beira de Abaetetuba, Pará. Rev. FSA 2013, 10, 44–66. [Google Scholar] [CrossRef]
- Mariotti, P.R. Transformação da Paisagem na Zona de Transição Amazônia e Cerrado, Vila Bela da Santíssima Trindade, Mato Grosso, Amazônia Meridional; Dissertação (Mestrado em Ciências Ambientais)—Universidade do Estado de Mato Grosso: Cáceres, Spain, 2015. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. What kind of seed dormancy might palms have? Seed Sci. Res. 2014, 24, 17. [Google Scholar] [CrossRef]
- Luzuriaga, A.L.; Escudero, A.; Pérez-Garcia, F. Environmental maternal effects on seed morphology and germination in Sinapis arvensis (Cruciferae). Weed Res. 2006, 46, 163–174. [Google Scholar] [CrossRef]
- Singh, D. The relative importance of characters affecting genetic divergence. Indian J. Genet. Plant Breed. 1981, 41, 237–245. [Google Scholar]
- Santos, M.F. Variação Genética em Populações Naturais de Babaçu (Orbignya Phalerata Mart.) por Marcadores Morfoagronômicos e Moleculares; Dissertação (Mestrado)—Universidade Federal do Piauí: Teresina, Brazil, 2011. [Google Scholar]
- Qaderi, M.M.; Cavers, P.B.; Bernards, M.A. Seed bank dynamics of Onopordum acanthium: Emergence patterns and chemical attributes. J. Ecol. 2002, 90, 672–683. [Google Scholar] [CrossRef]
- Matos, F.; Nunes, Y.R.F.; Silva, M.A.P.; de Sena Oliveira, I. Variação biométrica de diásporos de buriti (Mauritia flexuosa Lf–ARECACEAE) em veredas em diferentes estágios de conservação. Ciênc. Florest. 2014, 24, 833–842. [Google Scholar] [CrossRef]
- Abdelbasit, H.; Mahgoup, S.; ElDoma, A. Variation in seed morphometric characteristics and germination of Acacia tortilis subspecies raddiana and subspecies spirocarpa among three provenances in Sudan. Glob. J. Biotechnol. 2014, 3, 191–196. [Google Scholar]
- Barbosa, R.I.; Lima, A.D.; Mourão Junior, M. Biometria de Frutos do Buriti (Mauritia flexuosa L.f.—Arecaceae): Estimativas de Produtividade de Polpa e óleo Vegetal em uma área de Savana em Roraima; Instituto Nacional de Pesquisa da Amazônia—INPA: Boa Vista, Brazil, 2009; 23p.
- BRASIL. Regras para análise de sementes. In Ministério da Agricultura e da Reforma Agrária; Secretaria Nacional de Irrigação: Brasília, Brazil, 1992. [Google Scholar]
- Braz, M.I.G.; Portela, R.D.C.Q.; Cosme, L.H.M.; Marques, V.G.C.; de Mattos, E.A. Germination niche breadth differs in two co-occurring palms of the Atlantic Rainforest. Nat. Conserv. 2014, 12, 124–128. [Google Scholar] [CrossRef]
- Da Silva, C.J.; Sousa, K.N.S.; Ikeda-Castrillon, S.K.; Lopes, C.R.A.S.; da Silva Nunes, J.R.; Carniello, M.A.; Façanha, C.L. Biodiversity and its drivers and pressures of change in the wetlands of the Upper Paraguay–Guaporé Ecotone, Mato Grosso (Brazil). Land Use Policy 2015, 47, 163–178. [Google Scholar] [CrossRef]
- Leao, N.V.M.; Felipe, S.H.S.; Emidio-Silva, C.; dos Santos Moraes, A.C.; Shimizu, E.S.C.; Gallo, R.; Kato, O.R. Morphometric diversity between fruits and seeds of mahogany trees (‘Swietenia macrophylla’King.) from Parakana Indigenous Land, Para State, Brazil. Aust. J. Crop Sci. 2018, 12, 435. [Google Scholar] [CrossRef]
- Venable, D.L.; Lawlor, L. Delayed germination and dispersal in desert annuals: Escape in space and time. Oecologia 1980, 46, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S. Solos em Sistema Agroflorestal na Amazônia Meridional; Dissertação (Mestrado em Solos e Nutrição de Plantas)—Universidade Federal de Viçosa: Viçosa, Brazil, 2006. [Google Scholar]
- SEPLAN–MT, 2000h. Mapa de Solos do Projeto de Desenvolvimento Agroambiental do Estado De Mato Grosso—PRODEAGRO. In ZONEAMENTO SÓCIO-ECONÔMICO-ECOLÓGICO: Diagnóstico Sócio-Econômico-Ecológico do Estado de Mato Grosso e Assistência Técnica na Formulação Da 2ª Aproximação; Memória Técnica—Pedologia: Cuiabá/MT, Brazil, 2000. [Google Scholar]
- Ribeiro, J.F.; Walter, B.M.T. As principais fitofisionomias do bioma cerrado. In Cerrado: Ecologia e Flora; Sano, S.M., Almeida, S.P., Ribeiro, J.F., Brasília, B.R., Eds.; Embrapa-Cerrados: Brasília, Brazil, 2008. [Google Scholar]
- Pinto, J.R.R.; Oliveira-Filho, A.T. Perfil florístico e estrutura da comunidade arbórea de uma floresta de vale no Parque Nacional da Chapada dos Guimarães, Mato Grosso, Brasil. Rev. Bras. Bot. 1999, 22, 53–67. [Google Scholar] [CrossRef]
- Ab’Saber, A.N. Domínios morfoclimáticos e províncias fitogeográficas do Brasil. Orientação 1967, 3, 45–48. [Google Scholar]
- Shimizu, J.Y.; Kageyama, P.Y.; Higa, A.R. Procedimentos e Recomendações para Estudos de Progênies de Essências Florestais; EMBRAPA/ URPFCS: Curitiba, Brazil, 1982. [Google Scholar]
- Cruz, C.D.; Regazzi, A.J. Divergência genética. In Modelos Biométricos Aplicados ao Melhoramento Genético; Cruz, C.D., Regazzi, A.J., Viçosa, B.R., Eds.; da UFV: Abbotsford, British Columbia, 2014. [Google Scholar]
- Cruz, C.D. GENES—A software package for analysis in experimental statistics and quantitative genetics. Acta Sci. 2013, 35, 271–276. [Google Scholar]
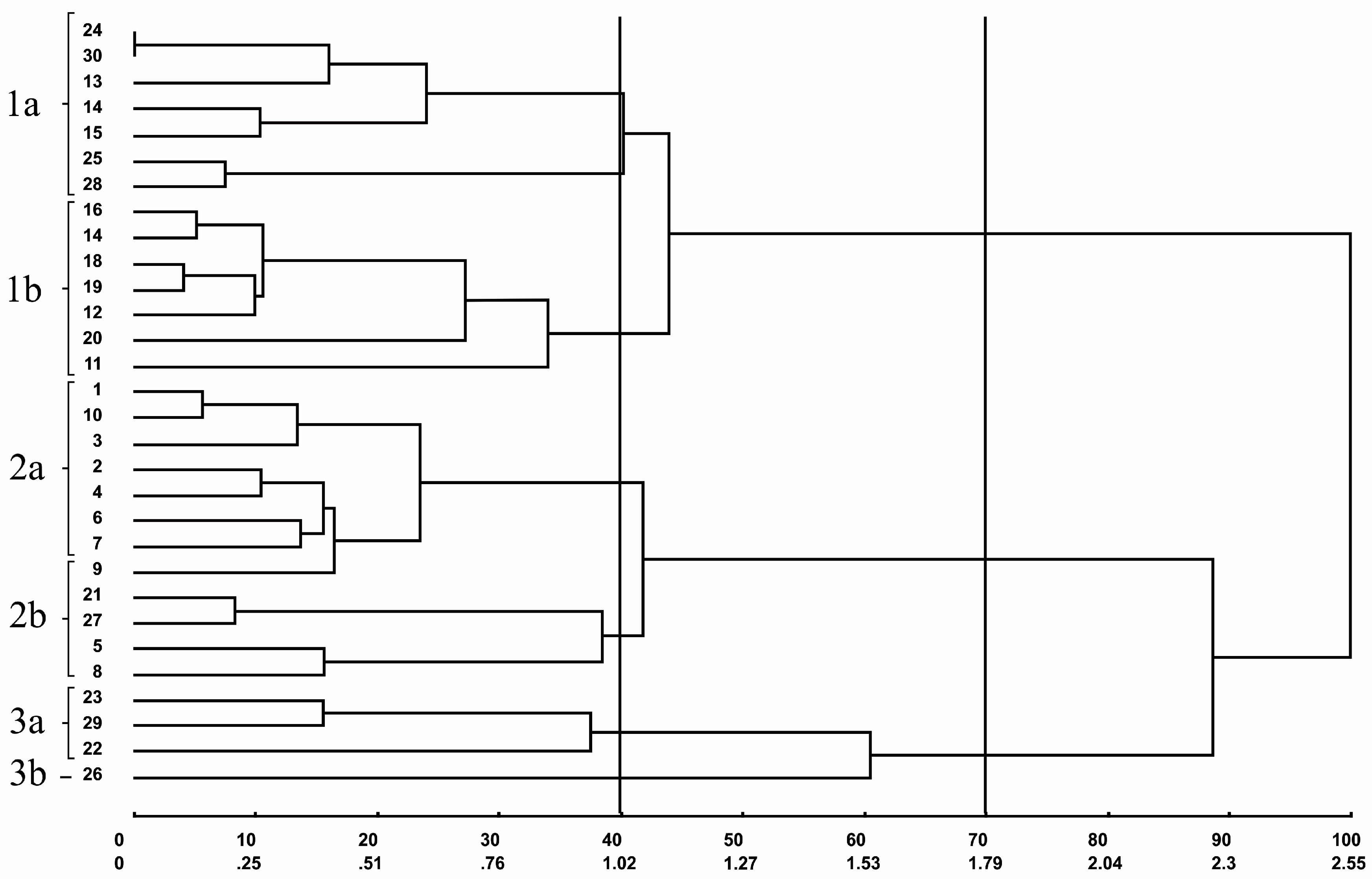

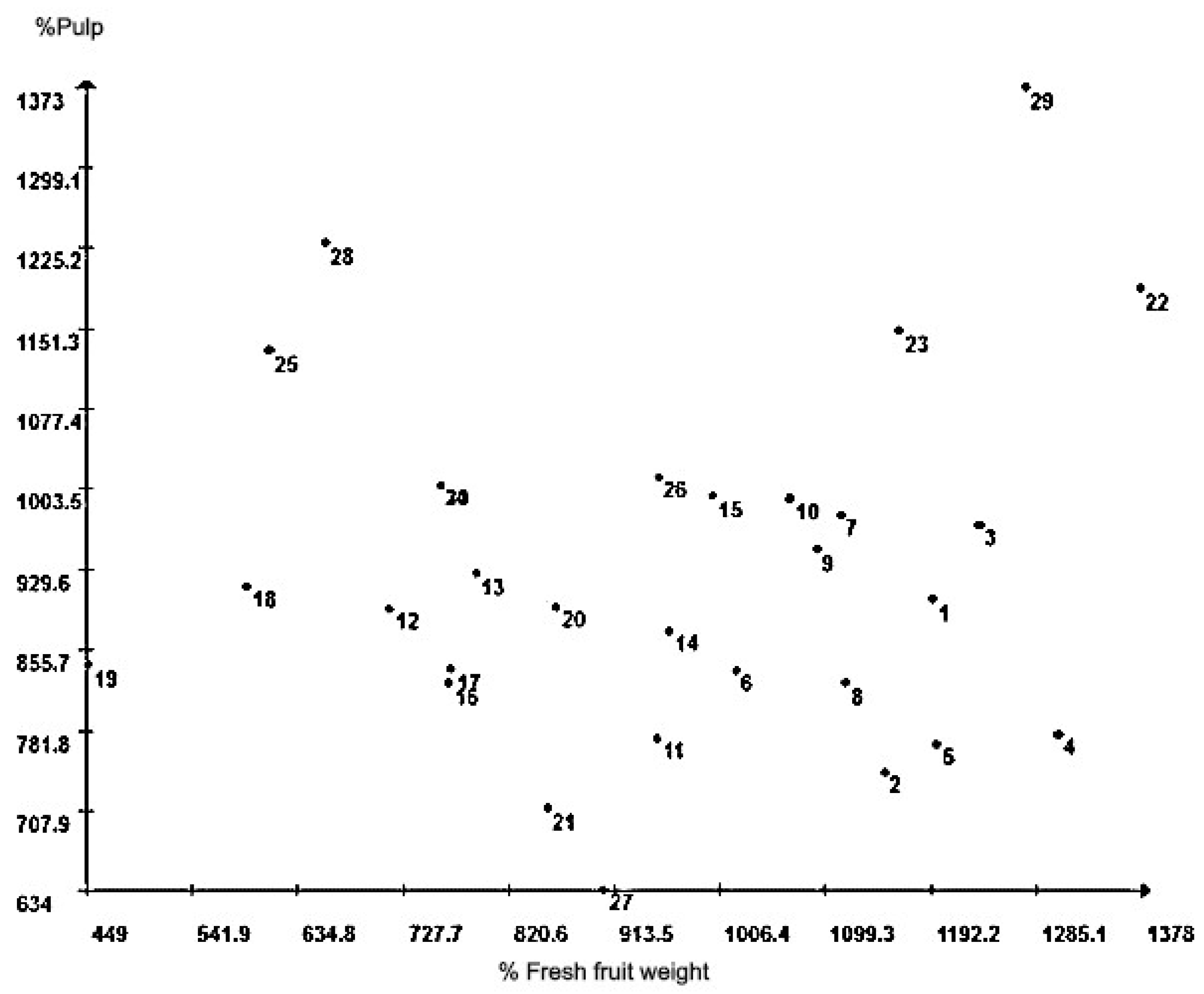

| Group | Individuals | (%) |
|---|---|---|
| I | 24, 30, 13, 25, 28, 12, 19, 16, 18, 17, 20 | 36.67 |
| II | 01, 10, 03, 02, 09, 04, 07, 06, 08 | 30.00 |
| III | 21, 27, 14, 15, 11 | 16.67 |
| IV | 23, 29, 22, 26 | 13.33 |
| V | 05 | 3.33 |
| Total | 100.00 |
| Trait | S.j’ | Contribution (%) |
|---|---|---|
| Fresh fruit weight (FFW) (g) | 12,1054.08 | 37.50 |
| Pulp rate (PR) | 57,663.50 | 17.86 |
| Fresh pulp weight (FPuW) (g) | 47,393.07 | 14.68 |
| Moisture rate (MC) | 38,449.07 | 11.91 |
| Fresh seed weight (FSW) (g) | 20,033.90 | 6.20 |
| Dry fruit weight (DFW) (g) | 17,810.58 | 5.51 |
| Dry seed weight (DSW) (g) | 6798.02 | 2.10 |
| Fresh peel weight (FPW) (g) | 4802.09 | 1.48 |
| Other fresh structures weight (OFS) (g) | 4708.57 | 1.45 |
| Dry peel weight (DPW) (g) | 1476.64 | 0.45 |
| Dry pulp weight (DPuW) (g) | 1248.68 | 0.38 |
| Other dry structures weight (ODS) (g) | 1078.60 | 0.33 |
| Fruit height (H) (mm) | 169.10 | 0.05 |
| Fruit diameter (D) (mm) | 80.78 | 0.02 |
| Total | 100.00 |
| Locality | Coord. X; Y | Rainfall (mm). | No. Dry Months | Altitude (m) | Temp. (min) | Temp. (max) |
|---|---|---|---|---|---|---|
| Chapada dos Guimarães | 15°23’1.05” S; 55°49’46.8” O | 1.300–1.400 | 6 | 600–800 | 18 | 30 |
| Vila Bela da Santíssima Trindade | 15°3’37.3” S; 59°54’18.5” O | 2.150 | 3–4 | 250 | 17 | 33 |
| Alta Floresta | 9°47’15.56” S; 56° 4’1.73” O | 2.000–2.300 | 4 | 200–300 | 18 | 32 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sander, N.L.; da Silva, C.J.; Duarte, A.V.M.; W. Zago, B.; Galbiati, C.; G. Viana, I.; de Arruda, J.C.; E. Dardengo, J.; P. Poletine, J.; H. Siqueira Leite, M.; et al. The Influence of Environmental Features on the Morphometric Variation in Mauritia flexuosa L.f. Fruits and Seeds. Plants 2020, 9, 1304. https://doi.org/10.3390/plants9101304
Sander NL, da Silva CJ, Duarte AVM, W. Zago B, Galbiati C, G. Viana I, de Arruda JC, E. Dardengo J, P. Poletine J, H. Siqueira Leite M, et al. The Influence of Environmental Features on the Morphometric Variation in Mauritia flexuosa L.f. Fruits and Seeds. Plants. 2020; 9(10):1304. https://doi.org/10.3390/plants9101304
Chicago/Turabian StyleSander, Nilo L., Carolina J. da Silva, Aline V. M. Duarte, Bruno W. Zago, Carla Galbiati, Iris G. Viana, Joari C. de Arruda, Juliana E. Dardengo, Juliana P. Poletine, Marcelo H. Siqueira Leite, and et al. 2020. "The Influence of Environmental Features on the Morphometric Variation in Mauritia flexuosa L.f. Fruits and Seeds" Plants 9, no. 10: 1304. https://doi.org/10.3390/plants9101304
APA StyleSander, N. L., da Silva, C. J., Duarte, A. V. M., W. Zago, B., Galbiati, C., G. Viana, I., de Arruda, J. C., E. Dardengo, J., P. Poletine, J., H. Siqueira Leite, M., de Souza, M. H. S., de Oliveira, R. F., S. Guimarães, T., P. da Silva, V., & A. A. Barelli, M. (2020). The Influence of Environmental Features on the Morphometric Variation in Mauritia flexuosa L.f. Fruits and Seeds. Plants, 9(10), 1304. https://doi.org/10.3390/plants9101304





